Abstract
Jak3, one of the four members comprising the Jak family of cytosolic tyrosine kinases, has emerged as a promising target for nontoxic immunotherapies. Although a number of Jak inhibitors has already demonstrated efficacy, they suffer from secondary effects apparently associated to their pan-Jak activity. However, whether selective Jak3 inhibition would afford therapeutic efficacy remains unclear. To address this question we have investigated the immunosuppressive potential of selective Jak3 intervention in lymphocytes using RNA interference (RNAi) technology in vitro and in vivo. Using synthetic small interference RNA (siRNA) sequences we achieved successful transfections into human and mouse primary T lymphocytes. We found that Jak3 knockdown was sufficient to impair not only interleukin-2 (IL-2) and T cell receptor (TCR)-mediated cell activation in vitro, but also antigen-triggereds welling, inflammatory cell infiltration, and proinflammatory cytokine raise in vivo. Furthermore, Jak1 (which mediates γc cytokine signaling in conjunction with Jak3) cosilencing did not provide higher potency to the aforementioned immunosuppressant effects. Our data provides direct evidences indicating that Jak3 protein plays an important role in γc cytokine and antigen-mediated T cell activation and modulates Th1-mediated inflammatory disorders, all in all highlighting its potential as a target in immunosuppressive therapies.
Keywords: cytokines, inflammation, RNA interference, T-lymphocyte, therapy
Introduction
The main drawback of current immunosuppressive therapies is their pleiotropic side-effects due to the ubiquitous distribution and involvement in multiple functions of their molecular targets. A plausible alternative seems to be the Jak family of cytoplasmatic protein kinases (Jak1, Jak2, Jak3, and Tyk2) that associate with type I and type II cytokine receptors. Among them, two essential characteristics make Jak3 particularly appealing: (i) Jak3 is predominantly expressed in hematopoietic linage, while Jak1, Jak2, and Tyk2 are ubiquitously expressed, and (ii) Jak3 specifically binds the γ common subunit (γc) cytokine receptor and it is uniquely activated by γc family cytokines [interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, IL-21], unlike the other family members which bind multiple cytokine receptors.1,2,3
The importance of Jak3 in lymphoid system development is highlighted in gene targeted mice and in humans with primary immunodeficiency: Jak3 knockout mice and Jak3-SCID human patients suffer from a profound immunodeficiency.4,5,6 Furthermore, increased Jak3 expression has been associated with immune system disorders such as rheumatoid arthritis in animal models and human patients.7,8,9
Jak inhibitors currently under clinical development have demonstrated efficacy in the treatment of rheumatoid arthritis, allograft rejection and psoriasis.10,11,12 Nevertheless safety concerns arise from their lack of selectivity versus other kinases13 and Jak family members.14,15 A pan-Jak inhibition could lead to undesirable side-effects because of the roles of Jak1 in innate immunity Jak2 in erythropoiesis: systemic inhibition of Jak1 would lead to increased susceptibility to viral and bacterial pathogens, whereas Jak2 systemic inhibition would lead to anemia, thrombocytopenia, and leukopenia.16,17 Indeed, clinical studies have revealed increased incidence of infections, mild reduction in hemoglobin levels and neutropenia most likely due to the pan-Jak activity of inhibitors tested.11,12,18
All γc cytokines exert their effect through the Jak1-Jak3 tandem, whose relative contribution to the signaling pathway is not fully understood. In this regard, whether a putative selective Jak3 inhibition would disrupt γc cytokine signaling and, if so, would it afford immunosuppressive activity, remains controversial. On the one hand, a virtually Jak3-selective compound has reported efficacy in mouse and rat arthritis models.9,19 However, the scope of the results obtained with small molecules is limited by their unavoidable lack of selectivity. On the other hand, data from reconstituted systems in vitro has suggested that Jak1 plays a dominant role in the γc cytokine signal transduction.20 Nevertheless, the consequences of an acute-specific intervention on endogenous Jak over γc cytokine signaling and T cell activation have not been investigated to date.
Here, we sought to discriminate the direct implications of individual Jak enzymes involved in γc cytokine (i.e., IL-2) signaling and T cell activation, as well as the immunosuppressive potential of Jak3 downregulation in vivo. We have used RNA interference (RNAi) technology to knockdown Jak1 and Jak3 in primary T lymphocytes in vitro and studied the responsiveness of these cells to several activation stimuli. Furthermore, we have tested whether silencing of Jak3 in T cells would provide an anti-inflammatory benefit in vivo.
The main challenge of RNAi applications is its successful delivery into the cytoplasm of target tissues or cells,21 which is particularly convoluted in lymphoid lineage.22 Furthermore, RNAi machinery itself works inefficiently in T lymphocytes, as compared with other cell types.23 Due to the aforementioned problems with small interference RNA (siRNA) delivery and functionality, studies involving target silencing in primary T lymphocytes have been seldom reported.
In the present study, we have used synthetic, chemically modified siRNA sequences to successfully transduce human and murine T lymphocytes without associated toxicity. We have demonstrated that specific Jak3 knockdown in primary T lymphocytes is sufficient to impair not only the Jak-Stat pathway, but also TCR activation and its downstream consequences. Most importantly, Jak3 knockdown in Th1-polarized effector cells prevented delayed type hypersensibility (DTH) reaction in vivo, highlighting the importance of Jak3 in Th1-driven inflammatory process. Furthermore, a dual Jak1–Jak3 intervention did not contribute to a deeper effect over the described phenotype. Altogether, the data presented in this manuscript supports the advisability of Jak3-selective inhibition as immunomodulatory strategy in pathologies with a strong Th1 component, such as rheumatoid arthritis.
Results
siRNA delivery and target silencing in human primary T lymphocytes
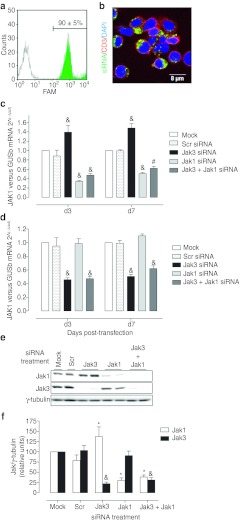
To overcome the difficulties associated with lymphoid cell transfection, we have used the synthetic, chemically modified ACCELL siRNA sequences that allow for a vector-free passive transfection. Our experimental conditions reported an exceptionally efficient (90 ± 5%, n = 4) and homogeneous siRNA uptake in primary human T lymphocytes (Figure 1a). Confocal analysis confirmed efficient internalization and cytoplasmic localization of the siRNA sequences, as indicated by green fluorescence localization (corresponding to FAM-Scr-siRNA) between plasmatic membrane (red) and nuclei (blue) markers (Figure 1b).
Figure 1.
Efficient small interference RNA (siRNA) transfection and target gene knockdown in human primary T lymphocytes. (a) Primary human T lymphocytes were incubated with 1 µmol/l Scrambled siRNA fluorescently labeled with FAM (FAM-Scr-siRNA) for 3 days. Fluorescence incorporation in Mock (empty histogram) and FAM-Scr-siRNA transfected T cells (green histogram) was assessed by flow cytometry. A representative histogram of four independent experiments is shown. (b) Cytoplasmatic localization of FAM-Scr-siRNA (green) sequence was confirmed by confocal microscopy. Cy3-CD3 (red) mAb was used as membrane marker. Nuclei were labeled with DRAQ5 (blue). A representative view of three independent experiments is shown. (c) JAK1 and (d) JAK3 mRNA content at day 3 (d3) and 7 (d7) post-transfection with 1 µmol/l indicated siRNA sequence was assessed by quantitative reverse transcription-PCR (RT-PCR). GUSb was used as housekeeping gene. Results are expressed as mean ± SEM relative to Mock; n = 3; &P < 0.001 versus Scr. (e) Western blot from protein extracts obtained on d3. A representative blot is shown. (f) Blots were quantified by subsequent densitometry. Results are expressed as mean ± SEM; n = 6–10; *P < 0.05; #P < 0.01; &P < 0.001 versus Scr. Two sequences (described in Materials and Methods section) were tested for each target, which performed equally (data not shown). Results are expressed as the mean values of the two sequences.
Next, knockdown efficiency against Jak1 and Jak3 in primary human T lymphocytes was tested. Dose response experiments were performed with Jak1 and Jak3 siRNA sequences and we established 1 µmol/l as the dose reporting maximal silencing with no toxicity (data not shown). Under these conditions, a consistent and stable target mRNA knockdown was observed up to 10 days after transfection. JAK1 mRNA was reduced to 34 ± 3% and 51 ± 3%, while JAK3 mRNA was reduced to 43 ± 3% and 51 ± 0% on day 3 and 7 post-transfection respectively, as compared to the control Mock group (Figure 1c,d). These results demonstrate good specificity, functionality and stability of silencing provided by ACELL siRNA sequences. Accordingly, a sequence-specific knockdown was noteworthy at the protein level by western blot (Figure 1e). Densitometry revealed a 30 ± 6% and 22 ± 4% Jak1 and Jak3 residual protein content, respectively (Figure 1f). Surprisingly, Jak1 mRNA and protein content was slightly increased in response to Jak3 knockdown (Figure 1c,e,f).
Jak3 is essential for the IL-2-mediated Jak-Stat signaling pathway
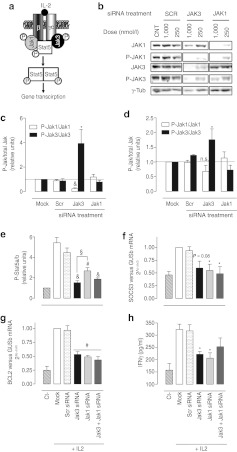
To elucidate whether an acute Jak3 knockdown is sufficient to impair the γc cytokine-mediated Jak-Stat pathway (represented in Figure 2a), the steady-state phosphorylation status of Jak1, Jak3, and Stat5a/b was analyzed. A high (1,000 nmol/l; Figure 2b,c) and mild (250 nmol/l; Figure 2b,d) siRNA concentration treatments were tested to ascertain the trend in phosphorylation ratios. We found that Jak1 knockdown was accompanied by a concomitant reduction of phospho-Jak1, whereas phospho-Jak3 was not significantly decreased, resulting in sustained phospho-Jak1 versus total Jak1 and phospho-Jak3 versus total Jak3 ratio. Conversely, after Jak3 silencing, both Jak3 and Jak1 phosphorylated forms dropped. When normalized by total amount of Jak3 protein, it was evidenced that Jak3 knockdown markedly repressed Jak1 phosphorylation, while residual Jak3 became highly phosphorylated, resulting in a significant reduction of the P-Jak1/Jak1 ratio and a striking increase in P-Jak3/Jak3 ratio (Figure 2b,c,d). Taken together, these results suggest that Jak3 phosphorylation is a first step in the IL-2-induced trans-phosphorylation cascade. Furthermore, Stat5a/b phosphorylation was impaired after either Jak1 or Jak3 knockdown (Figure 2e). Jak3 silencing almost completely prevented IL-2-induced Stat5a/b phosphorylation; while in Jak1 silenced cells a remainder Stat5a/b phosphorylation over basal levels (Ct) was evident, suggestive of a prominent role of Jak3 in IL-2 signal transduction. Additionally, Jak1-Jak3 cosilencing did not potentiate the effect of Jak3 silencing in terms of Stat5a/b phosphorylation. Equivalent results regarding Jak and Stat5a/b phosphorylation were obtained with partial Jaks silencing (circa 50%) achieved after 250 nmol/l siRNA treatments (data not shown). These results indicate that both kinases are necessary for effective IL-2 signal transduction and there is not an additive effect in the cytokine signaling after dual Jak1 and Jak3 intervention.
Figure 2.
Single Jak depletion compromises the Jak-Stat signaling pathway. Human primary T lymphocytes were cultured in the absence (Ct) or presence of interleukin-2 (IL-2) stimulus during either Mock, Scrambled (Scr), Jak3, Jak1, or Jak1+Jak3 small interference RNA (siRNA) treatments at 1 µmol/l or 250 nmol/l. After 3 days, IL-2 signal transduction was evaluated. (a) Scheme of γc cytokine-mediated Jak-Stat pathway. (b) Jak1-P Tyr1022–1023 and Jak3-P Tyr980–981 were analyzed by western blot. Representative blot of six independent experiments is shown. (c) Densitometric analysis of blots obtained from cells treated with 1 µmol/l siRNA; (n = 6). (d) Densitometric analysis of blots obtained from cells treated with 250 nmol/l siRNA; (n = 4). Data are expressed as phospho-Jak/total Jak ratio relative to Mock treatment. (e) Stat5a/b phosphorylation was analyzed using the Luminex xMAP technology (n = 4). Data are expressed as phospho-Stat5a/b relative to nonstimulated control treatment (Ct). In addition, the expression of downstream targets genes as (f) suppressor of cytokine signaling 3 (SOCS3) (n = 4) and (g) B-cell leukemia/lymphoma 2 (BCL2) (n = 4) was examined. Gene expression was quantified by quantitative reverse transcription-PCR (RT-PCR). As housekeeping gene β-glucoronidase (GUSb) was used. Each value represents the mean relative amount of mRNA with respect to Mock treatment. (h) IL-2 induced interferon-γ (IFNγ) secretion was evaluated as IFNγ on the culture supernatant determined by enzyme-linked immunosorbent assay (ELISA) (n = 4). Data are expressed as mean ± SEM; *P < 0.05; #P < 0.01; &P < 0.001 versus Scr; §P < 0.05 versus Jak3 siRNA.
As a result of Jak-Stat pathway blockade, expression of the downstream target genes suppressor of cytokine signaling 3 (SOCS3) (Figure 2f) and B-cell leukemia/lymphoma 2 (BCL2) (Figure 2g) were similarly reduced close to basal levels (no IL-2 stimulus) after either Jak1 and/or Jak3 knockdown, with no additive effect upon dual silencing. Accordingly, an end point product of the pathway, namely IL-2-induced interferon-γ (IFNγ) secretion, was reduced to a 47 ± 6% and 41 ± 11% after Jak3 and Jak1 silencing, respectively (Figure 2h), confirming that a single Jak knockdown is enough to disrupt the Jak-Stat pathway.
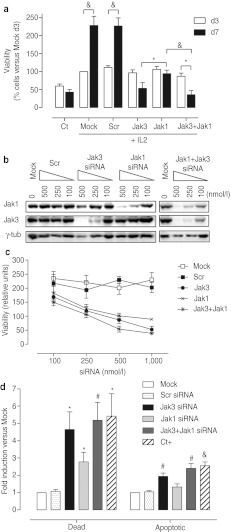
To further evaluate the functional significance of Jak1 and/or Jak3 knockdown, cell viability was assessed on day three (d3) and day seven (d7) after transfection as an indicator of IL-2-dependent T-cell proliferation. As expected, in absence of IL-2 stimulus (Ct) there was no increment in cell viability at d7 versus d3. We found no signs of altered cell viability between different treatments on d3, whereas on d7 a striking increment in viable cell number was evident in control groups (Mock and Scr), indicative of IL-2-induced cell proliferation. Conversely, on d7 cell viability was stagnated or even declined in Jak3 and/or Jak1 siRNA-treated groups, suggesting a blockade of IL-2-induced cell proliferation and/or cell death induction (Figure 3a).It is remarkable that, albeit equivalent target knockdown efficiency, Jak3 silencing had a deeper impact in proliferation than Jak1 silencing. Furthermore, we did not observe any additive effect over cell viability after dual compared with single Jak3 silencing, but we did so when comparing dual versus single Jak1 silencing. To test whether the role of Jak1 in IL-2 signaling could be emphasized with a mild Jak3 inhibition, dose-response siRNA treatment was performed to obtain partial Jak knockdown (Figure 3b). No additive effect over cell viability was found at any dose tested (Figure 3c), supporting the idea that Jak3 plays a prominent role in IL-2-induced cell proliferation.
Figure 3.
Jak3 and Jak1 are essential to maintain cell viability in interleukin-2 (IL-2) activated T lymphocytes. Human primary T lymphocytes were cultured in absence (Ct) or presence of IL-2 stimulus during either Mock or 1 µmol/l Scrambled (Scr), Jak3, Jak1, or Jak1+Jak3 small interference RNA (siRNA) treatments. (a) Cell viability was measured on day three (d3) and seven (d7) after siRNA incubation. Data are represented as mean ± SEM % viability versus control Mock group on d3. n = 8; *P < 0.05 and &P < 0.001. (b) Dose-response silencing in primary human T lymphocytes. Cells were treated with 500, 250, and 100 nmol/l indicated siRNA sequences. On d3 protein extracts were obtained and analyzed by western blot. A representative blot from at least three independent experiments is shown. (c) Cell viability was measured on d7 after 1,000 nmol/l (n = 8), 500 nmol/l (n = 5), 250 nmol/l (n = 8), or 100 nmol/l (n = 7) indicated siRNA sequences treatment. Data are expressed as mean ± SEM relative to control Mock group on d3. (d) Dead and/or apoptotic cell population secondary to Jak knockdown was assessed on d7 by cytometry using Alexa Fluor488-Annexin V and propidium iodide (PI) double staining. Death (AnnV+/PI+) and apoptotic (Annexin V+/PI) populations were quantified. As positive control for apoptotic stimulus (Ct+), cells were incubated with 1.5 µmol/l camptothecin for 24 hours. Data are expressed as mean ± SEM relative to Mock control group. n = 4; *P < 0.05; #P < 0.01; &P < 0.001 versus Scr.
In order to dissect the mechanism responsible for the decreased cell viability, apoptosis was measured by flow cytometry. In agreement with viability data, siRNA treatments did not induce cell death or apoptosis on d3 (data not shown), whereas on d7 we did observe apoptosis and cell death induction in Jak1 and/or Jak3 siRNA-treated cells (Figure 3d). Accordingly, reduced expression of the anti-apoptotic gene BCL2 was found in these groups (Figure 2e). Interestingly, Jak3 knockdown resulted in higher cell death and apoptosis induction compared with Jak1 knockdown, while dual intervention resembled Jak3 knockdown phenotype.
Altogether, these results indicate that both enzymes are required for a complete γc cytokine signal transduction in T cells and that a single intervention is sufficient to impair downstream effects like IFNγ secretion and cell proliferation, resulting in apoptosis and cell death.
Jak3 and Jak1 are involved in early TCR activation
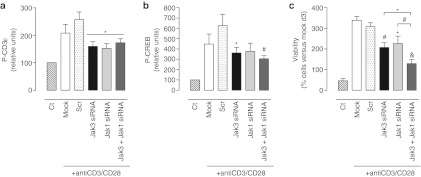
To understand further the impact of Jak3 knockdown in lymphocyte activation, the putative relationship between Jak3 and TCR, whose interaction is controversial,24,25,26 was analyzed. Jak3, as well as Jak1 and dual knockdown impaired early TCR signaling, as indicated by a slightly reduced phosphorylation of CD3ε-chain and CREB after CD3-CD28 mAb challenge (Figure 4a, b). Furthermore, homotypical cell clustering and proliferation, ultimate phenotypical hallmarks of TCR activation, were accordingly altered: CD3-CD28 mAb-activated T cells exhibited prominent cell clusters, while single Jak3, Jak1, or dual siRNA treatments almost completely prevented clustering (data not shown) and diminished cell proliferation (Figure 4c).
Figure 4.
Jak1 and Jak3 targeting equally impairs early T cell receptor (TCR) signaling events and cell activation in vitro. On day 3 after either Mock (empty bars) or 1 µmol/l Scrambled (dotted bars), Jak3 (black bars), Jak1 (light gray bars), or Jak3 +Jak1 (dark gray bars) small interference RNA (siRNA) treatments, human T lymphocytes were stimulated with CD3-CD28 mAb for 10 minutes. (a) Phospho-CD3ε and (b) phospho-CREB were detected using the Luminex xMAP system. Data are expressed as mean ± SEM relative phosphorylation versus non-CD3-CD28-stimulated control (Ct). n = 4, *P < 0.05; #P < 0.01 versus Scr. (c) Cellular viability was assessed before and 72 hours after CD3-CD28 challenge. Data are expressed as mean ± SEM relative to control Mock group before stimulus. n = 3, *P < 0.05; #P < 0.01; &P < 0.001 versus Scr.
Overall, these results suggest that not only Jak3, but also Jak1 play a dual role in the integration of TCR and γc cytokine-mediated T cell activation. These results prompted us to further investigate whether Jak3 intervention could be used to interfere with T cell activation and antigen-driven inflammation in vivo.
siRNA delivery and Jak silencing in murine T cells
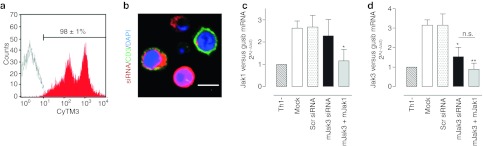
The ex vivo transfection of murine Th1 effector cells with ACCELL siRNA sequences was optimized in order to obtain Jak-silenced Th1 cells to be used later in our adoptive cell transfer model (described in Materials and Methods section). We established optimal transfection conditions that report good cellular viability (73 ± 3%, n = 4, measured as FSC-Height-SSC-Height, data not shown) and optimal transfection rates (98 ± 1%, n = 4). An heterogeneous siRNA uptake and cytoplasmatic localization was evidenced by the double-peak histogram (Figure 5a) and confocal images (Figure 5b). Interestingly, as seen in confocal sections, the low-range fluorescence peak corresponds to a CD3+ population. This may be due to the lower rates of siRNA uptake characteristic of murine T lymphocytes, as compared with the other cell types (i.e., dendritic cells (DC) and macrophages) present in the splenocyte:lymph node suspension.
Figure 5.
Murine lymphocytes small interference RNA (siRNA) transfection setup and endogenous target silencing. (a) BALB/c Th1 cells were incubated in the absence (empty histogram) or presence of 1 µmol/l Scrambled ACCELL siRNA fluorescently labeled with CyTM3 (red histogram) for 3 days. On the third day, fluorescence incorporation was assessed by flow cytometry. A representative histogram of four independent experiments is shown. (b) Cytoplasmatic localization of CyTM3-labeled scrambled siRNA sequences (red) was confirmed by confocal microscopy in the same cells. FITC-CD3 mAb (green) was used as a T cell membrane marker. Nuclei were labeled with DRAQ5 (blue). A representative view of three independent experiments is shown. For silencing efficiency experiments, cells were left unstimulated (Th1-), or stimulated with Th1 cytokines during incubations in absence (Mock) or presence of 1 µmol/l Scrambled (Scr), mJak3 ormJak1 siRNA sequences. On the third day (c) jak1 and (d) jak3 mRNA levels were assessed by quantitative reverse transcription-PCR (RT-PCR). gusb was used as housekeeping gene. Data are expressed as mean ± SEM relative to nonstimulated cells (Th1-); n = 5; *P < 0.05 versus Scr; n.s.; nonsignificative; one-tailed Student's t-test.
Furthermore, a satisfactory silencing efficiency was obtained. After sequence-specific siRNA treatment against mouse Jak1 (mJak1 siRNA) and mouse Jak3 (mJak3 siRNA), Th1 cytokine-induced increase in jak1 and jak3 mRNA levels was attenuated close to the baseline, non-Th1-stimulated conditions (Th1-). As compared to nontargeting siRNA treatment (Scr), jak1 (Figure 5c) and jak3 (Figure 5d) mRNAs were reduced in a 42 ± 6% and 49 ± 1% (n = 5), respectively.
Specific Jak3 targeting prevents Th1-mediated inflammation in vivo
Rheumatoid arthritis is an inflammatory disorder with a strong Th1 component. To test the potency of selective Jak3 downregulation in preventing inflammatory process in vivo we used a DTH model elicited by ex vivo-generated OVA323–339-activated Th1 effector cells from DO11.10 TCR transgenic mice (OVA-TCRtg Th1 cells). After adoptive transfer of OVA-TCRtg Th1 cells into naive wild type recipient, a single OVA323–339 challenge induce a traceable local inflammation with a strong Th1 component which is mediated by the transferred cells.27,28,29
Transfection of the OVA-TCRtg Th1 cells with mJak1 and mJak3 siRNA sequences resulted in a 59 ± 2% and 48 ± 2% (n = 4, P < 0.01) jak1 and jak3 sequence specific mRNA drop, respectively, as compared to control, nontargeting siRNA transfected cells (Scr). In correlation, densitometric analysis of western blots revealed a Jak1 and Jak3 protein content fall to 58 ± 8% (n = 5, P < 0.01) and 50 ± 4% (n = 5, P < 0.01), respectively (data not shown).
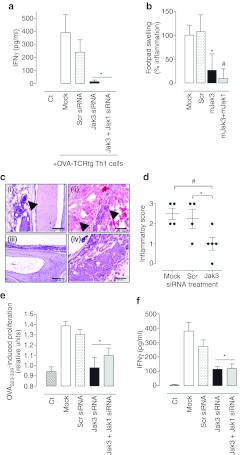
Consistently with Th1 inflammatory processes, OVA323–339 challenge elicited a dramatic IFNγ rise in mice adoptively transferred with Mock and Scr-treated OVA-TCRtg Th1 cells, but not in nonadoptively transferred mice (Ct). As expected, IL-4 levels remained unaltered (data not shown). Concomitant with plasmatic IFNγ levels, swelling was evident in OVA323 339 challenged footpad from Mock- and ScrsiRNA-treated groups. Importantly, Jak3 knockdown rendered resistance to antigen-triggered plasmatic IFNγ rise (Figure 6a) and showed a substantially reduced footpad swelling (Figure 6b). It is noteworthy to point out that dual mJak3-mJak1 siRNA treatments did not provide any additional anti-inflammatory effect, as compared to single Jak3 silencing (Figure 6a,b).
Figure 6.
Specific Jak3 knockdown alleviates inflammation in vivo. OVA-TCRtg stimulated Th1 cells were Mock, Scrambled small interference RNA (siRNA), mJak3 siRNA or mJak3+mJak1 siRNA transfected at 1 µmol/l each as stated in Materials and Methods section. After 72 hours, 5 × 106 cells were adoptively transferred into naive syngeneic recipient mice. Twenty four hours after adoptive cell transfer, mice were challenged by s.c. injection of 5 µg OVA323–339/incomplete Freund's adjuvant (IFA) emulsion in the right footpad. As a control, nonadoptively transferred naive mice were used (Ct). (a) Plasmatic interferon-γ (IFNγ) levels 24 hours after OVA323–339 challenge were determined by enzyme-linked immunosorbent assay (ELISA). Data are mean ± SEM; n = 6; *P < 0.05 versus Scr. (b) Footpad swelling was measured 24 hours after challenge. Results were defined as % inflammation relative to the background in 0% control (Ct) and the 100% control (Mock), respectively. Data are mean ± SEM of 12 animals analyzed in two independent experiments. *P < 0.05; #P < 0.01 versus Scr. (c) Cell migration in the delayed type hypersensibility (DTH) test site was evaluated in hematoxylin-eosin stained sections from Mock (panels i), Scr (panel ii), and Jak3 groups (panels iii and iv). Representative images of five samples analyzed per group are shown. Arrows point to cellular infiltrate indicative of inflammation. Size bars represent 200 µm. (d) Histological score for inflamed footpads was awarded as stated in Material and Methods. n = 4–5; *P < 0.05, #P < 0.01, one tailed Student's t-test versus Scr. Finally, 24 hours after in vivo OVA323–339 challenge, draining lymph node cells were collected and rechallenged in vitro with 10 µg/ml OVA323–339 further 48 hours. At this point, proliferation and IFNγ secretion were evaluated. (e) Proliferation response was estimated as relative cell viability versus nonchallenged cells (0 µg/ml OVA323–339) in each group. (f) Supernatant IFNγ was measured by enzyme-linked immunosorbent assay (ELISA). Data are mean ± SEM of 4–7 measurements from two independent experiments. *P < 0.05 versus Scr. TCR, T cell receptor.
DTH reaction is characterized by a strong cellular infiltrate. Microscopic analysis of the footpads correlated with inflammation severity. In line with cytokine profile and footpad swelling, substantial inflammatory cell migration into subcutaneous adipose and muscular tissue was observed in footpads of Mock and Scr-treated mice in response to OVA323–339 challenge (Figure 6c; panels (i) and (ii)). In contrast, overall inflammatory infiltration and tissue damage at the antigen-challenged site was significantly ameliorated in the mJak3 knocked-down group, with only mild subcutaneous infiltrate, or even complete absence of inflammation (Figure 6c; panel (iii) and (iv)). Inflammatory histological scores of evaluated sections are shown in Figure 6d. These data, in conjunction with macroscopic observations and plasmatic cytokine profile, strongly advocate a crucial role of Jak3 in Th1 inflammatory response.
To further analyze antigen-specific response at a cellular level, draining lymph nodes were isolated and restimulated in vitro with OVA323–339. As expected, lymphoid cells from naive mice (Ct, not adoptively transferred with Th1 effector cells) did not responded to in vitro OVA323–339 exposure in terms of proliferation and IFNγ secretion (Figure 6e,f). Cells from mice that had been adoptively transferred with control OVA-TCRtg Th1 cells (Mock and ScrsiRNA-treated) were capable of proliferating and secreting IFNγ after in vitro OVA323–339 re-challenge. In contrast, Jak-silenced groups poorly proliferated and secreted lower IFNγ levels, similarly to lymphoid cells from naive, nonadoptively transferred mice (Figure 6e,f). Together, these data suggest that T cells are unresponsive to antigen activation in the absence of Jak3 activity.
Overall, our results strongly reinforce the concept that Jak3-specific intervention could provide therapeutic benefits in immunomodulatory therapies and that dual Jak1-Jak3 intervention would not account for a synergistic effect in Th1-mediated inflammatory disorders.
Discussion
Herein, we describe reliable and selective Jak3 knockdown in lymphocytes using RNAi technology and investigate its direct implications in IL-2-mediated signaling and T cell activation in vitro and in antigen-driven inflammation in vivo.
Since its discovery,30 RNAi technology has rapidly emerged, not only as a new strategy for target validation and functional genomics, but also as a promising therapeutic approach for otherwise “undruggable” targets (i.e., molecules without ligand binding domains or those that have structural homology with other family members or critical proteins in the cell).31 However, applied to the lymphoid lineage, there are still several technical limitations namely siRNA delivery, RNAi pathway functionality and off-target effects.21,23,32,33,34
To tackle these challenges, in this study we have used ACCELL siRNA sequences, that include chemical modifications to improve stability and target specificity, avoid off-target effects and allow vector-free delivery efficiency into difficult-to transfect cells. We have achieved an almost absolute siRNA delivery in human and murine primary T lymphocytes. Furthermore, specific and stable target knockdown (Jak1 and Jak3) was observed at the RNA and protein level. Interestingly, Jak1 mRNA and protein content slightly increased in response to Jak3 knockdown, likely due to the loss of negative regulation exerted by SOCS3 preferentially over Jak1, rather than Jak3.35,36 However, the consequences of the little Jak1 upregulation observed could be diverse as long as Jak1 is associated with cytokine receptors other than γc in which Jak3 is not associated: on the one hand Jak1 associates with IFNα and IFNγ receptors. These cytokines are involved in innate immune response; therefore maintaining a Jak1 residual activity may be positive in order to be able to respond against pathogens. This might be crucial to prevent the susceptible to pathogens seen in clinical trials testing pan-Jak inhibitors.11,12,18 On the other, Jak1 also associates with IL-6 cytokine receptor. IL-6 is one of the most abundant proinflammatory cytokines found in both the joint and blood of patients with active rheumatoid arthritis;37 but whether this would translate on an exacerbated IL-6 signal transduction is not clear. The effect of an increase of Jak1 in this scenario is uncertain and would need to be further evaluated in a rheumatoid arthritis model.
Importantly, no signs of toxicity (cell death or apoptosis) intrinsic to the transfection method or siRNA sequences was observed at any experimental time, in contrast to some recent studies that have reported activation of the innate immune system due to so called immunostimulatory RNA.34 The secretion of markers like IFNγ and IL-6 secretion,38 as well as 2′-5′ oligoadenylate synthetase 2 (Oas2) and suppressor of cytokine signaling 1 (SOCS1) mRNA levels33,39 was unaltered after siRNA treatments (data not shown), dismissing both sequence-dependent and independent immunostimulatory RNA -trigger off-target effects.
In summary, these results are a significant step forward to efficient and nontoxic siRNA transfection experiments in lymphoid cells, opening new avenues into target validation studies in leukocyte-implicated diseases. Current pharmacological approaches for autoimmune disorders are focusing on small molecule inhibitors for molecular targets involved in either T cell or B cell activation,40 including Jak3, that has positioned itself as one of the most desirable targets.2,4,5,7,8
The clinical efficacy of Jak inhibition has been recently proven in clinical trials for rheumatoid arthritis and kidney allograft rejection.10,11,12 However, these studies revealed secondary side-effects (anemia, neutropenia, dyslipidemia) most likely due topan-Jak inhibition. Although the selectivity pattern of Jak inhibitors in vitro has been recently improved without harming the in vivo activity in mouse models,9,19 these data should be carefully interpreted because (i) the specificity profile can vary considerably depending on the assay format used to gauge selectivity, and (ii) the interpretation of the in vivo efficacy of compounds with different selectivity profile is biased by their different kinetic properties. In this scenario, it remains unanswered whether an acute Jak3-specific inhibition is sufficient to disrupt γc cytokine signaling and, if so, could it result in efficacious amelioration of immune-related disorders?
In our in vitro experiments we have found that a single (either Jak1 or Jak3) intervention was enough to disrupt IL-2 signal transduction (i.e., Stat 5a/b phosphorylation) and downstream effects (i.e., target genes transcription, IFNγ secretion and proliferation) in primary T lymphocytes. There are several implications on these results: (i) Jaks have obligatory and nonredundant roles in cytokine-mediated responses, as stated in knockout strains.4,17,26 (ii) Since Jak3 pair Jak1 in all γc receptor-mediated signaling pathways, Jak3 inhibition may interfere not only with IL-2 but also with all γc cytokine signaling, some of which play an important role in the pathogenesis of autoimmune diseases.37 (iii) Jak1 inhibition would also interfere with other cytokine-mediated pathways, such as IL-6 family (whose relevance in autoimmunity has been demonstrated37) and the type II receptors such as the IFNs and the IL-10 family.1,2,3 This represents a double-edged sword: while inhibiting Jak family more broadly might potentially provide stronger efficacy since different cytokine signaling pathways will be blocked, the adverse effects of a systemic multi-cytokine inhibition should be carefully evaluated.
Furthermore, we have found evidence suggesting that, in the IL-2 signaling cascade, there is a hierarchy led by Jak3. Firstly, Jak3 knockdown abrogates Jak1 phosphorylation in response to IL-2 while residual Jak3 is hyperactivated (probably due to signaling cut-off), but not vice-versa. These results advocate an upstream function of Jak3 in a trans-phosphorylation phenomenon triggered by IL-2, and suggest that Jak1 may be a substrate of Jak3. These observations agree with those made by others in different cell lines or heterologous systems.41,42 Secondly, in spite of equivalent Jak1 and Jak3 knockdown efficiency, Jak3 abrogation resulted in a small but consistent tendency toward a stronger phenotype in specific readouts (less Stat5a/b phosphorylation and greater cell viability loss). This could be suggestive of Jak3 supremacy in IL-2 signal transduction in T lymphocytes. This fact has also been described by others in megacarioblastic43 and fibroblast44 cell lines, and in other γc cytokines like IL-4.45 Taken together, evidence shown here reinforce the importance of cross-talk among receptor-associated Jak kinases and discards a functional redundancy in this system. Furthermore, our results suggest a first-step and dominant role of Jak3 in common-γc receptors signal transduction and discard the need of a dual Jak1–Jak3 intervention to fully disrupt γc cytokine signaling.
Relationship between Jak3 and T cell receptor (TCR) is controversial. Some have described intact TCR signaling in Jak3–/– T cells,26 whereas others have found impairment in CD3 mAb-induced TCR activation in Jak3–/– T cells and direct association of Jak3 with CD3ζ subunit.25 Furthermore, uncoupled early TCR-triggered signalling from essential downstream events after Jak3 pharmacological inhibition has been described.24 However, these studies were made using either transgenic knockout mice strains, that could reflect an adaptive response,46 or pharmacological inhibitors which are neither specific nor potent and should be carefully interpreted. Results here presented provide a direct link between Jaks and TCR: we have found that both Jak3 and Jak1 knockdown compromises CD3-CD28 mAb-induced phosphorylation of TCR CD3ε subunit, as well as the downstream mediator CREB. Cell clustering and proliferation, phenotypic hallmarks of TCR activation, were concurrently affected. These results support a plausible role of Jak kinases in the TCR-pathway which would be acting as cross-talking molecules for T cell activation at different points: antigen recognition (signal I and II) and cell growth signals transduction (signal III).
However, inflammation is a complex process mediated by multiple cytokines where a number of cell types interact. Therefore, we next aimed at assessing to what extent-specific Jak3 knockdown in effector T cells would be sufficient to prevent an inflammatory process in vivo. Th1-mediated DTH allowed us to recreate a complex inflammatory scenario mediated by endogenous counterparts while modulating the expression of Jak proteins. We observed a significant alleviation of the inflammatory process was obtained in response to approximately 50% Jak3 knockdown in our model. In correlation to in vitro observations, Jak1 cosilencing did not cooperate to obtain a greater anti-inflammatory effect. Very noteworthy our in vivo results sustained that selective Jak3 inhibition could provide immunosuppressive effect in inflammatory disorders avoiding potential secondary effects due to pan-Jak inhibition outside immune system.
Jak3 silencing could impair the inflammatory process by different mechanisms: (i) T cell activation. As seen in our in vitro restimulation experiments, T cells could remain refractory to antigen-mediated activation in vivo in absence of Jak3, due to either disruption of γc cytokine signaling, TCR pathway or both. (ii) Antigen priming: besides T cells, DC from the synovial tissue of rheumatoid arthritis patients have been found to express Jak38 although its functional role is unknown. Taking into account that Th1 cultures started up from a mixture of splenocytes and lymph nodes cell suspension, where there are many DC, we cannot rule out the possibility that Jak3 knockdown efficiency in vivo might be, at least in part, due to impaired DC triggering. (iii) Th1 differentiation: Jak3 has been described to play a role in Th1 differentiation by means of epigenetic modifications.47
To sum up, in this study we have demonstrated that Jak3-specific suppression in lymphocytes is enough to damper IL-2 and CD3-CD28-mediated T cell activation in vitro and Th1 antigen-driven inflammation in vivo. The data here presented strongly supports the hypothesis that specific Jak3 inhibition could be effective to treat autoimmune disorders where inflammatory pathways such as IL-2 signaling, TCR activation, antigen presentation and a Th1 cellular response has been found to be major insights.7,8 Such therapeutic strategy would balance potential adverse effects versus efficacy.
A remaining question to answer, however, is the feasibility to synthesize a Jak3-selective small molecule inhibitor, as many kinase inhibitors work by interaction with the ATP-binding cleft.13 Since the generation of selective kinase inhibitors versus other kinase families seems to be feasible, there are still many challenges in synthesizing a specific Jak3 antagonist versus other family members, although significant improvements are being made.19,48 As long as T cell-selective systemic siRNA delivery has recently been described,49 a plausible alternative would be the development of a Jak3 siRNA-based immunosuppressive therapy.
Materials and Methods
siRNA sequences. ACCELL siRNA were purchased from Thermo Scientific Dharmacon (Lafayette, CO). Selected mRNA target sequences tested were: human Jak1: 5′ccagaauguuuaaugcaau3′ and 5′ggaugagguucuauuucac3′ human Jak3: 5′ccauggugcaggaauuugu3′ and 5′ggguccuucacc aagauuu3′ mouse Jak1: 5′gcuuugugcugaaacgaug3′ mouse Jak3: 5′uccuugacuuacacguuuu3′. As negative controls, siRNA-free treatment (Mock) and nontargeting siRNA sequence (Scrambled; Scr) were used.
Human primary T lymphocytes isolation, culture, and transfection. Peripheral blood T lymphocytes from healthy volunteers were isolated using Ficoll (Biochrom, Berlin, Germany) density gradient centrifugation. Thereafter, T cells were further isolated with the MACS pan T cell Isolation kit by negative selection in MACS LD depletion magnetic columns (MiltenyiBiotec, Bergisch Gladbach, Germany). The T-cell isolation procedure yielded >92% CD3+ cells. T lymphocytes were cultured at a density of 1 × 106/ml for 3 days in complete RPMI (RPMI 1640, 2 mmol/l glutamine, 10 mmol/l Na/Pyruvate, 100 U Penicillin, 100 µg/ml Streptomycin and 10% inactivated fetal bovine serum, all from Gibco, Paisley, UK) supplemented with 10 µg/ml PHA (Roche, Indianapolis, IN). Afterwards, cells were washed and further cultured in complete RPMI supplemented with 50 ng/ml human recombinant IL-2 (BD Bioscience, San Diego, CA) during all experimental processes.
Transfections were conducted on the fourth day of IL-2 incubation in ACCELL medium w/o fetal bovine serum at a cell density 4 × 106 cells/ml with 1 µmol/l ACCELL siRNA for 3 days to allow passive transfection. Afterwards, cells were either collected, or diluted to 1 × 106 cells/ml in complete RPMI.
Confocal microscopy. Cells were fixed in 4% paraformaldehyde on d3 after transfection. The T cell membrane was highlighted using anti-FITC or Cy3TM labeled CD3mAb (BD Pharmingen, San Diego, CA). Nuclei were labeled with 5 µmol/l DRAQ5 (Biostatus, Leicestershire, UK). Cells were pelleted using a cytospin and mounted in Mowiol-0.1% DABCO (Sigma-Aldrich, St Louis, MO). Fluorescence was detected using a spectral confocal microscope (Leica TCS-SL) at the Research Support Services from the Biology Unit of Bellvitge, University of Barcelona (Barcelona, Spain).
RNA extraction and quantitative reverse transcription-PCR. Total RNA was extracted using RNAeasy mini kit (Qiagen, Mannheim, Germany) or Tryzol reagent (Invitrogen, Carlsbad, CA). RNA quality was assessed using a Bioanalyzer 2100 system at the Transcriptomic Platform from Research Support Services, Scientific Parc from Barcelona (PCB) (Barcelona, Spain). To obtain cDNA 50–200 ng of RNA was reverse transcribed using random hexamers plus either the reverse transcription reagents kit (Applied Biosystems, Branchburg, NJ) or SuperScriptVILO (Invitrogen) for human and mouse samples, respectively. Expression levels of mRNA for selected genes were quantified using TaqMan probes and Gene Expression Master Mix in a 7500HT Real Time-PCR system (all from Applied Biosystems). Gene expression was normalized with β-glucoronidase (Gusb) and 18S as a housekeeping gene with equivalent results. Data analysis is based on the ΔΔCt method.
Western blot. Cells were lysed in RIPA buffer containing protease and phosphatase inhibitor cocktail (Roche, Mannheim, Germany) and 1 mmol/l phenylmethylsulfonyl fluoride. Protein concentration was measured by BCA (Pierce, Rockford, IL). Equivalent amounts of proteins from whole cell lysates (20–50 µg) were resolved by electrophoresis in 7.5% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE). Gels were electro-transferred to PVDF membranes (Millipore, Billerica, MA). Membranes were then incubated with primary antibody followed by horseradish peroxidase-conjugated secondary antibody. Jak1 primary antibody (Chemicon, Temecula, CA) was used at 1/500 dilution, while human Jak3 (Cell Signaling, Danvers, MA) and mouse Jak3 (B12) (Santa Cruz Biotechnology, Heidelberg, Germany) specific antibodies were diluted 1/1,000 and 1/200, respectively. Phosphorylation of the kinase domain (JH1) was detected using the phospho-specific antibody P-JAK1 (Tyr1022–1023) (Santa Cruz Biotechnology) at 1/200 dilution, which recognizes P-Tyr1022/1023 and P-Tyr980–981 from Jak1 and Jak3 respectively. Blots were normalized using γ-tubulin antibody, diluted 1/10,000 (Sigma-Aldrich). Corresponding secondary antibodies were diluted 1/20,000. The membranes were developed with enhanced chemiluminescence (SuperSignal West Dura; Thermo Scientific, Rockford, IL) and visualized in a Molecular Imager ChemiDoc XRS System from Bio-Rad (Barcelona, Spain). Densitometric scanning of immunoblots was performed using the Quantity One analysis software.
Cell viability. Cell viability was indirectly measured using the CellGlo viability assay (Promega, Madison, WI) following manufacturer's guidelines.
Flow cytometry analysis. Cytometric analysis was performed on fresh cells using a FACS calibur cytometer and CellQuest software (BD Bioscience, Bedford, MA).
Luminex. Stat5a/b and TCR activation were investigated by means of xMAP technology in a LIFECODES Life Match Fluoro Analyzer apparatus (Tepnel, Stamford, TP) equipped with Luminex 100 IS 2.3 software. On the third day after transfection 12.5 µg of cell lysates were obtained for analysis following supplier guidelines. Steady-state Stat5a/b phosphorylation levels were analyzed using MILLIPLEX Phospho Stat5a/b (Tyr694/699) MAPMates (Millipore). TCR signaling pathway (CD3 and CREB phosphorylation levels) was analyzed using a Beadlyte 7-plex human T receptor signaling kit (Millipore) after 10-minute stimulation with 1 µg/ml CD3 and 0.5 µg/ml CD28 mAb (BD Biosciences). Data are expressed as relative phosphorylation levels versus nonstimulated control cells.
Mice. Female BALB/c mice were purchased from Harlan Interfauna Ibérica SI (Barcelona, Spain) and female DO11.10 mice from Charles River Laboratories (Barcelona, Spain). All mice were used between seven to fourteen weeks-old and maintained in a constant 12 hours light-dark cycle, fed a standard rodent chow and water ad libitum. All animal protocols were approved by the Animal Experimentation Committee from the Generalitat de Catalunya.
Generation and transfection of murine Th1 cells. Single cell suspensions from mouse splenocytes and mesenteric lymph nodes were prepared by 100-µm mesh filtration followed by erythrocytes lysis with AKC buffer. Cell concentration was adjusted to 2 × 106 cells/ml and lymph node cells were cocultured with splenocytes at a 1:2.5 ratio. Cells were grown in VLE-RPMI 1640 medium with stable glutamine (Biochrom) containing 10% heat-inactivated fetal bovine serum (Gibco), 50 µmol/l β-mercaptoethanol (Sigma-Aldrich), 100 U/ml penicillin and 100 µg/ml streptomycin sulfate (Gibco). To induce Th1 differentiation, cells were incubated with a Th1 cytokine cocktail consisting of recombinant mouse IFNγ (20 ng/ml), IL-12 (10 ng/ml), IL-2 (5 ng/ml), all of them purchased from PeproTech EC (London, UK). After 2 days, cells were incubated with 1 µmol/l siRNA in serum depleted (2%) complete VLE-RPMI 1640 plus Th1 cytokine cocktail. Cells were left in cultured for three more days to allow passive transfection prior to either knockdown assessment or adoptive cell transfer.
Th1-mediated DTH in vivo model. T cells from DO11.10 mice express a transgenic TCR recognizing OVA323–339 peptide.50 OVA323–339-specific Th1 effector cells were generated ex vivo by antigen-specific activation of Th1 culture with 2 µg/ml OVA323–339 peptide (GenScript, Piscataway, NJ). After 5 days in culture, these cells were used for adoptive cell transfer. 5 × 106 activated DO11.10 Th1 cells (OVA-TCRtg Th1) were intravenously injected into naive BALB/c mice. After 24 hours, a DTH response was induced by subcutaneous injection of 5 µg OVA323–339 peptide emulsified in incomplete Freund's adjuvant (Sigma-Aldrich) into the right footpad. As a control, the left footpad was injected with an equivalent volume of phosphate-buffered saline-incomplete Freund's adjuvant. This treatment induces a strong DTH reaction within the footpad containing the OVA323–339 peptide, with a peak response 24 hours after the challenge.28 At this time point, the inflammatory reaction was measured using a Dial Thickness Gauge (0.01–10 mm) (Peacock, Ozaki MFG, Japan). Footpad swelling was calculated by subtracting footpad thickness measured before, and 24 hours after challenge from each individual animal. The degree of footpad swelling was calculated as: percentage increase = [(right footpad thickness after antigen challenge/uninjected left footpad thickness after antigen challenge) – 1] × 100.
In vitro antigen-specific restimulation of Th1 cells. To analyze antigen-specific cellular response, popliteal lymph nodes were isolated 24 hours after in vivo OVA323–339 challenge and 4 × 105 cells cultured in complete VLE-RPMI medium in presence or absence of 10 µg OVA323–339 for 48 hours. Cellular viability and IFNγ secretion in the supernatant was assessed.
Histology. Histological evaluation of inflamed footpad tissue was performed 24 hours after DTH induction. For hematoxylin and eosin staining formalin-fixed samples were decalcified, paraffin-embedded and 4-µm sections were obtained. Evaluation was performed by microscopic analysis by an experienced pathologist in a blind fashion (ANAPATH, Granada, Spain). For overall infiltration and tissue damage a 0 to 4 score was used (footpad score) as follows: 0, no infiltrates, no edema; 1, scarce infiltrates within subcutaneous tissue, little edema; 2, moderate edema, mild infiltrates within subcutaneous fatty tissue; 3, moderate edema, mild infiltrates in skeletal muscle; and 4, abscess formation, with severe infiltrates in skeletal muscle.
Statistical analysis. Results are expressed as the mean ± SEM. Statistical analysis was performed, unless otherwise stated, by two tailed Student t-test on two group comparison and one-way ANOVA for multiple comparisons. Data were considered statistically significant when P < 0.05.
Acknowledgments
A.G.G.-V. was supported by the Torres y Quevedo programfomtheMinisterio de Cienciae Innovación (Spain). We thank José Antonio Garcia and Ana Esther for their skillful assistance with animal handling, Mónica Porras for her helpful advice with the in vivo model and Àngela Roca and Noelia Ardanaz for their invaluable assistance reviewing the manuscript (all from Palau Pharma S.A). We thank Daniel W. Stuckey (Cellular Stress Group, MRC, CSC, Imperial College) for language and grammar reviewing. The authors declared no conflict of interest.
REFERENCES
- Johnston JA, Kawamura M, Kirken RA, Chen YQ, Blake TB, Shibuya K.et al. (1994Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2 Nature 370151–153. [DOI] [PubMed] [Google Scholar]
- Kawamura M, McVicar DW, Johnston JA, Blake TB, Chen YQ, Lal BK.et al. (1994Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes Proc Natl Acad Sci USA 916374–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JJ, Pesu M, Borie DC., and, Changelian PS. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat Rev Drug Discov. 2004;3:555–564. doi: 10.1038/nrd1441. [DOI] [PubMed] [Google Scholar]
- Baird AM, Thomis DC., and, Berg LJ. T cell development and activation in Jak3-deficient mice. J Leukoc Biol. 1998;63:669–677. doi: 10.1002/jlb.63.6.669. [DOI] [PubMed] [Google Scholar]
- Nosaka T, van Deursen JM, Tripp RA, Thierfelder WE, Witthuhn BA, McMickle AP.et al. (1995Defective lymphoid development in mice lacking Jak3 Science 270800–802. [DOI] [PubMed] [Google Scholar]
- Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ.et al. (1995Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development Science 270797–800. [DOI] [PubMed] [Google Scholar]
- Soto H, Hevezi P, Roth RB, Pahuja A, Alleva D, Acosta HM.et al. (2008Gene array analysis comparison between rat collagen-induced arthritis and human rheumatoid arthritis Scand J Immunol 6843–57. [DOI] [PubMed] [Google Scholar]
- Walker JG, Ahern MJ, Coleman M, Weedon H, Papangelis V, Beroukas D.et al. (2007Characterisation of a dendritic cell subset in synovial tissue which strongly expresses Jak/STAT transcription factors from patients with rheumatoid arthritis Ann Rheum Dis 66992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, BH, Kim, M, Yin, CH, Jee, JG, Sandoval, C, Lee, H.et al. (2011Inhibition of JAK3 alleviates inflammation in monoarthritic rats Br J Pharmacol 164106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JH, Bloom BJ, Breedveld FC, Fletcher MP, Gruben D, Kremer JM.et al. (2010Improved pain, physical functioning and health status in patients with rheumatoid arthritis treated with CP-690,550, an orally active Janus kinase (JAK) inhibitor: results from a randomised, double-blind, placebo-controlled trial Ann Rheum Dis 69413–416. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D.et al. (2009The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo Arthritis Rheum 601895–1905. [DOI] [PubMed] [Google Scholar]
- Busque S, Leventhal J, Brennan DC, Steinberg S, Klintmalm G, Shah T.et al. (2009Calcineurin-inhibitor-free immunosuppression based on the JAK inhibitor CP-690,550: a pilot study in de novo kidney allograft recipients Am J Transplant 91936–1945. [DOI] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT.et al. (2008A quantitative analysis of kinase inhibitor selectivity Nat Biotechnol 26127–132. [DOI] [PubMed] [Google Scholar]
- Changelian PS, Moshinsky D, Kuhn CF, Flanagan ME, Munchhof MJ, Harris TM.et al. (2008The specificity of JAK3 kinase inhibitors Blood 1112155–2157. [DOI] [PubMed] [Google Scholar]
- Jiang JK, Ghoreschi K, Deflorian F, Chen Z, Perreira M, Pesu M.et al. (2008Examining the chirality, conformation and selective kinase inhibition of 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile (CP-690,550) J Med Chem 518012–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S.et al. (1998Jak2 is essential for signaling through a variety of cytokine receptors Cell 93385–395. [DOI] [PubMed] [Google Scholar]
- Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD.et al. (1998Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses Cell 93373–383. [DOI] [PubMed] [Google Scholar]
- van Gurp E, Weimar W, Gaston R, Brennan D, Mendez R, Pirsch J.et al. (2008Phase 1 dose-escalation study of CP-690 550 in stable renal allograft recipients: preliminary findings of safety, tolerability, effects on lymphocyte subsets and pharmacokinetics Am J Transplant 81711–1718. [DOI] [PubMed] [Google Scholar]
- Lin TH, Hegen M, Quadros E, Nickerson-Nutter CL, Appell KC, Cole AG.et al. (2010Selective functional inhibition of JAK-3 is sufficient for efficacy in collagen-induced arthritis in mice Arthritis Rheum 622283–2293. [DOI] [PubMed] [Google Scholar]
- Haan C, Rolvering C, Raulf F, Kapp M, Drückes P, Thoma G.et al. (2011Jak1 has a dominant role over Jak3 in signal transduction through ?c-containing cytokine receptors Chem Biol 18314–323. [DOI] [PubMed] [Google Scholar]
- Akhtar S., and, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–3632. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S., and, Peer D. RNAi nanomedicines: challenges and opportunities within the immune system. Nanotechnology. 2010;21:232001. doi: 10.1088/0957-4484/21/23/232001. [DOI] [PubMed] [Google Scholar]
- Oberdoerffer P, Kanellopoulou C, Heissmeyer V, Paeper C, Borowski C, Aifantis I.et al. (2005Efficiency of RNA interference in the mouse hematopoietic system varies between cell types and developmental stages Mol Cell Biol 253896–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Säemann MD, Zeyda M, Diakos C, Szekeres A, Böhmig GA, Kelemen P.et al. (2003Suppression of early T-cell-receptor-triggered cellular activation by the Janus kinase 3 inhibitor WHI-P-154 Transplantation 751864–1872. [DOI] [PubMed] [Google Scholar]
- Tomita K, Saijo K, Yamasaki S, Iida T, Nakatsu F, Arase H.et al. (2001Cytokine-independent Jak3 activation upon T cell receptor (TCR) stimulation through direct association of Jak3 and the TCR complex J Biol Chem 27625378–25385. [DOI] [PubMed] [Google Scholar]
- Thomis DC., and, Berg LJ. Peripheral expression of Jak3 is required to maintain T lymphocyte function. J Exp Med. 1997;185:197–206. doi: 10.1084/jem.185.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Sato N, Yahata T, Ohmi Y, Santa K, Sato T.et al. (1997Manipulation of Th1/Th2 balance in vivo by adoptive transfer of antigen-specific Th1 or Th2 cells J Immunol Methods 20985–92. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Eulenburg K, Loddenkemper C, Hamann A., and, Huehn J. Self-limitation of Th1-mediated inflammation by IFN-gamma. J Immunol. 2006;176:2857–2863. doi: 10.4049/jimmunol.176.5.2857. [DOI] [PubMed] [Google Scholar]
- Gust TC, Neubrandt L, Merz C, Asadullah K, Zügel U., and, von Bonin A. RNA interference-mediated gene silencing in murine T cells: in vitro and in vivo validation of proinflammatory target genes. Cell Commun Signal. 2008;6:3. doi: 10.1186/1478-811X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE., and, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Novobrantseva TI, Akinc A, Borodovsky A., and, de Fougerolles A. Delivering silence: advancements in developing siRNA therapeutics. Curr Opin Drug Discov Devel. 2008;11:217–224. [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K., and, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Sledz CA, Holko M, de Veer MJ, Silverman RH., and, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- Schlee M, Hornung V., and, Hartmann G. siRNA and isRNA: two edges of one sword. Mol Ther. 2006;14:463–470. doi: 10.1016/j.ymthe.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Cohney SJ, Sanden D, Cacalano NA, Yoshimura A, Mui A, Migone TS.et al. (1999SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation Mol Cell Biol 194980–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey, JB, Nadia JK, James, MM, Leila, NV, Artem L.et al. (2012Suppression of cytokine signaling by SOCS3: Characterization of the mode of inhibition and the basis of its specificity Immunity 36239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FM., and, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourquin C, Anz D, Zwiorek K, Lanz AL, Fuchs S, Weigel S.et al. (2008Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity J Immunol 1812990–2998. [DOI] [PubMed] [Google Scholar]
- Cekaite L, Furset G, Hovig E., and, Sioud M. Gene expression analysis in blood cells in response to unmodified and 2′-modified siRNAs reveals TLR-dependent and independent effects. J Mol Biol. 2007;365:90–108. doi: 10.1016/j.jmb.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Hegen M, Keith JC, Jr, Collins M., and, Nickerson-Nutter CL. Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann Rheum Dis. 2008;67:1505–1515. doi: 10.1136/ard.2007.076430. [DOI] [PubMed] [Google Scholar]
- Kirken RA, Rui H, Malabarba MG, Howard OM, Kawamura M, O'Shea JJ.et al. (1995Activation of JAK3, but not JAK1, is critical for IL-2-induced proliferation and STAT5 recruitment by a COOH-terminal region of the IL-2 receptor beta-chain Cytokine 7689–700. [DOI] [PubMed] [Google Scholar]
- Zhou YJ, Magnuson KS, Cheng TP, Gadina M, Frucht DM, Galon J.et al. (2000Hierarchy of protein tyrosine kinases in interleukin-2 (IL-2) signaling: activation of syk depends on Jak3; however, neither Syk nor Lck is required for IL-2-mediated STAT activation Mol Cell Biol 204371–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner JW, Walters DK, Willis SG, Luttropp M, Oost J, Loriaux M.et al. (2008RNAi screening of the tyrosine kinome identifies therapeutic targets in acute myeloid leukemia Blood 1112238–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H. Cell type-specific roles of Jak3 in IL-2-induced proliferative signal transduction. Biochem Biophys Res Commun. 2007;354:825–829. doi: 10.1016/j.bbrc.2007.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malabarba MG, Kirken RA, Rui H, Koettnitz K, Kawamura M, O'Shea JJ.et al. (1995Activation of JAK3, but not JAK1, is critical to interleukin-4 (IL4) stimulated proliferation and requires a membrane-proximal region of IL4 receptor alpha J Biol Chem 2709630–9637. [DOI] [PubMed] [Google Scholar]
- De Souza AT, Dai X, Spencer AG, Reppen T, Menzie A, Roesch PL.et al. (2006Transcriptional and phenotypic comparisons of Ppara knockout and siRNA knockdown mice Nucleic Acids Res 344486–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Lin TH, Appell KC., and, Berg LJ. Janus-kinase-3-dependent signals induce chromatin remodeling at the Ifng locus during T helper 1 cell differentiation. Immunity. 2008;28:763–773. doi: 10.1016/j.immuni.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BH, Oh SR, Yin CH, Lee S, Kim EA, Kim MS.et al. (2010MS-1020 is a novel small molecule that selectively inhibits JAK3 activity Br J Haematol 148132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer D, Park EJ, Morishita Y, Carman CV., and, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Heimberger AB., and, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]