Abstract
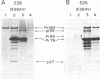
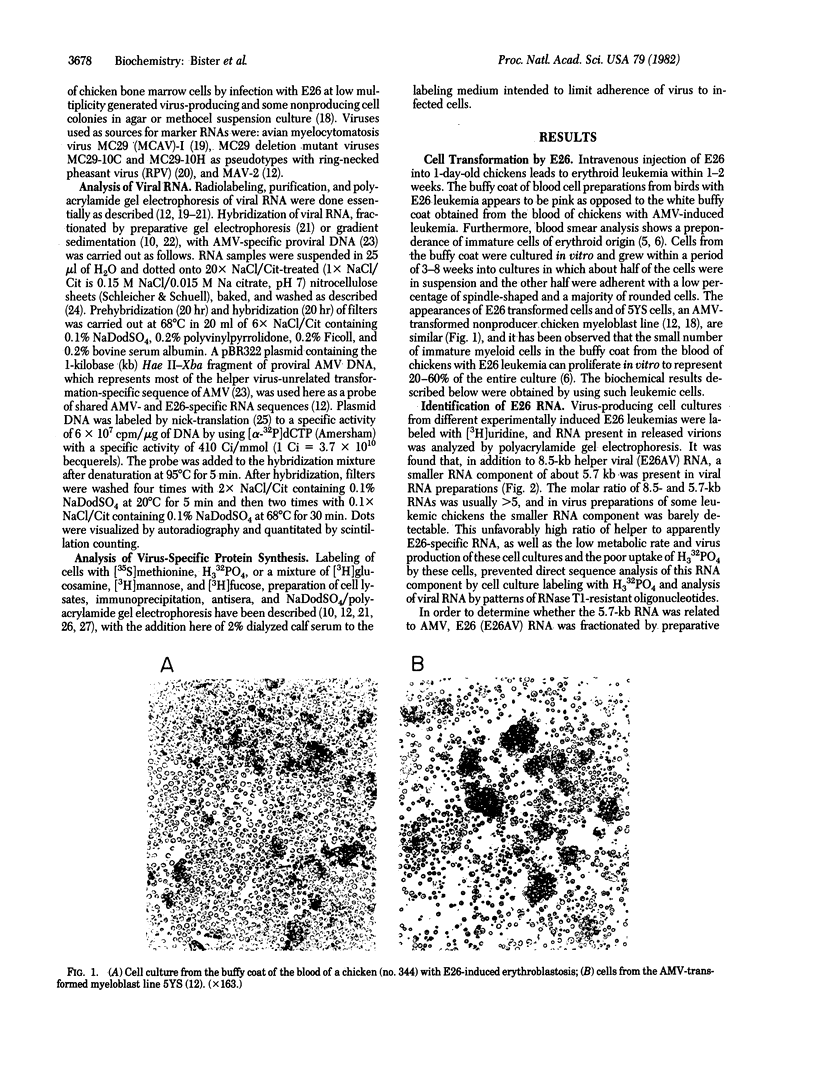
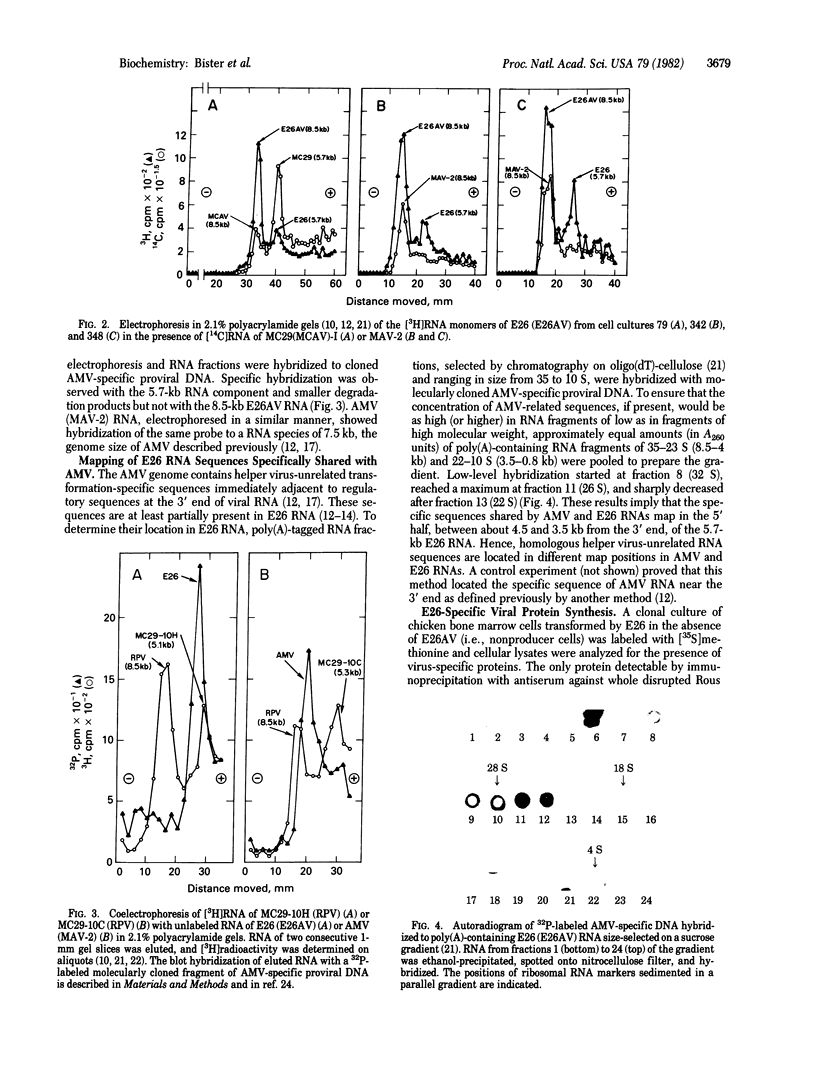
Replication-defective acute leukemia viruses E26 and myeloblastosis virus (AMV) cause distinct leukemias although they belong to the same subgroup of oncogenic avian tumor viruses based on shared transformation-specific (onc) RNA sequences. E26 causes predominantly erythroblastosis in chicken and in quail, whereas AMV induces a myeloid leukemia. However, upon cultivation in vitro for >1 month, a majority of surviving hemopoietic cells of E26-infected animals bear myeloid markers similar to those of AMV-transformed cells. We have analyzed the genetic structure and gene products of E26 virus for a comparison with those of AMV. An E26/helper virus complex was found to contain two RNA species: a 5.7-kilobase (kb) RNA that hybridizes with cloned AMV-specific proviral DNA and hence is probably the E26 genome; and an 8.5-kb RNA that is unrelated to AMV and represents helper virus RNA. Thus, E26 RNA is smaller than 7.5-kb AMV RNA. Hybridization of size-selected poly(A)-terminating E26 RNA fragments with AMV-specific DNA indicated that the shared specific sequences are located in the 5′ half of the E26 genome as opposed to a 3′ location in AMV RNA. In nonproducer cells transformed in vitro by E26, a gag-related nonstructural 135,000-dalton protein (p135) was found. No gag(Pr76) or gag-pol (Pr180) precursors of essential virion proteins, which are present in AMV nonproducer cells, were observed. p135 was also found in cultured E26 virus producing cells of several leukemic chickens, and its intracellular concentration relative to that of the essential virion proteins encoded by the helper virus correlates with the ratio of E26 to helper RNA in virions released by these cells. p135 is phosphorylated but not glycosylated; antigenically it is not related to the pol or env gene products. It appears to be coded for by a partial gag gene and by E26-specific RNA sequences, presumably including those shared with AMV. Hence, AMV and E26 appear to use different strategies for the expression of related onc sequences: AMV is thought to encode a transforming protein via a subgenomic mRNA, whereas E26 codes for a gag-related polyprotein via genomic RNA. It is speculated that differences in the oncogenic properties of E26 and AMV are due to differences in their genetic structures and gene products.
Keywords: erythroid and myeloid leukemia, transforming genes and proteins, RNA and protein gel electrophoresis, nucleic acid hybridization
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. M., Chen J. H. In vitro translation of avian myeloblastosis virus RNA. J Virol. 1981 Oct;40(1):107–117. doi: 10.1128/jvi.40.1.107-117.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Genetic structure of avian acute leukemia viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):801–822. doi: 10.1101/sqb.1980.044.01.086. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Structure and specific sequences of avian erythroblastosis virus RNA: evidence for multiple classes of transforming genes among avian tumor viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5023–5027. doi: 10.1073/pnas.76.10.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bister K., Lee W. H., Duesberg P. H. Phosphorylation of the nonstructural proteins encoded by three avian acute leukemia viruses and by avian fujinami sarcoma virus. J Virol. 1980 Nov;36(2):617–621. doi: 10.1128/jvi.36.2.617-621.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Löliger H. C., Duesberg P. H. Oligoribonucleotide map and protein of CMII: detection of conserved and nonconserved genetic elements in avian acute leukemia viruses CMII, MC29, and MH2. J Virol. 1979 Oct;32(1):208–219. doi: 10.1128/jvi.32.1.208-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Ramsay G. M., Hayman M. J. Deletions within the transformation-specific RNA sequences of acute leukemia virus MC29 give rise to partially transformation-defective mutants. J Virol. 1982 Mar;41(3):754–766. doi: 10.1128/jvi.41.3.754-766.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Hayward W. S., Moscovici C. Size and genetic content of virus-specific RNA in myeloblasts transformed by avian myeloblastosis virus (AMV). Virology. 1981 Apr 15;110(1):128–136. doi: 10.1016/0042-6822(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Moscovici C. Avian acute leukemia virus MC29: conserved and variable RNA sequences and recombination with helper virus. Virology. 1979 Nov;99(1):121–134. doi: 10.1016/0042-6822(79)90043-6. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Moscovici C. Genetic structure of avian myeloblastosis virus, released from transformed myeloblasts as a defective virus particle. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5120–5124. doi: 10.1073/pnas.77.9.5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Transforming genes of retroviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):13–29. doi: 10.1101/sqb.1980.044.01.005. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Fanshier L., Bishop J. M., Moscovici C., Moscovici M. G. The genome and the intracellular RNAs of avian myeloblastosis virus. Cell. 1981 Jan;23(1):279–290. doi: 10.1016/0092-8674(81)90292-0. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Oker-Blom N., Todorov T. G., Beug H. Transforming capacities and defectiveness of avian leukemia viruses OK10 and E 26. Virology. 1979 Dec;99(2):431–436. doi: 10.1016/0042-6822(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C. Leukemic transformation with avian myeloblastosis virus: present status. Curr Top Microbiol Immunol. 1975;71:79–101. doi: 10.1007/978-3-642-66193-8_2. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Samarut J., Gazzolo L., Moscovici M. G. Myeloid and erythroid neoplastic responses to avian defective leukemia viruses in chickens and in quail. Virology. 1981 Sep;113(2):765–768. doi: 10.1016/0042-6822(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Perbal B., Baluda M. A. Avian myeloblastosis virus transforming gene is related to unique chicken DNA regions separated by at least one intervening sequence. J Virol. 1982 Jan;41(1):250–257. doi: 10.1128/jvi.41.1.250-257.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Schulz R. A., Chirikjian J. G., Papas T. S. Analysis of avian myeloblastosis viral RNA and in vitro synthesis of proviral DNA. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2057–2061. doi: 10.1073/pnas.78.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotirov N. Histone H5 in the immature blood cells of chickens with leukosis induced by avian leukosis virus strain E26. J Natl Cancer Inst. 1981 Jun;66(6):1143–1149. doi: 10.1093/jnci/66.6.1143. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Strommer J. N., Hillyard R. L., Komaromy M. C., Baluda M. A. Cellular sequences are present in the presumptive avian myeloblastosis virus genome. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5177–5181. doi: 10.1073/pnas.77.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stéhelin D., Saule S., Roussel M., Sergeant A., Lagrou C., Rommens C., Raes M. B. Three new types of viral oncogenes in defective avian leukemia viruses. I. Specific nucleotide sequences of cellular origin correlate with specific transformation. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1215–1223. doi: 10.1101/sqb.1980.044.01.132. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]