ABSTRACT
Bisphenol A (BPA) is an estrogenic chemical used to manufacture many commonly used plastic and epoxy resin-based products. BPA ubiquitously binds to estrogen receptors throughout the body, including estrogen receptor alpha (ESR1) in the ovary. Few studies have investigated the effects of BPA on ovarian antral follicles. Thus, we tested the hypothesis that BPA alters cell cycle regulators and induces atresia in antral follicles via the genomic estrogenic pathway, inhibiting follicle growth. To test this hypothesis, we isolated antral follicles from 32- to 35-day-old control and Esr1-overexpressing mice and cultured them with vehicle control (dimethylsulfoxide [DMSO]) or BPA (1–100 μg/ml). Additionally, antral follicles were isolated from 32- to 35-day-old FVB mice and cultured with DMSO, BPA (1–100 μg/ml), estradiol (10 nM), ICI 182,780 (ICI; 1 μM), BPA plus ICI, or BPA plus estradiol. Follicles were measured for growth every 24 h for 96–120 h and processed either for analysis of estrogen receptor, cell cycle, and/or atresia factor mRNA expression, or for histological evaluation of atresia. Results indicate that estradiol and ICI do not protect follicles from BPA-induced growth inhibition and that estradiol does not protect follicles from BPA-induced atresia. Furthermore, overexpressing Esr1 does not increase susceptibility of follicles to BPA-induced growth inhibition. Additionally, BPA up-regulates Cdk4, Ccne1, and Trp53 expression, whereas it down-regulates Ccnd2 expression. BPA also up-regulates Bax and Bcl2 expression while inducing atresia in antral follicles. These data indicate that BPA abnormally regulates cell cycle and atresia factors, and this may lead to atresia and inhibited follicle growth independently of the genomic estrogenic pathway.
Keywords: atresia, bisphenol A, cell cycle, follicle growth
Bisphenol A inhibits antral follicle growth by aberrant up-regulation cell cycle regulators, inducing the cell cycle inhibitor transformation-related protein 53 and inducing atresia, independently of the genomic estrogenic pathway.
INTRODUCTION
Bisphenol A (BPA) is a synthetic estrogen used in the manufacture of commonly used consumer products such as polycarbonate plastics and epoxy resins for food and beverage cans. Levels of human exposure to BPA have been reported in various tissues, including but not limited to adult sera (0.2–20 ng/ml), fetal sera (averaging 8.3 ng/ml), placental tissues (11.2 ng/g), human breast milk (0.28–0.97 ng/ml), human colostrum (1–7 ng/ml), and urine (1.12 ng/ml in women) [1]. BPA exposure has also been measured in Chinese men (sera, 2.84 μg/ml; median personal airborne level, 450 μg/ml) working in factories producing BPA and products containing BPA, such as epoxy resins [2, 3]. Furthermore, the estimated daily intake of BPA is thought to range from 0.6–71.4 μg/day based on urine samples taken in the morning from human study subjects [1, 4]. In a government funded study (National Health and Nutrition Examination Survey III [5]), BPA has been found to be present in 95% of human urine samples, indicating that humans are constantly exposed to measurable levels of BPA [5].
BPA is an endocrine-disrupting chemical and can interact with various endocrine receptors in the female reproductive tract, including estrogen receptors alpha (ESR1) and beta (ESR2) [6, 7]. BPA has also been shown to interact with the membrane-bound estrogen receptor (GPR30) in human breast cancer cells [8], as well as with estrogen-related receptor gamma in competitive receptor-binding assays [9], indicating that BPA may work through genomic and nongenomic estrogen-related pathways. Previous studies indicate that BPA can affect facets of the reproductive and endocrine systems, including behavior [10, 11], sexual development in the brain [12, 13], and prostate tumor cell proliferation [14]. Also, in the ovary, BPA exposure impairs early oogenesis; induces meiotic incompetence, arrest, and aneuploidy; increases chromosomal congression failure during meiosis; and alters Ca2+ oscillations within oocytes isolated from antral follicles [15–18]. However, while these studies investigated the effects of BPA on oocytes isolated from antral follicles, few studies have focused on the effects of BPA on whole antral follicles. Furthermore, it is not known whether BPA works through Esr pathways in antral follicles. Thus, one goal of this study was to test the hypothesis that BPA inhibits antral follicle growth through Esr pathways.
Follicles, which are the functional units of the ovary, are responsible for growing and housing the oocyte and for producing sex steroid hormones. Follicles grow and develop from the primordial stage to the antral and preovulatory stages throughout the reproductive life of a female [19]. Each stage of follicle development is accompanied by an increase in the number of cell layers present in the follicle. For example, primordial and primary follicles have a single layer of granulosa cells surrounding the oocyte. Preantral, antral, and preovulatory follicles all have multiple layers of granulosa cells and outer cell layers called thecal layers. Follicle growth is required for the follicle to ovulate and release the oocyte for fertilization. Any perturbations in follicle growth can lead to improper oocyte development, anovulation, and abnormal sex steroid hormone levels. In turn, this can result in subfertility or infertility [20, 21]. Without proper growth, the follicle will also be marked for programmed cell death and undergo atresia, preventing ovulation [20, 21]. Over 99% of the finite follicles in the follicular pool at birth will undergo atresia, leaving 1% of follicles to properly develop and ovulate for fertilization during the reproductive lifespan [22, 23]. Exposure to reproductive toxicants that decrease the proper development of antral follicles can hinder normal reproduction.
BPA has been shown to inhibit mouse antral follicle growth in vitro [24]. Follicle growth is dependent on the proliferation of granulosa and thecal cells [25]. Like most other cells in the body, granulosa and thecal cell proliferation is dependent upon the cell cycle [26]. The cell cycle is controlled in a directional and sequential pattern by cell cycle regulators, complexes of proteins called cyclins and cyclin-dependent kinases (CDK) [26, 27]. These cyclin:CDK complexes are responsible for promoting cell survival by protecting the cell from replicating with damaged DNA or from mutations that may lead to aberrant and uncontrolled cell growth [20]. Cyclin D2 (CCND2) and cyclin-dependent kinase 4 (CDK4) form a complex that monitors the progression of a cell through early G1 phase. This complex directly up-regulates cyclin E1 (CCNE1), a cell cycle regulator part of another cyclin:Cdk complex that monitors the progression of a cell at the end of G1 phase and prevents entry into S phase if necessary [20]. Each cyclin:CDK complex in the cell cycle acts as a checkpoint and can, if necessary, halt the cells' progression through the cell cycle and either arrest progression or program cells for death. Transformation-related protein 53 (TRP53) is a specific factor that controls whether the cyclin:Cdk complexes allow cells to progress through the cycle [20]. TRP53, after induction by tissue damage or release from binding inactivation, can down-regulate and inactivate the cyclin:Cdk complex, arresting cell growth or inducing cell death [20, 27]. Given the fact that BPA inhibits antral follicle growth [24] and antral follicle growth is partially controlled by cell cycle regulators [27], a second goal of this study was to test the hypothesis that BPA alters expression of cell cycle regulators in antral follicles, possibly leading to inhibition in growth.
In addition to inhibiting follicle growth by reducing expression of cell cycle regulators, it is possible that BPA inhibits follicle growth by inducing atresia. Atresia, or programmed cell death of antral follicles, is complementary to the cell cycle and follicle growth [27]. Thus, a third goal of this study was to test the hypothesis that BPA induces atresia of antral follicles.
MATERIALS AND METHODS
Chemicals
BPA powder (99%) was purchased from Sigma-Aldrich. A stock solution of BPA was dissolved and diluted in dimethylsulfoxide (DMSO; Sigma-Aldrich) to achieve various BPA treatment concentrations (1.3, 13.3, 133 mg/ml) for final working concentrations of 1, 10, and 100 μg of BPA per milliliter of culture medium. Using these treatment concentrations allowed each working concentration to contain the same volume of chemical and vehicle.
BPA concentrations were also chosen based on studies showing the effects of BPA on ovarian cells and antral follicles [24, 28]. For example, BPA exposure of between 100 fM and 100 μM for 24–72 h results in an increase in apoptosis and G2-to-M arrest in cultured mouse ovarian granulosa cells [28]. Additionally, BPA exposure of between 10 and 100 μg/ml for 120 h results in a decrease in antral follicle growth, steroidogenesis, and expression of steroidogenic enzymes in vitro [24]. Our concentrations of BPA were relevant to current regulatory levels set for BPA. The lowest observable adverse effect level (LOAEL) is 50 mg/kg/day. This concentration equates to 50 μg/ml. Doses used in these experiments were 1, 10, and 100 μg/ml, encompassing the LOAEL concentration.
Estradiol was obtained from Sigma-Aldrich. A stock solution of estradiol was dissolved and diluted in DMSO (20 μM) for a final concentration in culture of 10 nM. ICI 182,780 (ICI), a high-affinity ESR antagonist, was obtained from Tocris-Cookson. Stock solutions of ICI were dissolved in DMSO (2 mM) for a final concentration in culture of 1 μM. Final concentrations of estradiol and ICI in culture were chosen based on previous studies in our laboratory [29].
Animals
Adult, cycling, female sensitivity to Friend leukemia virus B (FVB) strain mice were purchased from Jackson Laboratory and allowed to acclimate to the facility for at least 5 days before use. Esr1-overexpressing (Esr1 OE) and control mice used in this study were generated as previously described and were obtained from a breeding colony at the University of Illinois [30, 31]. Esr1 OE mice were validated for overexpression of the Esr1 gene and protein levels in ovaries and antral follicles and compared control mice as previously described [30]. All mice were housed at the University of Illinois at Urbana-Champaign, Veterinary Medicine Animal Facility. Food (catalog no. 8626; Harlan Teklad) and water were provided for ad libitum consumption. Temperature was maintained at 22° ± 1°C, and animals were subjected to 12L:12D cycles. The Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collection.
Follicle Culture
Female FVB mice were euthanized on Postnatal Days (PND) 32–35, and their ovaries were removed using aseptic technique. Antral follicles were mechanically isolated from the ovary based on relative size (250–400 μm), cleaned of interstitial tissue by using fine watchmaker forceps [23, 32], and individually placed in wells of 96-well culture plates containing unsupplemented α-minimal essential medium (α-MEM) prior to treatment. Sufficient numbers of antral follicles for statistical power were isolated from unprimed mouse ovaries; follicles from 2–3 mice were isolated per experiment, providing approximately 20–40 antral follicles from each mouse. Each experiment contained a minimum of 8–16 follicles per treatment group. Concentrations of vehicle control (DMSO), BPA (1–100 μg/ml), estradiol (10 nM), and ICI (1 μM) were individually prepared in supplemented α-MEM. Supplemented α-MEM was prepared with 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium), 100 U/ml penicillin, 100 mg/ml streptomycin, 5 IU/ml human recombinant follicle-stimulating hormone (FSH [provided by Dr. A.F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA]), and 5% fetal calf serum (Atlanta Biologicals) [23, 32]. An equal volume of chemical was added for each treatment to control for the amount of vehicle in each preparation. In some cultures, antral follicles were cotreated with BPA plus estradiol. In follicle cultures with ICI, antral follicles were pretreated with ICI in supplemented α-MEM for 2 h prior to subsequent treatments with BPA, ICI, and BPA plus ICI. All antral follicles were cultured for 24–120 h in an incubator supplying 5% CO2 at 37°C.
Follicle growth was measured at 24-h intervals beginning at 0 hr by measuring follicle diameters on a perpendicular axis with an inverted microscope equipped with a calibrated ocular micrometer. Follicle measurements were averaged among treatment groups at each time point, and data were presented as percent change in growth over time per treatment group. Images of the follicles were taken using a digital microscope camera (ProgRes CT3 model; Jenoptik) and captured using image acquisition software (ProgRes CapturePro; Jenoptik).
Analysis of Atresia
At the end of follicle culture (48 or 120 h), supplemented α-MEM was removed from each well, and Dietrick solution was added to fix the follicles. Follicles were fixed for at least 24 h in Dietrick solution and transferred in histology cassettes to 70% ethanol. Tissues were dehydrated, embedded using a methyl methacrylate-based resin kit (Technovit; Heraeus Kulzer GmbH), serially sectioned (2 μm), mounted on glass slides, and stained with Lee methylene blue-basic fuchsin stain. Each follicle section was examined for the level of atresia as shown by the presence of apoptotic bodies and reported at the highest level observed throughout the follicle. Follicle sections were rated on a scale of 1–4 for the presence of apoptotic bodies, where 1 = 0 apoptotic bodies (healthy); 2 = ≤10% apoptotic bodies (early atresia); 3 = 10%–30% apoptotic bodies (mid atresia); and 4 = ≥30% apoptotic bodies (late atresia), as previously described [32]. All analyses were conducted without knowledge of treatment group. Ratings were averaged and plotted to compare the effects of BPA treatments on atresia levels. Images of the follicles were taken at 40× magnification, using a digital microscopy camera (model DFC290; Leica Microsystems) and captured with image acquisition software (ImagePro; Leica).
Analysis of Gene Expression
Female FVB mouse antral follicles were cultured as described above for 24–96 h. At the end of culture, follicles were collected and snap frozen at –80°C for real-time quantitative PCR (qPCR) analysis. Total RNA was extracted from follicles using the RNeasy micro kit (Qiagen, Inc.) according to the manufacturer's protocol. Reverse transcriptase generation of cDNA was performed with 0.3–1 μg of total RNA, using an iScript RT kit (Bio-Rad Laboratories, Inc.). qPCR was conducted using the CFX96 real-time PCR detection system (Bio-Rad Laboratories, Inc.) and accompanying software (CFX Manager software) according to the manufacturer's instructions. The CFX96 system quantifies the amount of PCR product generated by measuring a dye (SYBR Green) that fluoresces when bound to double-stranded DNA. A standard curve was generated from five serial dilutions of one of the samples, thus allowing analysis of the amount of cDNA in the exponential phase. qPCR analysis was performed using 2 μl of cDNA, forward and reverse primers (5 pmol) for estrogen receptor alpha (Esr1; GenBank accession no. NM_007956), estrogen receptor beta (Esr2; GenBank accession no. NM_207707), cyclin-dependent kinase 4 (Cdk4; GenBank accession no. NM_009870.3), cyclin D2 (Ccnd2; GenBank accession no. NM_009829.3), cyclin E1 (Ccne1; GenBank accession no. NM_007633.2), transformation-related protein 53 (Trp53; GenBank accession no. NM_011640.3), B-cell lymphoma 2 (Bcl2; GenBank accession no. NM_009741.3), Bcl2-associated X protein (Bax; GenBank accession no. NM_007527.3), or beta-actin (Actb; GenBank accession no. NM_007393), in conjunction with a SsoFast EvaGreen Supermix qPCR kit (Bio-Rad Laboratories). An initial incubation of 95°C for 10 min was followed by denaturing at 94°C for 10 sec, annealing from 60°C for 10 sec, and extension at 72°C for 10 sec for 40 cycles (β-actin), followed by final extension at 72°C for 10 min. A melting curve was generated at 55°–90°C to monitor the generation of a single product. The software also generated a standard curve. The Actb gene was used as a reference for each sample. Final values were calculated and expressed as the ratio normalized to Actb. All analyses were performed in duplicate for at least three separate experiments.
Statistical Analysis
Data were expressed as means ± standard errors of the means (SEM), and multiple comparisons between experimental groups were made using general linearized model and ANOVA as applicable, followed by Tukey post hoc comparisons. At least three separate experiments were conducted for each treatment prior to data analysis. Statistical significance was assigned at a P value of ≤0.05.
RESULTS
Effect of BPA Treatment on Follicle Growth
Previous studies indicate that BPA inhibits follicle growth in mouse antral follicles [24]. In the current studies, we expanded on previous work by testing the hypothesis that BPA inhibits follicle growth through ESR pathways. Thus, it was first important to determine whether BPA inhibited follicle growth as was found similarly in previous studies. Exposure to BPA (100 μg/ml) significantly decreased follicle growth compared to exposure to DMSO beginning at 72 h and continuing throughout culture (Fig. 1). BPA at 1 μg/ml and BPA at 10 μg/ml did not significantly affect growth compared to DMSO at any time point.
FIG. 1. .
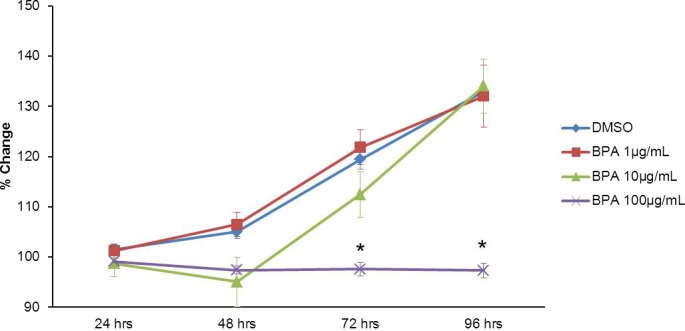
Effect of BPA on follicle growth. Antral follicles were mechanically isolated from FVB mice and exposed in vitro to DMSO or 10–100 μg/ml BPA for 24–96 h. Growth of follicles was monitored during culture and recorded in micrometers and reported as percent change. Data are means ± SEM from at least three separate experiments. Lines with asterisks (*) show data that are significantly different from those of DMSO controls (n = 8–16 follicles per treatment per experiment from at least three separate experiments; P ≤ 0.05).
Morphological changes in follicle growth were consistent with data indicating follicle growth is significantly inhibited after exposure to 100 μg/ml BPA compared to DMSO beginning at 72 h and continuing throughout culture (Fig. 1). In DMSO-treated follicles, follicle size steadily increased over time, suggesting that cells were proliferating, resulting in a “fuzzy” appearance (Fig. 2A). In 100 μg/ml BPA-treated follicles, size did not significantly change over time, suggesting no significant proliferation within the follicle (Fig. 2B).
FIG. 2. .
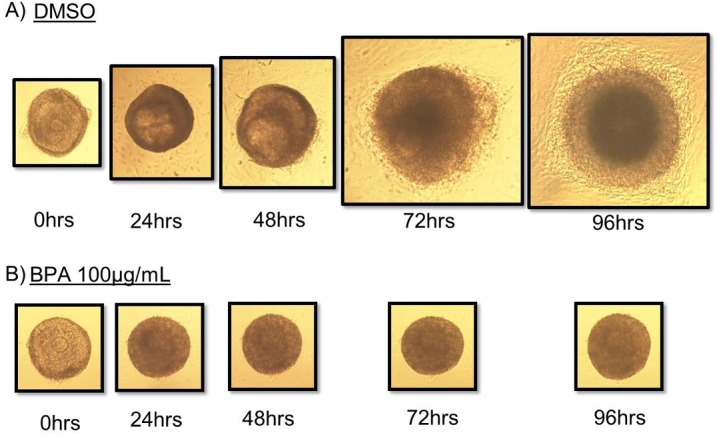
Effect of BPA on morphology. Antral follicles were mechanically isolated from FVB mice and exposed in vitro to DMSO or 100 μg/ml BPA for 24–96 h. Growth of follicles was observed as described in Materials and Methods. Original magnification ×10.
Effects of BPA Treatment on Esr Pathways in Antral Follicles
To examine whether BPA used ESR pathways in antral follicles, we first examined the effect of BPA on Esr1 and Esr2 expression levels in antral follicles. Exposure to 1 μg/ml BPA decreased Esr1 expression at 24 h compared to DMSO, but expression returned to control levels at 48, 72, and 96 h. Exposure to 100 μg/ml BPA significantly increased Esr1 expression at 24 h but decreased Esr1 expression at 72 h compared to DMSO controls (Fig. 3A). BPA at 10 μg/ml did not alter Esr1 expression at any time point. Exposure to 10 μg/ml BPA significantly increased Esr2 expression compared to that in DMSO controls beginning at 24 h and continuing through 72 h of culture (Fig. 3B). BPA at 1 μg/ml and BPA at 100 μg/ml did not significantly alter expression of Esr2 at any time point.
FIG. 3. .
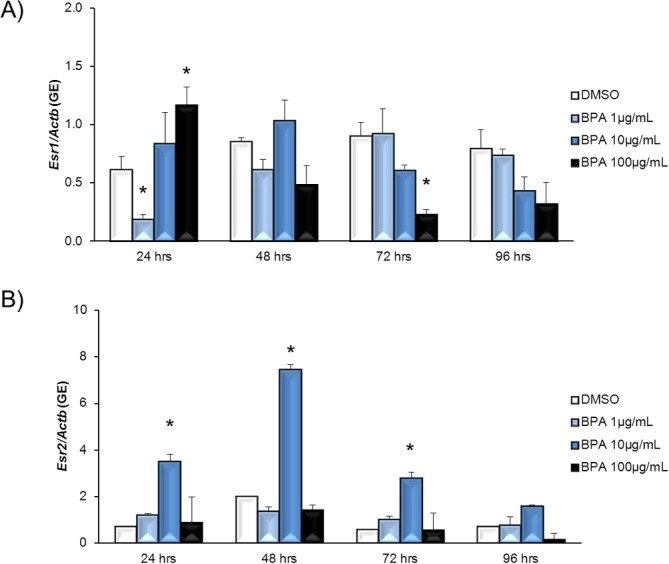
Effect of BPA exposure on Esr1 and Esr2 mRNA expression levels. After antral follicles were exposed to DMSO control or 1–100 μg/ml BPA for 24–96 h in vitro, the follicles were collected and subjected to qPCR analysis for Esr1 (A) and Esr2 (B) mRNA expression levels. All values were normalized to those of beta-actin as a loading control. Graphs show means ± SEM from at least three separate experiments. *P ≤ 0.05 from DMSO control.
To determine whether BPA works through the ESR pathway, we next cotreated antral follicles with BPA and estradiol to examine whether estradiol could block BPA-induced growth inhibition. Exposure to 100 μg/ml BPA significantly decreased follicle growth compared to exposure to DMSO and estradiol controls (Fig. 4A). Estradiol cotreatment did not protect antral follicles from BPA-induced inhibition of follicle growth (Fig. 4A).
FIG. 4. .
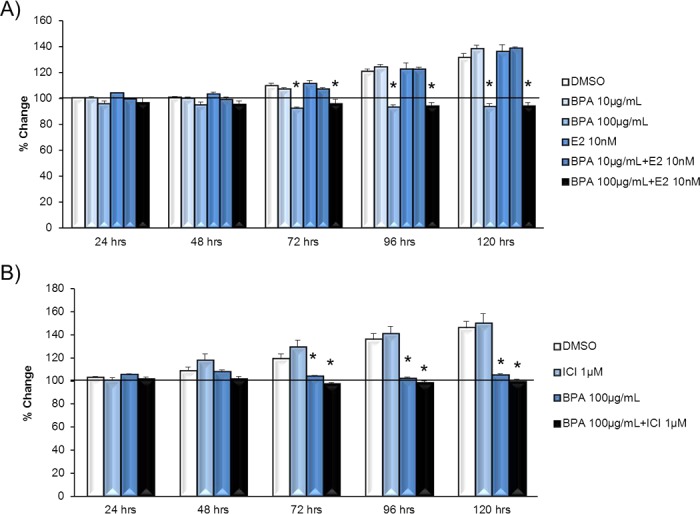
Effect of estradiol and ICI on BPA-induced follicle growth inhibition. Antral follicles were mechanically isolated from FVB mice and exposed in vitro to DMSO; 10–100 μg/ml BPA; 10 nM estradiol or 10–100 μg/ml BPA plus 10 nM estradiol (A) or DMSO; 100 μg/ml BPA, 1 μM ICI; or 100 μg/ml BPA plus 1 μM ICI (B) for 24–120 h. Growth of follicles was monitored during culture, recorded in micrometers, and reported as percent change. Graphs show means ± SEM from at least three separate experiments. Lines with asterisks (*) show data that are significantly different from those of DMSO controls (n = 8–16 follicles per treatment per experiment from at least three separate experiments; P ≤ 0.05).
Furthermore, to determine whether blocking ESRs in antral follicles could protect antral follicles from BPA-induced follicle growth inhibition, we pretreated antral follicles with the ESR antagonist ICI to examine whether ICI blocked BPA-induced growth inhibition. Exposure to BPA at 100 μg/ml significantly decreased follicle growth compared to DMSO and ICI controls (Fig. 4B). Pretreating follicles with ICI did not protect antral follicles from BPA-induced inhibition of follicle growth (Fig. 4B).
Finally, because previous studies have shown that overexpressing ESR1 increases the susceptibility of follicles to growth inhibition induced by other estrogenic endocrine-disrupting chemicals [30], we treated Esr1 OE mouse antral follicles with BPA to determine whether overexpressing Esr1 increases the susceptibility of follicles to BPA-induced growth inhibition. Exposure to 100 μg/ml BPA significantly decreased follicle growth compared to DMSO in controls (Fig. 5A) and Esr1 OE (Fig. 5B) follicles beginning at 72 h and continuing throughout culture. BPA at 1 μg/ml and BPA at 10 μg/ml did not significantly affect growth in either control or Esr1 OE follicles.
FIG. 5. .
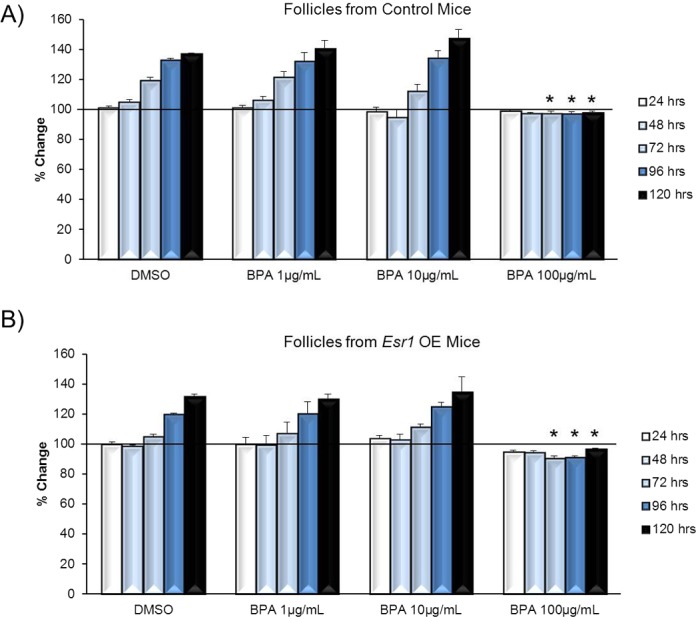
Effect of overexpressing Esr1 on susceptibility to BPA-induced follicle growth inhibition. Antral follicles were mechanically isolated from control (A) or Esr1 OE (B) mice and exposed in vitro to DMSO or BPA at 10–100 μg/ml for 24–120 h. Growth of follicles was monitored during culture, recorded in micrometers, and reported as percent change. Graphs show means ± SEM from at least three separate experiments. Lines with asterisks (*) show data that are significantly different from those of DMSO controls (n = 8–16 follicles per treatment per experiment from at least three separate experiments; P ≤ 0.05).
Effect of BPA on Cell Cycle Regulators
Even though BPA does not work through ESR1 to inhibit follicle growth, BPA still inhibits follicle growth (Fig. 1). Thus, we next examined whether BPA alters the expression of cell cycle regulators in antral follicles, because cell cycle regulators have been shown to control granulosa cell proliferation and, thus, control follicle growth [26]. Exposure to 100 μg/ml BPA significantly increased Cdk4 expression compared to DMSO controls beginning at 24 h and continuing throughout culture (Fig. 6A). BPA at 1 μg/ml and BPA at 10 μg/ml did not significantly affect Cdk4 expression compared to DMSO at any time point. Exposure to 100 μg/ml BPA significantly decreased Ccnd2 expression compared to DMSO at 48 h (Fig. 6B). BPA at 1 μg/ml and BPA at 10 μg/ml did not significantly affect Ccnd2 expression compared to DMSO at any time point. Exposure to 100 μg/ml BPA significantly increased Ccne1 expression compared to DMSO beginning at 24 h and continuing throughout culture (Fig. 7A). BPA at 1 μg/ml and BPA at 10 μg/ml did not significantly affect Ccne1 expression compared to DMSO at any time point. Exposure to 100 μg/ml BPA significantly increased Trp53 expression compared to DMSO beginning at 24 h and continuing throughout culture (Fig. 7B). BPA at 1 μg/ml and BPA at 10 μg/ml did not significantly affect Trp53 expression compared to DMSO at any time point.
FIG. 6. .
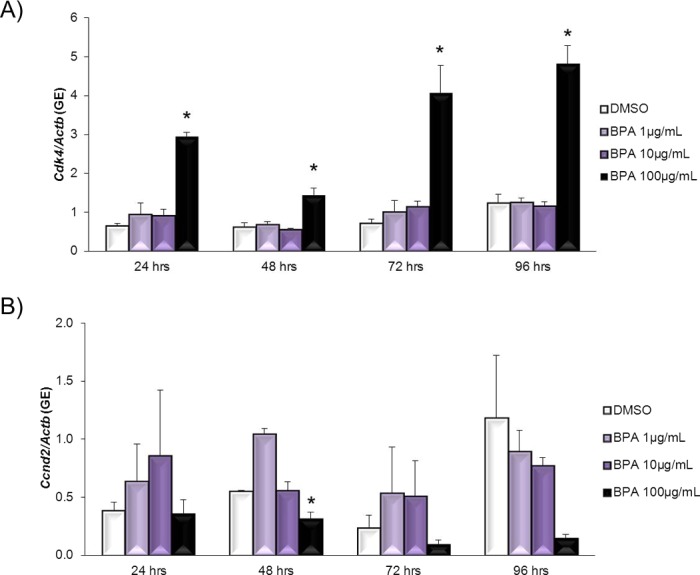
Effect of BPA on Cdk4 and Ccnd2 mRNA expression levels. After antral follicles were exposed to DMSO control or BPA at 1–100 μg/ml for 24–96 h in vitro, the follicles were collected and subjected to qPCR analysis for Cdk4 (A) and Ccnd2 (B) mRNA expression levels. All values were normalized to those of beta-actin as a loading control. Graphs show means ± SEM from at least three separate experiments. *P ≤ 0.05 from DMSO control.
FIG. 7. .
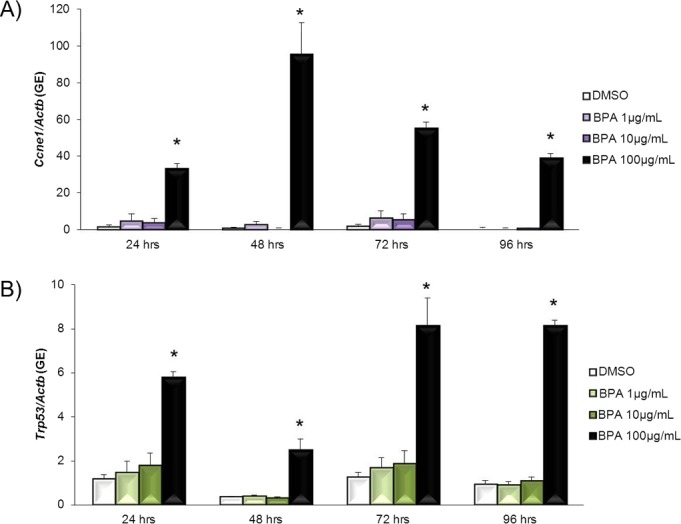
Effect of BPA on Ccne1 and Trp53 mRNA expression levels. After antral follicles were exposed to DMSO control or BPA at 1–100 μg/ml for 24–96 h in vitro, the follicles were collected and subjected to qPCR analysis for Ccne1 (A) and Trp53 (B) mRNA expression levels. All values were normalized to those of beta-actin as a loading control. Graphs show means ± SEM from at least three separate experiments. *P ≤ 0.05 from DMSO control.
Effect of BPA on Atresia of Antral Follicles
Another mechanism by which BPA might inhibit follicle growth may include inducing atresia. If BPA induces atresia, follicles would not be able to grow normally. Thus, we conducted studies to determine whether BPA induces atresia. Atresia was rated by quantifying the percentage of apoptotic bodies present in the follicles. These morphological changes in the follicle were used to determine whether BPA at 100 μg/ml induced atresia compared to DMSO beginning at 48 h and continuing to 120 h in culture (Fig. 8, A–D). Apoptotic bodies also were used to determine whether estradiol cotreatment does not protect follicles from BPA-induced atresia (Fig. 8, E and F). In DMSO- and estradiol-treated follicles at 48 and 120 h, granulosa cells were tightly organized in layers, and no apoptotic bodies were present within the follicle (Fig. 8, A, C, and E). In follicles treated with 100 μg/ml BPA and those treated with 100 μg/ml BPA plus estradiol (10 nM), beginning at 48 h and continuing at 120 h, granulosa cells were no longer tightly organized into multiple layers, and apoptotic bodies were present (Fig. 8, red arrows) in greater than 30% of the follicles. Furthermore, after follicles were exposed to BPA at 100 μg/ml or to BPA at 100 μg/ml plus estradiol (10 nM) at 120 h, few granulosa cells remained (Fig. 8, B, D, and F). Exposure to BPA at 100 μg/ml significantly induced atresia at 48 and 120 h compared to DMSO (Fig. 9A). Cotreatment with estradiol did not protect antral follicles from BPA-induced atresia (Fig. 9B) at any time point.
FIG. 8. .
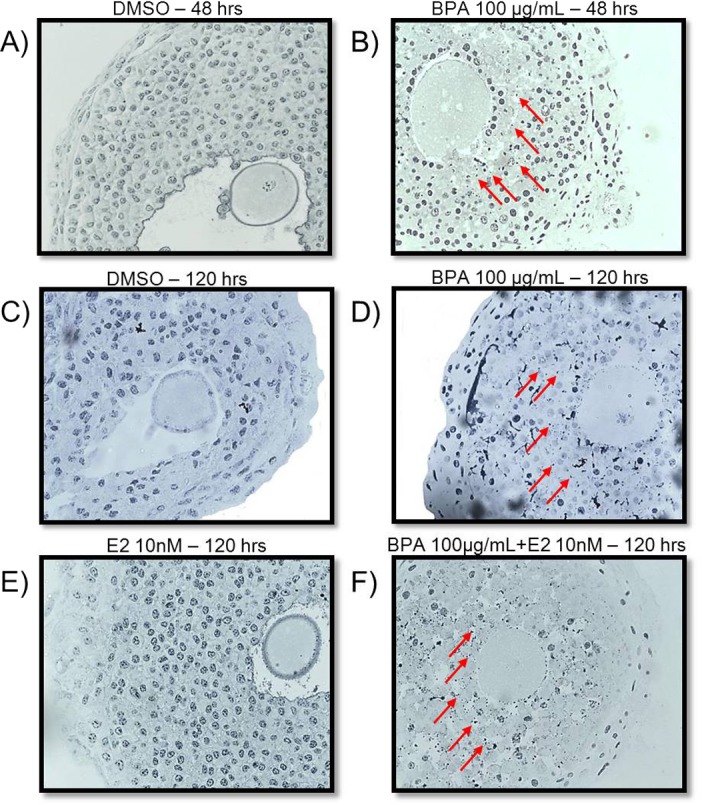
Effect of BPA on morphology. After antral follicles were exposed to DMSO (A, C), BPA at 100 μg/ml (B,D), estradiol at 10 nM (E), or BPA at 100 μg/ml plus estradiol at 10 nM (F) for 48 and/or 120 h, the follicles were removed from culture, and atresia was observed as described in Materials and Methods. Red arrows indicate apoptotic bodies. *P ≤ 0.05 from DMSO control. Original magnification ×40.
FIG. 9. .
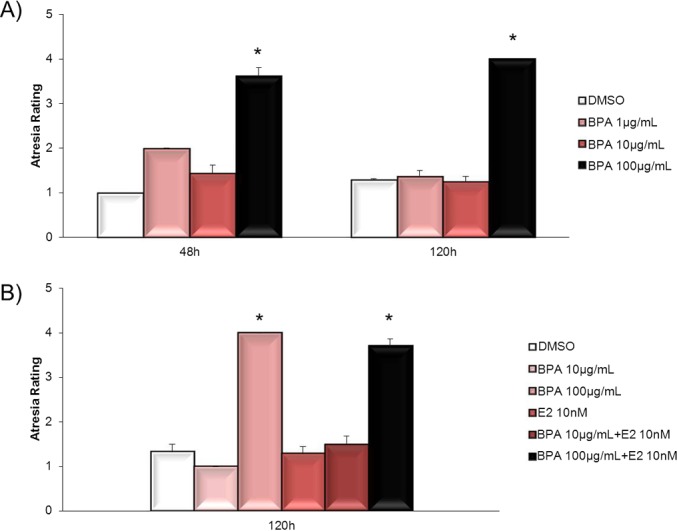
Effect of BPA on atresia. After antral follicles were exposed to DMSO or BPA at 100 μg/ml for 48–120 h (A) or to DMSO, BPA at 10–100 μg/ml, estradiol at 10 nM, or BPA at 10–100 μg/ml plus estradiol at 10 nM for 120 h (B), the follicles were removed from culture and processed for histological evaluation of atresia as described in Materials and Methods. Atresia of follicles was reported as a rating per treatment group. Graph shows means ± SEM from at least three separate follicles per treatment group. *P ≤ 0.05 from DMSO control.
Because BPA induces atresia in antral follicles (Fig. 9), we next determined whether BPA alters the expression of selected regulators of atresia. Exposure to BPA at 100 μg/ml significantly increased expression of Bax, a proatretic factor, compared to DMSO beginning at 24 h and continuing throughout culture (Fig. 10A). BPA at 1 μg/ml and BPA at 10 μg/ml did not significantly affect Bax expression compared to DMSO at any time point. Similarly, exposure to BPA at 100 μg/ml significantly increased expression of Bcl2, an antiatretic factor, compared to DMSO beginning at 24 h and continuing throughout culture (Fig. 10B). BPA at 1 μg/ml and BPA at 10 μg/ml did not significantly affect Bcl2 expression compared to DMSO at any time point.
FIG. 10. .
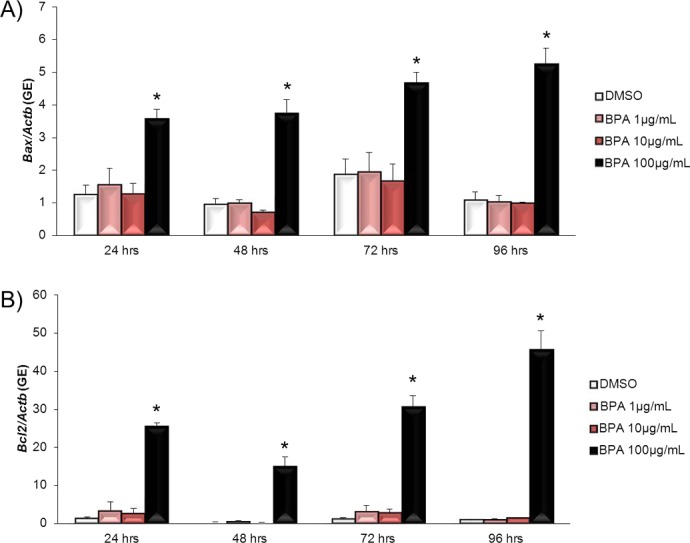
Effect of BPA on Bax and Bcl2 mRNA expression levels. After antral follicles were exposed to DMSO control or BPA at 1–100 μg/ml for 24–96 h in vitro, the follicles were collected and subjected to qPCR analysis for Bax (A) and Bcl2 (B) mRNA expression levels. All values were normalized to those of beta-actin as a loading control. Graphs show means ± SEM from at least three separate experiments. *P ≤ 0.05 from DMSO control.
DISCUSSION
Consistent with this study, previous work by our group has shown that in vitro BPA exposure inhibits antral follicle growth [24]. However, the current study expands on previous studies by showing that BPA does not work through genomic estrogen receptors to inhibit follicle growth. Although BPA binds with less affinity than estradiol to ESR1 [33–35] and ESR2 [34, 34], studies have shown that BPA acts through genomic estrogen receptors in other cell types. BPA induces Esr1 expression in fetal mouse mesenchyme cells [36], induces Esr1 expression and estrogen responsive proteins in the female rat uterus [33], and induces activation of estrogen response elements in Hep2G cells [33] and pituitary, stimulating ESR-dependent prolactin release and cell proliferation in the pituitary [33]. In our study, Esr1 expression was up-regulated in the follicles with inhibited growth at 24 h but then down-regulated at 72 h, indicating BPA has an effect on Esr1 but that this effect is partially time-dependent. Furthermore, although 10 μg/ml BPA did not significantly inhibit follicle growth, exposure to 10 μg/ml BPA increased Esr2 expression beginning at 24 h and continuing throughout culture. Granulosa cell proliferation and follicle growth are driven by ESR2 [37], thus up-regulation of Esr2 may have protected these follicles from BPA-induced growth inhibition by promoting granulosa cell proliferation.
After identifying the fact that BPA increases expression of Esr1 in follicles with inhibited growth, we wanted to determine whether BPA works through this receptor. Previous studies have shown that estradiol can displace BPA from ESR1 in competitive binding assays [34] and that ICI can block Esr1 activation by BPA in Hep2G cells transfected with Esr1 [33]. Furthermore, overexpression of Esr1 can increase the susceptibility of follicles to growth inhibition after exposure to other endocrine-disrupting chemicals, such as methoxychlor [30]. Thus, we hypothesized that inhibition of follicle growth is mediated through Esr1 and that addition of estradiol and ICI would protect the follicles from BPA-induced inhibition of follicle growth. Furthermore, overexpression of Esr1 would make follicles more susceptible to BPA. However, neither cotreatment with estradiol nor pretreatment with ICI prevented BPA from inhibiting follicle growth. Additionally, overexpression of Esr1 did not increase the sensitivity of follicles to BPA-induced follicle growth inhibition.
Although our data indicate that BPA does not inhibit follicle growth directly through the genomic estrogenic pathway, BPA does affect Esr1 and Esr2 expression and thus may mediate estrogenic effects in the follicle. Other studies have shown that BPA quickly acts through the nongenomic estrogen responsive GPR30 and ERK pathways in breast cancer cells and that similar to our results, these effects could not be prevented by ICI [8]. Therefore, we speculate that BPA may inhibit follicle growth through a nongenomic estrogenic pathway, although further studies are needed to confirm this hypothesis.
Previous studies have shown that follicle growth is not always dependent on hormone production by follicles. Specifically, previous studies indicate BPA at 10 μg/ml inhibits steroidogenesis without affecting follicle growth [24]. Thus, we examined other mechanisms by which BPA inhibits follicle growth. Morphological examination of the follicles over time indicated striking differences between control and BPA-treated follicles (Fig. 2). Control follicles readily changed in shape and expanded in size, whereas follicles treated with 100 μg/ml BPA seemed suspended in time or frozen; they did not grow or change in size or appearance outside of becoming more opaque. Follicle growth is dependent on granulosa cell proliferation, and this is regulated, in part, by the cell cycle [26]. Therefore, we considered the possibility that this suspension/inhibition of growth by BPA was due to BPA affecting the cell cycle. The cell cycle is a dynamic process of DNA replication and cell division and is separated by growth phases [26, 27]. In these phases, cells grow in size and replicate their cytosolic contents in preparation for cell division and, thus, proliferation [26]. Estrogenic endocrine disruptors can inhibit follicle growth by down-regulating expression of cell cycle regulators during the G1-S transition [38]. However, in this study, while BPA down-regulated Ccnd2 expression at 48 h, BPA up-regulated the cell cycle regulators Cdk4 and Ccne1 and the inhibitor Trp53 beginning at 24 h and continuing throughout culture (Fig. 11). Similar to our studies, others have shown that BPA affects the cell cycle of follicular cells. BPA inhibits cell cycle progression in oocytes [39] and reduces granulosa cell proliferation of early preantral follicles in vitro [40]. Although the up-regulation of Cdk4 and Ccne1 should favor cell proliferation and, therefore, these factors should be down-regulated in quiescent follicles, up-regulation of Trp53 coupled with down-regulation of Ccnd2 might be preventing cell proliferation, explaining the inhibited growth and stalled appearance in BPA treated follicles [26].
FIG. 11. .
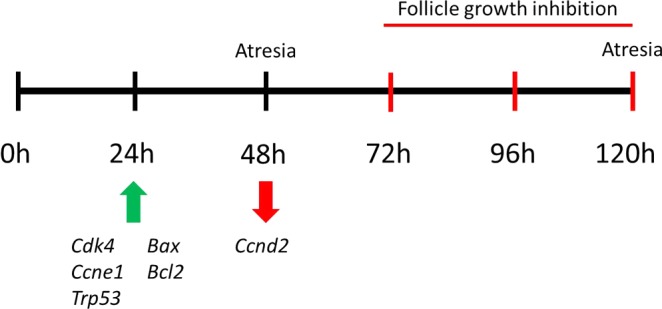
Schematic of observed effects of BPA on gene expression, follicle growth inhibition, and atresia. At 24 h, BPA significantly increases expression of Cdk4, Ccne1, Trp53, Bax, and Bcl2. At 48 h, BPA significantly decreases Ccnd2 and induces atresia. Between 72 and 120 h, BPA significantly inhibits follicle growth and induces atresia.
In addition to examining whether BPA inhibited follicle growth by affecting cell cycle regulators, we examined whether BPA could inhibit growth because it induced atresia. We found that BPA at 100 μg/ml induced atresia beginning at 48 h in culture, 24 h before follicle growth was inhibited, compared to DMSO controls (Fig. 11). This is the same concentration that inhibits follicle growth, suggesting that follicles are not growing because they are dying. The BCL2 family mediates atresia and helps determine whether cells within the follicle will remain healthy or undergo atresia [19, 27, 28, 41]. The family consists of numerous proteins that are either proapoptotic, such as BAX, or antiapoptotic, such as BCL2. We found that BPA at 100 μg/ml induced Bax beginning at 24 h and continuing throughout culture (Fig. 11). Unexpectedly, BPA at 100 μg/ml also induced Bcl2 expression beginning at 24 h (Fig. 11). These findings correspond with those of other studies showing that BPA arrests the cell cycle and dysregulates atresia factors and induces cell death in isolated granulosa cells [28]. This increase in Bcl2 as well as Bax was surprising because it is well known that a higher Bax:Bcl2 ratio will promote atresia, whereas a high Bcl2:Bax ratio promotes survival because BCL2 binds to and inactivates BAX [42]. However, in this study, a higher Bcl2:Bax ratio did not protect the follicles from atresia. It is possible that follicles might up-regulate Bcl2 to try and prevent atresia but that this rescue attempt was unsuccessful. We also found that BPA at 100 μg/ml induced Trp53 expression, a direct transcriptional regulator of Bax [42], beginning at 24 h and continuing throughout culture. Therefore, BPA may trigger the atresia pathway, stimulating the up-regulation of Trp53 and the proatresia pathway. These actions ultimately inhibit follicle growth and induce atresia despite attempts to promote survival by up-regulating Bcl2.
It is well understood that the oocyte and somatic cells of ovarian follicles bidirectionally regulate each other for proper oocyte maturation and somatic cell proliferation and differentiation [43]. In these studies, the effects of BPA on the oocyte were not measured. Although the effects of BPA in both the oocytes and the surrounding follicles have been investigated separately, there are no studies investigating whether the effects of BPA originate in the oocyte or the somatic cells. In isolated granulosa cells, BPA exposure within 24–72 h resulted in arrested mitosis of granulosa cells [26]. Numerous studies have shown BPA affects the oocytes, although these effects ranged from 16 h [13] to 7 days [16]. Further studies are needed to accurately assess whether BPA effects originate in the somatic cells or in the oocyte.
The implications of our findings may be relevant to humans. Although the highest dose used in this study, 100 μg/ml BPA, is higher than some other concentrations observed in other fluids and placental tissues, it is not known how much BPA is found in antral follicles, and it is possible that concentrations could reach these levels. Antral follicles are highly vascularized structures, potentially exposed constantly to BPA released from bioaccumulation in fatty tissues surrounding the ovaries [44]. Studies have shown that levels of BPA in fatty tissues can be higher than those measured in serum [45, 46]. Furthermore, humans are constantly exposed to BPA, as indicated by the presence of BPA in 93%–96% of urine samples taken in various studies. Estimates of daily BPA intake range from 0.6 to 71.4 μg/day, although this is only an estimate using urine samples taken in the morning from human study subjects and could be higher [1, 4]. Although this is only speculation, bioaccumulation in fat is highly likely, making levels of BPA used in our study plausible for human exposure. This may be particularly important because studies indicate that some women undergoing in vitro fertilization treatment have high levels of BPA [47].
Overall, this study shows that exposure to BPA inhibits growth and induces atresia in antral follicles. Future studies should examine whether BPA affects follicle growth and atresia in humans.
Footnotes
Supported by National Institutes of Health grants R01ES019178 and P20ES018163.
REFERENCES
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007; 24: 139 177 [DOI] [PubMed] [Google Scholar]
- He Y, Miao M, Wu C, Yuan W, Gao E, Zhou Z, Li D-K. Occupational exposure levels of bisphenol A among Chinese workers. J Occup Health 2009; 51: 432 436 [DOI] [PubMed] [Google Scholar]
- He Y, Miao M, Herrinton LJ, Wu C, Yuan W, Zhou Z, Li D-K. Bisphenol A levels in blood and urine in a Chinese population and the personal factors affecting the levels. Environ Res 2009; 109: 629 633 [DOI] [PubMed] [Google Scholar]
- Ouchi K, Watanabe H. Measurement of bisphenol A in human urine using liquid chromatography with multi-channel coulometric electrochemical detection. J Chromatogr B 2002; 780: 365 370 [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 2005; 113: 391 395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol 2007; 24: 178 198 [DOI] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 2006; 147: S56 S69 [DOI] [PubMed] [Google Scholar]
- Dong S, Terasaka S, Kiyama R. Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ Pollut 2011; 159: 212 218 [DOI] [PubMed] [Google Scholar]
- Matsushima A, Teramoto T, Okada H, Liu X, Tokunaga T, Kakuta Y, Shimohigashi Y. ERRgamma tethers strongly bisphenol A and 4-alpha-cumylphenol in an induced-fit manner. Biochem Biophys Res Commun 2008; 373: 408 413 [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res 2006; 1068: 49 55 [DOI] [PubMed] [Google Scholar]
- Yoneda T, Hiroi T, Osada M, Asada A, Funae Y. Non-genomic modulation of dopamine release by bisphenol-A in PC12 cells. J Neurochem 2003; 87: 1499 1508 [DOI] [PubMed] [Google Scholar]
- Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res 2003; 45: 345 356 [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology 2006; 148: 116 127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill YB, Petre CE, Monk KR, Puga A, Knudsen KE. The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostatic adenocarcinoma cells. Mol Cancer Ther 2002; 1: 515 524 [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Vogt E, Cukurcam S, Sun F, Pacchierotti F, Parry J. Exposure of mouse oocytes to bisphenol A causes meiotic arrest but not aneuploidy. Mutat Res 2008; 651: 82 92 [DOI] [PubMed] [Google Scholar]
- Mohri T, Yoshida S. Estrogen and bisphenol A disrupt spontaneous [Ca2+]i oscillations in mouse oocytes. Biochem Biophys Res Commun 2005; 326: 166 173 [DOI] [PubMed] [Google Scholar]
- Susiarjo M, Hassold TJ, Freeman E, Hunt PA, Bisphenol A. Exposure in utero disrupts early oogenesis in the mouse. PLoS Genet 2007; 3: 63 70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr Biol 2003; 13: 546 553 [DOI] [PubMed] [Google Scholar]
- Willians CJ, Erickson GF. Morphology and physiology of the ovary. In: Rebar RW. (ed.), Female Reproductive Endocrinology. Endotext.com: 2012.World Wide Web (URL: http://www.endotext.org/female/female1/femaleframe1.htm.) (July 2012)
- Crozet N, Ahmed-Ali M, Dubos MP. Developmental competence of goat oocytes from follicles of different size categories following maturation, fertilization and culture in vitro. J Reprod Fertil 1995; 103: 293 298 [DOI] [PubMed] [Google Scholar]
- Khatir H, Anouassi A, Tibary A. Effect of follicular size on in vitro developmental competence of oocytes and viability of embryos after transfer in the dromedary (Camelus dromedarius). Anim Reprod Sci 2007; 99: 413 420 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien M, Wigglesworth K. Mammalian oocyte growth and development in vitro. Mol Reprod Dev 1996; 44: 260 273 [DOI] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci 2006; 93: 382 389 [DOI] [PubMed] [Google Scholar]
- Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and down-regulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci 2010; 119: 209 217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MC, Midgley AR, Jr, Richards JS. Hormonal regulation of ovarian cellular proliferation. Cell 1978; 14: 71 78 [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. The cell cycle and programmed cell death. : Alberts B. (ed.), Molecular Biology of the Cell, 4 ed. New York: Garland Science; 2002; 983 1027 [Google Scholar]
- Quirk SM, Cowan RG, Harman RM, Hu CL, Porter DA. Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. J Anim Sci 2004; 82: E40 E52 [DOI] [PubMed] [Google Scholar]
- Xu J, Osuga Y, Yano T, Morita Y, Tang X, Fujiwara T, Takai Y, Matsumi H, Koga K, Taketani Y, Tsutsumi O. Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochem Biophys Res Commun 2002; 292: 456 462 [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci 2006; 93: 180 188 [DOI] [PubMed] [Google Scholar]
- Paulose T, Hernández-Ochoa I, Basavarajappa MS, Peretz J, Flaws JA. Increased sensitivity of estrogen receptor alpha overexpressing antral follicles to methoxychlor and its metabolites. Toxicol Sci 2011; 120: 447 459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic D, Frech MS, Babus JK, Symonds D, Furth PA, Koos RD, Flaws JA. Effects of ERalpha overexpression on female reproduction in mice. Reprod Toxicol 2007; 23: 317 325 [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfield CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci 2005; 88: 213 221 [DOI] [PubMed] [Google Scholar]
- Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, Safe S, McDonnell DP, Gaido KW. Bisphenol A interacts with the estrogen receptor α in a distinct manner from estradiol. Mol Cell Endocrinol 1998; 142: 203 214 [DOI] [PubMed] [Google Scholar]
- Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors a and ß. Chem Res Toxicol 2001; 14: 149 157 [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 1993; 132: 2279 2286 [DOI] [PubMed] [Google Scholar]
- Richter CA, Taylor JA, Ruhlen RL, Welshons WV, vom Saal FS. Estradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ Health Perspect 2007; 115: 902 908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele-Hartung C, Siebel P, Peters O, Kosemund D, Müller G, Hillisch A, Walter A, Kraetzschmar J, Fritzemeier KH. Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc Natl Acad Sci U S A 2004; 101: 5129 5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Meachum S, Hernández-Ochoa I, Peretz J, Yao HH, Flaws JA. Methoxychlor inhibits growth of antral follicles by altering cell cycle regulators. Toxicol Appl Pharmacol 2009; 240: 1 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Semiz O, Cinar O. Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol Hum Reprod 2005; 11: 389 396 [DOI] [PubMed] [Google Scholar]
- Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat Res 2008; 651: 71 81 [DOI] [PubMed] [Google Scholar]
- Markström E. Svensson ECh, Shao R, Svanberg B, Billig H. Survival factors regulating ovarian apoptosis-dependence on follicle differentiation. Reproduction 2002; 123: 23 30 [DOI] [PubMed] [Google Scholar]
- Basu A, Haldar S. The relationship between Bcl2, Bax, and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod 1998; 4: 1099 1109 [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001; 122: 829 838 [DOI] [PubMed] [Google Scholar]
- Tillet T, Bisphenol A. chapter 2: new data shed light on exposure, potential bioaccumulation. Environ Health Perspect 2009; 117 (5): A210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez AA, Kannan K, Giesy JP, Fang J, Clemens LG. Effects of bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere 2001; 42: 917 922 [DOI] [PubMed] [Google Scholar]
- Fernandez MF, Arrebola JP, Taoufiki J, Navalón A, Ballesteros O, Pulgar R, Vilchez JL, Olea N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol 2007; 24: 259 264 [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, Ye X, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect 2012; 120 (7): 978 983 [DOI] [PMC free article] [PubMed] [Google Scholar]


