ABSTRACT
Accumulating evidence strongly supports the premise that testosterone may be a key player in fetal programming on hypertension. Studies have shown that gestational protein restriction doubles the plasma testosterone levels in pregnant rats. In this study, we hypothesized that elevated testosterone levels in response to gestational protein restriction were caused by enhanced expression of steroidogenic enzymes or impaired expression of Hsd17b2, a known testosterone inactivator that converts testosterone to androstenedione in placenta. Pregnant Sprague-Dawley rats were fed normal (20% protein, control; n = 10) or a low-protein diet (6% protein, PR; n = 10) from Day 1 of pregnancy until killed at Days 14, 18, or 21. Junctional (JZ) and labyrinth (LZ) zones of placenta were collected for expression assay on steroidogenic genes (Cyp11a1, Hsd3b1, Cyp17a1, Hsd17b2, and Srd5a1) by real-time PCR. The main findings include the following: 1) expressions of Cyp11a1, Hsd3b1, and Cyp17a1 in JZ were not affected by diet but were affected by day of pregnancy; 2) expression of Hsd17b2 in both female and male JZs was remarkably increased by PR at Days 18 and 21 of pregnancy; 3) expressions of Hsd17b2 were reduced by PR in both female and male LZ at Day 18 of pregnancy and in female LZ at Day 21 of pregnancy; and 4) expression of Srd5a1in LZ was not affected by day of pregnancy, gender, or diet. These results indicate that in response to gestational protein restriction, Hsd17b2 may be a key regulator of testosterone levels and associated activities in placental zones, apparently in a paradoxical manner.
Keywords: androgen, gestational protein restriction, Hsd17b2, junctional zone, labyrinth zone, placenta, pregnancy, rat, steroidogenic gene, testosterone
In the rat, gestational protein restriction reduces expression of Hsd17b2 in the placental labyrinth zone in late pregnancy, allowing testosterone to infuse into fetal circulation and contribute to fetal programming of hypertension.
INTRODUCTION
Accumulating evidence indicates that offspring from dams with gestational protein restriction develop hypertension and other cardiovascular diseases in adulthood, and this occurs in a gender- and time-dependent manner, with an earlier onset and more severe hypertension in males compared with females [1–7]. Among the potential multiple factors responsible for the fetal programming on adulthood hypertension and cardiovascular diseases, the placenta may contribute largely to the process of programming, apparently because of its critical roles in hormone production and nutrient transport [8–11] and susceptibility to external stimuli, including nutritional and hormonal interruptions and stress during pregnancy [12–14].
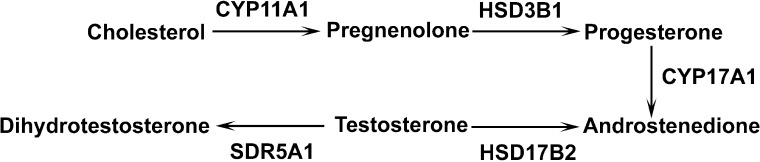
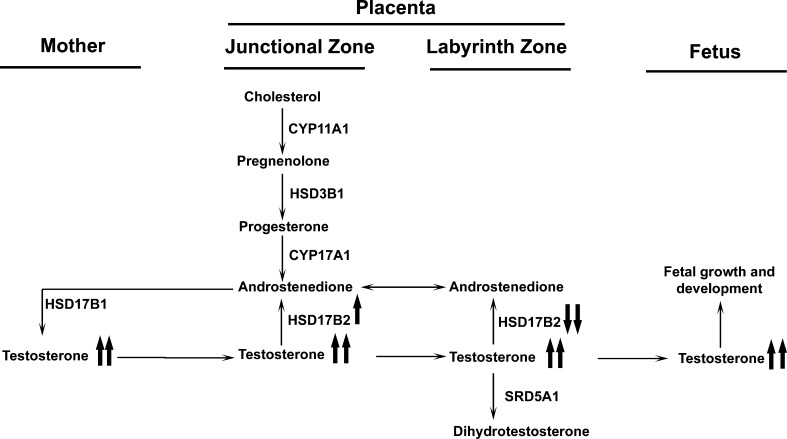
Two distinct placental zones in rodents, the junctional (JZ) and the labyrinth zones (LZ), execute different functions. The JZ is responsible mainly for hormone production, while the LZ represents the main place for maternal-fetal hemotrophic exchange in rodents [15]. In JZ, the presence of the steroidogenic enzymes provides the machinery for androgen production (Fig. 1). The rodent placenta, in particular, provides the majority of androstenedione in late pregnancy, and androstenedione is the sole substrate in estrogen production in the corpus luteum [16–19]. Therefore, the androgen production in the placenta is of great significance in maintaining a normal pregnancy in rodents. Similarly, LZ is of great importance in fetal survival and development. Maternal blood flows through irregularly shaped spaces, while fetal blood circulates within the fetal capillaries [20]. The LZ undergoes rapid and intense angiogenesis, resulting in expanded maternal blood space and progressive fetal capillary development in mid- and late pregnancy, which may be one of the mechanisms responsible for the remarkable increase in placental efficiency in late pregnancy [15, 20]. In addition to nutrient transport, the placenta, especially the LZ, builds functional barriers at the maternal and fetal interface to prevent fetal overexposure to certain bioactive reagents, such as glucocorticoids [21–23] and monoamines [24], and to maintain normal fetal growth. The insufficient placental barriers may be responsible for adulthood cardiovascular, metabolic, neuroendocrine, and psychiatric disorders in the progeny [22].
FIG. 1. .
Androgen production in rat placenta. The arrow indicates the direction of the converting process catalyzed by the enzyme. CYP11A1, cytochrome P450, family 11, subfamily a, polypeptide 1; HSD3B1, hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1; CYP17A1, cytochrome P450, family 17, subfamily A, polypeptide 1; HSD17B2, hydroxysteroid (17-beta) dehydrogenase 2; SRD5A1, steroid-5-alpha-reductase, alpha polypeptide 1 (3-oxo-5 alpha-steroid delta 4-dehydrogenase alpha 1).
Pregnant rats subjected to protein restriction have been widely used in the study of metabolic programming and associated offspring hypertension [25]. Although the underlying mechanisms remain unclear, the placenta, the mediator between mother and fetus, is affected by gestational protein restriction in many aspects. First, a microarray analysis with limited probe sets demonstrated that gestational protein restriction in the mouse has deleterious effects on placental development, with altered expression of genes related to cell growth and metabolism, apoptosis, and epigenetic control [26]. Second, placental nutrient transport including neutral amino acids [27, 28] was impaired by dietary protein restriction. Third, the renin-angiotensin system, which normally demonstrates dynamic changes in mid- and late pregnancy, was altered in both the LZ [29] and the uterine artery [30] by gestational protein restriction. The resultant reduction of uteroplacental blood flow and the associated uteroplacental insufficiency may finally cause intrauterine growth retardation [31]. Fourth, placental androgen production may be affected, as protein restriction alters the endocrine status of pregnant rats to include elevated plasma levels of testosterone and estradiol [32] and reduced plasma levels of progesterone [33, 34]. However, the reason for the elevated plasma testosterone in pregnant rats with protein restriction is unknown.
The elevated testosterone levels are associated with pregnancy-related complications, such as preeclampsia [35–37] and polycystic ovarian syndrome [38] in humans. More important, emerging evidence suggests that testosterone may play a role in fetal programming of hypertension by interrupting the renin-angiotensin system systemically or locally [39] or impairing vascular relaxation of nitric oxide in resistance artery [40]. The clinical importance of maintaining normal testosterone during pregnancy, together with the indispensability of placental androgen production in rodent pregnancy, inspired us to uncover the cause of elevated testosterone levels in pregnant rats with gestational protein restriction. In this study, we hypothesized that elevated testosterone levels in response to gestational protein restriction were caused by enhanced expression of steroidogenic enzymes or impaired expression of Hsd17b2, a known testosterone inactivator that converts testosterone to androstenedione in the placenta. The expression of steroidogenic genes (Cyp11a1, Cyp17a1, Hsd3b1, Hsd17b2, and Srd5a1) in LZ and JZ was analyzed by quantitative real-time PCR. Our study determined the effects of protein restriction, day of pregnancy, and/or gender of placenta on these gene expressions.
MATERIALS AND METHODS
Animals
All procedures were approved by the Animal Care and Use Committee at the University of Texas Medical Branch at Galveston and were in accordance with those guidelines published by the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). Virgin female Sprague-Dawley rats (Harlan Sprague Dawley, Houston, TX) weighing between 175 and 225 g were mated with male Sprague-Dawley rats. Day 1 of pregnancy was established when conception was confirmed by observation of a vaginal copulation plug or the presence of sperm in the vaginal flush. Pregnant rats were randomly divided into two dietary groups, housed individually, and fed a control (CT group, 20% protein) or low protein (PR group, 6% protein) diet until killed on Days 14, 18, or 21 of pregnancy (n = 10 per diet per day of pregnancy). The isocaloric low-protein and normal-protein diets were obtained from Harlan Teklad (Madison, WI; catalog nos. TD.90016 and TD.91352, respectively). The animals were housed in a room with a controlled temperature and a 12-h light:dark cycle. During 0800–1000 h on Days 14, 18, and 21 of pregnancy, rats were anesthetized with carbon dioxide. Maternal blood was collected by cardiac puncture into a BD vacuum tube containing K2-EDTA. The LZ and JZ were dissected as described by Ain et al. [41]. Liquid nitrogen was used to snap-freeze LZ and JZ, which were stored at −80°C until analyzed.
DNA Extraction from Fetal Extraembryonic Membrane and Sex Determination
Genomic DNA was extracted from frozen fetal membranes and tails of adult male and female rats with Qiagen DNeasy Blood & Tissue Kit (Qiagen Inc., Valencia, CA; catalog no. 69504), and all procedures were performed according to the instruction manual. Method used for sex determination were described previously [42].
RNA Extraction and RT-PCR
Total RNAs from frozen JZ and LZ tissues (n = 6 per diet per gender per day of pregnancy) were extracted using Qiagen RNeasy minikit (Qiagen; catalog no. 74104). The total RNAs were digested with RNA-free DNase I (Qiagen; catalog no. 79254), followed by a cleanup procedure. All procedures were performed according to the instruction manual. Complementary DNA (cDNA) was synthesized from 1 μg of total RNA by RT in a total volume of 20 μl using MyCycler Thermal Cycler (Bio-Rad Laboratories, Hercules, CA; catalog no. 170-9703) with the following conditions: 1 cycle at 28°C for 15 min, 42°C for 50 min, and 95°C for 5 min.
Quantitative Real-Time PCR
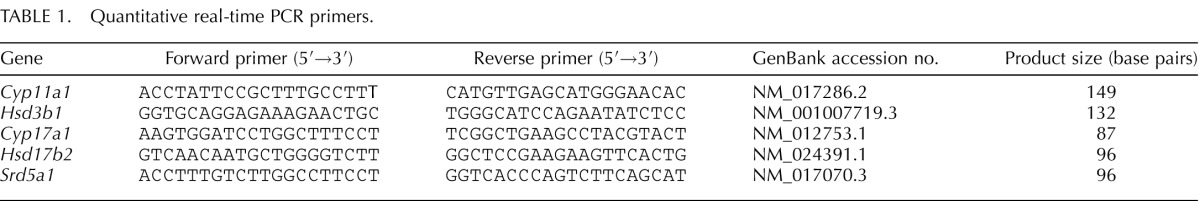
Real-time PCR detection was performed on a CFX96 real-time PCR Detection System (Bio-Rad; catalog no. 184-5096). The primers were designed using Primer 3 Version 4 and are shown in Table 1. Syber Green Supermix (Bio-Rad; catalog no.170-8882) was used for amplification of these genes. The mixture of reaction reagents was incubated at 95°C for 10 min and cycled according to the following parameters: 95°C for 30 sec and 60°C for 1 min for a total of 40 cycles. Negative control without cDNA was performed to test primer specificity.
TABLE 1. .
Quantitative real-time PCR primers.
Gapdh served as an endogenous control to standardize the amount of sample RNA added to a reaction. TaqMan Gene Expression Assays for rat Gapdh (Rn01775763_g1) and supermix reagents were from Applied Biosystems (Carlsbad, CA). The mixture of reaction reagents was incubated at 50°C for 2 min, heated to 95°C for 10 min, and cycled according to the following parameters: 95°C for 30 sec and 60°C for 1 min for a total of 40 cycles. The relative expression of target genes was calculated by use of the threshold cycle (CT) Gapdh/CT target gene.
Statistical Analysis
All quantitative data were subjected to least-squares analysis of variance using the general linear model procedures of the Statistical Analysis System (SAS Institute, Cary, NC). Data on gene expression were analyzed for effects of day of pregnancy, diet, gender of placenta, and their interaction. A P value of 0.05 or less was considered significant, whereas a P value greater than 0.05 but less than 0.10 was considered a trend toward significance. Data in gene expression are presented as least-squares means with overall standard errors.
RESULTS
Expressions of Steroidogenic Enzyme Genes in JZ in Response to Gestational Protein Restriction
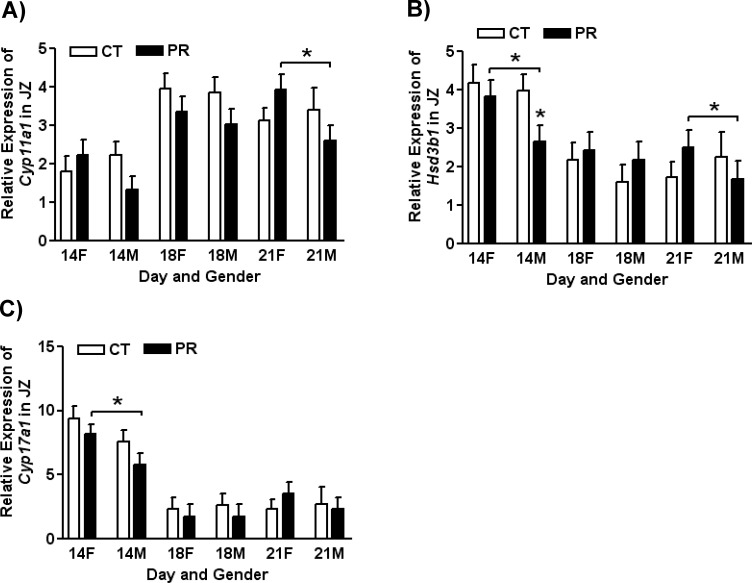
The expression of Cyp11a1 in JZ was affected by day of pregnancy (P < 0.0001), with higher expression at Days 18 and 21 of pregnancy compared with that at Day 14 of pregnancy. The mRNA levels of Cyp11a1 in JZ were not affected by diet; however, its expression in female JZ was higher than that in male JZ in PR rats at Day 21 of pregnancy (P < 0.05; Fig. 2A).
FIG. 2. .
Expressions of Cyp11a1 (A), Hsd3b1 (B), and Cyp17a1 (C) in placental junctional zones (JZ) in rats fed with the normal diet (CT) and low-protein diet (PR) at Days 14, 18, and 21 of pregnancy. The error bar represents the mean ± SEM. *P < 0.05.
The expression of Hsd3b1 in JZ was affected by day of pregnancy (P < 0.0001), with higher expression at Day 14 of pregnancy. The mRNA levels of Hsd3b1 were reduced by PR in male JZ at Day 14 of pregnancy, but they were not affected by diet. In addition, its expression in female JZ was higher than that in male JZ in PR rats at Days 14 and 21 of pregnancy (P < 0.05; Fig. 2B).
The expression Cyp17a1 in JZ was affected by day of pregnancy (P < 0.0001), with higher expression at Day 14 of pregnancy. The mRNA levels of Cyp17a1 in female JZ were higher than those in male JZ in PR rats at Days 14 and 21 of pregnancy (P < 0.05). Their expression in female JZ were decreased (P < 0.05) by PR at Day 14 of pregnancy (Fig. 2C).
Expression of Hsd17b2 in JZ and LZ in Response to Gestational Protein Restriction
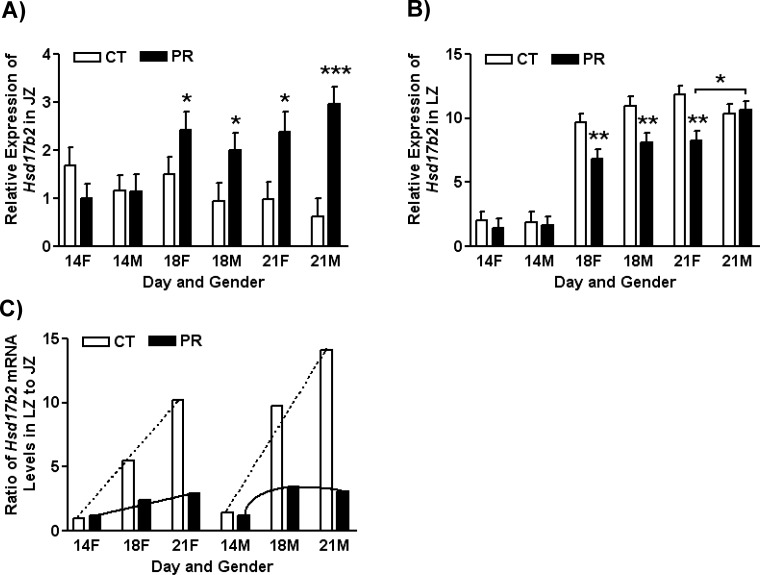
In JZ, the expression of Hsd17b2 was not affected by gender and diet at Day 14 of pregnancy, but its expression was remarkably increased in both female and male JZs by PR at Days 18 and 21 of pregnancy (P < 0.05). At Day 18 of pregnancy, mRNA levels of Hsd17b2 were increased 1.6- and 2.1-fold by PR in female and male JZ, respectively. Similarly, at Day 21 of pregnancy, mRNA levels of Hsd17b2 were increased 2.4- and 4.7-fold by PR in female and male JZ, respectively (Fig. 3A).
FIG. 3. .
Expression of Hsd17b2 in placental junctional (A) and labyrinth (B) zones in rats fed with the normal diet (CT) and low-protein diet (PR) at Days 14, 18, and 21 of pregnancy and the ratio of Hsd17b2 mRNA levels in LZ to JZ (C). The error bar represents the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
In LZ, the expression of Hsd17b2 was increased (P < 0.0001) 5.0- and 5.9-fold at Days 18 and 21 of pregnancy, respectively, compared with Day 14 of pregnancy. At Day 14 of pregnancy, the expression of Hsd17b2 was not affected by gender and diet. Its expression was significantly reduced (P < 0.01) in female and male JZs by PR at Days 18 of pregnancy and was also decreased (P < 0.01) in female JZ by PR at Day 21 of pregnancy. At Day 21 of pregnancy, in PR groups the expression of Hsd17b2 in LZ was higher in male than in female (P < 0.05; Fig. 3B).
To compare the relative abundance of Hsd17b2 mRNAs in LZ and JZ, the ratio of Hsd17b2 mRNA levels in LZ to JZ was calculated for each group defined by day of pregnancy, gender, and diet, as shown in Figure 3C. In female placentas in the CT group, the ratio of Hsd17b2 mRNA levels in LZ to JZ was linearly increased from Day 14 to Day 21 of pregnancy (y = 4.6x − 3.64; R2 = 0.99), while in female placentas in the PR group, the slope was reduced by 0.88 (y = 0.88x + 0.42; R2 = 0.96). Similarly, in male placentas in the CT group, the ratio of Hsd17b2 mRNA levels in LZ to JZ was linearly increased from Day 14 to Day 21 of pregnancy (y = 6.36x − 4.28; R2 = 0.96), while in male placentas in the PR groups, this ratio was increased from Day 14 to Day 18 of pregnancy but decreased thereafter (quadratic effect of day of pregnancy, y = −1.29x2 + 6.08x − 3.57; R2 = 1; Fig. 3C).
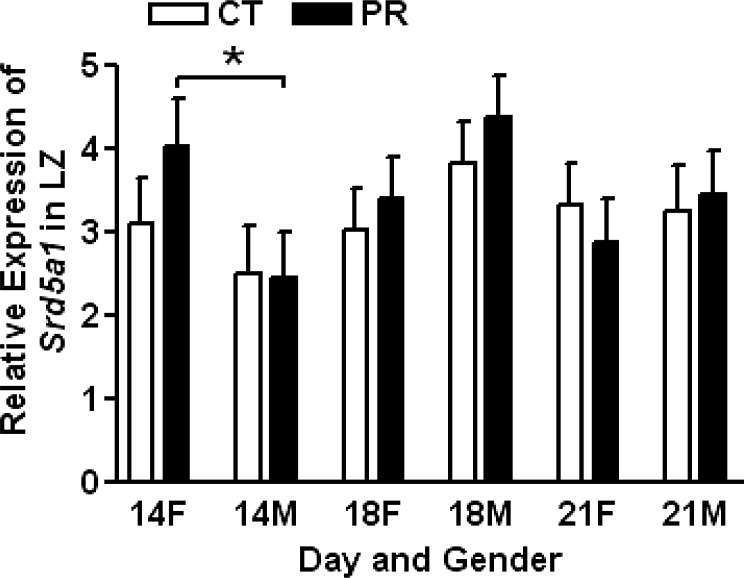
Expression of Srd5a1 in Labyrinth Zone in Response to Gestational Protein Restriction
The expression of Srd5a1 was not affected by gender, day of pregnancy, or diet. At Day 14 of pregnancy, the expression of Srd5a1 in PR female LZ was higher than that in PR male LZ (P < 0.05; Fig. 4).
FIG. 4. .
Expressions of Srd5a1 in placental labyrinth zones (LZ) in rats fed with the normal diet (CT) and low-protein diet (PR) at Days 14, 18, and 21 of pregnancy. The error bar represents the mean ± SEM. *P < 0.05.
DISCUSSION
This study for the first time reports that steroidogenic gene expressions in JZ were affected by maternal protein restriction in a gender- and time-dependent manner and that the expressions of Hsd17b2 in both JZ and LZ were affected by gestational protein restriction, with elevated expression in LZ and reduced expression in JZ. The finding that only Hsd17b2 among the enzymes for androgen production was affected by gestational protein restriction suggests the importance of Hsd17b2 in the placental programming on hypertension in response to maternal protein restriction during pregnancy.
The most striking finding in this study is that gestational protein restriction reduced the expression of Hsd17b2 in the labyrinth zone. The expression of Hsd17b2 was reduced in both female and male LZ at Day 18 of pregnancy and female LZ at Day 21 of pregnancy. Interestingly, this reduction in gene expression occurs when placenta synthesizes and secrets the largest amount of androgen in late pregnancy [16–19]. Not only is the LZ responsible for the nutrient transport from the maternal to the fetal side, but it is also emerging as a protective barrier at the maternal and fetal interface. For instance, the gradient expressions of Hsd11b2 [21] and Ido1 (indole 2,3-dioxygenase) [24] in the placenta build the corresponding barrier at the maternal and fetal interface to prevent fetal overexposure of glucocorticoids (cortisol) and monoamine, respectively. On the other hand, the impairment or insufficiency of these barriers results in fetal morbidity or mortality [21]. Similar to HSD11B2 and indole 2,3-dioxygenase in inactivating bioactive agents, in the rodents, placental HSD17B2 actively converts testosterone to androstenedione [43]. The fact that the expression of Hsd17b2 is interrupted by gestational protein restriction, with the assumption that HSD17B2 protein levels in the labyrinth are reduced by gestational protein restriction, supports the notion that more testosterone in the PR group can be synthesized and transported to the fetus because of the impaired function of HSD17B2. The increased testosterone levels may finally cause the fetal programming on hypertension, as our laboratory has accumulated data to support the role of testosterone in the fetal programming [40].
In contrast to its expression in LZ, the expression of Hsd17b2 in rat JZ was increased by gestational protein restriction at both Day 18 and Day 21 of pregnancy. If the function of this enzyme is restricted to inactivating the sex hormones, one may suggest that more testosterone will be converted into androstenedione in the PR group than in the control. In reality, in situ hybridization demonstrated that the expression of Hsd17b2 is much weaker in JZ compared to LZ in late pregnancy [44], which is consistent with the results of our real-time PCR analysis in this study. The mRNA levels of Hsd17b2 in JZ were comparable to those in LZ at Day 14 of pregnancy, and the ratios of mRNA levels in LZ to JZ were highly increased in both female and male placentas in CT groups at Days 18 and 21 of pregnancy, but the remarkable increase in the ratio was not seen in the PR groups (Fig. 3C) because of the reduced expression of Hsd17b2 in LZ (Fig. 3B) and elevated expression of Hsd17b2 in JZ (Fig. 3A) at Days 18 and 21 of pregnancy. In addition, the growth of JZ and LZ was impaired in the PR groups in late pregnancy [42]. Therefore, it is reasonable that the increased Hsd17b2 in JZ in gestational protein restriction could not compensate for the reduction in Hsd17b2 expression in LZ, and thus the availability of testosterone to the fetus is increased.
To date, the function of HSD17B2 in the placenta has not been completely understood. A recent mouse knockout study suggests the role of Hsd17b2 in the cellular organization in mouse placental development in addition to the canonical inactivation of sex hormones [45]. In our previous study, we did not find the obvious changes in the substructure of the rat placenta in response to gestational protein restriction [29], which is consistent with the findings from other researchers [46]. Thus, under the conditions of gestational protein restriction, the effect of the impaired Hsd17b2 expression in LZ may be achieved not by the involvement in cellular organization but by the regulation of sex hormone production.
The androgen production in the placenta may not be altered by gestational protein restriction in rats. In general, the expressions of key enzymes in androgen production, Cyp11a1, Hsd3b1, and Cyp17a1 were not affected by protein restriction, except for the reduced expression of Hsd3b1 in male JZ by PR at Day 14 of pregnancy (Fig. 2B). In addition, it is noteworthy that within the PR groups, the expression of Cyp11a1 in male JZ was lower than that in female JZ at Day 21 of pregnancy (Fig. 2A). Similarly, expression of Hsd3b1 was lower in PR male JZ at Days 14 and 21 of pregnancy (Fig. 2B), and expression of Cyp17a1 was lower in PR male JZ at Day 14 of pregnancy (Fig. 2C). Therefore, in response to gestational protein restriction, the substrates availability in this well-orchestrated cascade of enzymatic reactions (Fig. 1) may be less in male JZ than in female JZ. This phenomenon is apparently opposite to the earlier onset in occurrence and more severity of hypertension in male offspring programmed by gestational protein restriction [1–7]. The understanding of testosterone metabolism and function in fetal development, especially in late pregnancy, will be critical in revealing the mechanisms responsible for fetal programming on hypertension.
The limitation of this study is its lack of protein analysis due to there being no specific antibody for rat HSD17B2 available. The low sequence homology in the Hsd17b2 gene among different species, even between the mouse and the rat, contributes to the difficulty in studying this gene. Thus, the availability of an antibody for rat HSD17B2 protein will be very useful. In addition, conditional knockout and/or loss and gain function of Hsd17b2 may be an option in the study of this gene function.
In summary, results of the current study reveal that protein restriction reduced the expression of Hsd17b2 in rat LZ in late pregnancy, an effect that suggests the important role of Hsd17b2 in regulating the testosterone levels at the maternal and fetal interface. Based on this novel finding, we propose that the reduction in Hsd17b2 expression in LZ may allow more testosterone to reach the fetus, thus suggesting a mediating role for testosterone in the initiation of fetal programming on hypertension (Fig. 5).
FIG. 5. .
Schematic model of fetal overexposure to testosterone due to impaired placental HSD17B2 barrier in dams with gestational protein restriction. During the second half of pregnancy, the placental junctional zone synthesizes and releases androstenedione. In pregnant dams with gestational protein restriction, androstenedione of placental origin is converted to testosterone by HSD17B1, and maternal testosterone levels are elevated. Through the uteroplacental circulation, testosterone is transported into the placental junctional zone, where part of testosterone may be inactivated by HSD17B2. The rest of the testosterone in the placental labyrinth zone escapes the inactivation by HSD17B2 because of the reduced expression of Hsd17b2. Thus, presumably, the placental barrier of HSD17B2 that reduces testosterone availability to the fetus is compromised. As a result, testosterone at higher levels goes into fetal circulation and affects fetal growth and development, particularly cardiovascular development. The fetal overexposure to testosterone may initiate the process of fetal programming on hypertension. The thin arrow indicates the direction of the converting process catalyzed by the enzyme. The bold arrow denotes the changes in testosterone levels or Hsd17b2 expression, and the number of bold arrows indicates the magnitude of the changes.
Footnotes
Supported by the National Institutes of Health (NIH) through grants R01HL102866 and R01HL58144. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- Gangula PR, Reed L, Yallampalli C. Antihypertensive effects of flutamide in rats that are exposed to a low-protein diet in utero. Am J Obstet Gynecol 2005; 192: 952 960 [DOI] [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 2000; 127: 4195 4202 [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Gardner DS, Jackson AA. Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J Nutr 1996; 126: 1578 1585 [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 1999; 64: 965 974 [DOI] [PubMed] [Google Scholar]
- McMullen S, Langley-Evans SC. Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am J Physiol Regul Integr Comp Physiol 2005; 288: R85 R90 [DOI] [PubMed] [Google Scholar]
- McMullen S, Langley-Evans SC. Sex-specific effects of prenatal low-protein and carbenoxolone exposure on renal angiotensin receptor expression in rats. Hypertension 2005; 46: 1374 1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ, Wilkins A, Cunningham C, Perry VH, Seet MJ, Osmond C, Eckert JJ, Torrens C, Cagampang FR, Cleal J, Gray WP, Hanson MA. et al. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J Physiol 2008; 586: 2231 2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey K, Robinson S. Maternal nutrition, placental growth and fetal programming. Proc Nutr Soc 1998; 57: 105 111 [DOI] [PubMed] [Google Scholar]
- Jones HN, Powell TL, Jansson T. Regulation of placental nutrient transport–a review. Placenta 2007; 28: 763 774 [DOI] [PubMed] [Google Scholar]
- Myatt L. Placental adaptive responses and fetal programming. J Physiol 2006; 572: 25 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Wallace JM, Spencer TE. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci 2006; 84: 2316 2337 [DOI] [PubMed] [Google Scholar]
- Gheorghe CP, Goyal R, Mittal A, Longo LD. Gene expression in the placenta: maternal stress and epigenetic responses. Int J Dev Biol 2010; 54: 507 523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin I, Alvino G. Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta 2009; 30 (suppl A): S77 S82 [DOI] [PubMed] [Google Scholar]
- Godfrey KM. The role of the placenta in fetal programming—a review. Placenta 2002; 23 (suppl A): S20 S27 [DOI] [PubMed] [Google Scholar]
- Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 2002; 23: 3 19 [DOI] [PubMed] [Google Scholar]
- Jackson JA, Albrecht ED. The development of placental androstenedione and testosterone production and their utilization by the ovary for aromatization to estrogen during rat pregnancy. Biol Reprod 1985; 33: 451 457 [DOI] [PubMed] [Google Scholar]
- Sridaran R, Gibori G. Placental-ovarian relationship in the control of testosterone secretion in the rat. Placenta 1987; 8: 327 333 [DOI] [PubMed] [Google Scholar]
- Rembiesa R, Marchut M, Warchol A. Ovarian–placental dependency in rat. I. Biotransformation of C 21 steroids to androgens by rat placenta in vitro. Steroids 1972; 19: 65 84 [DOI] [PubMed] [Google Scholar]
- Rembiesa R. Biosynthesis, metabolism and regulation of steroid hormones secretion in the placental phase of gestation in the rat. Pol J Pharmacol Pharm 1984; 36: 187 197 [PubMed] [Google Scholar]
- Coan PM, Ferguson-Smith AC, Burton GJ. Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol Reprod 2004; 70: 1806 1813 [DOI] [PubMed] [Google Scholar]
- Brown RW, Diaz R, Robson AC, Kotelevtsev YV, Mullins JJ, Kaufman MH, Seckl JR. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology 1996; 137: 794 797 [DOI] [PubMed] [Google Scholar]
- Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal “programming” of adult pathophysiology. Nat Clin Pract Endocrinol Metab 2007; 3: 479 488 [DOI] [PubMed] [Google Scholar]
- Burton PJ, Waddell BJ. Dual function of 11beta-hydroxysteroid dehydrogenase in placenta: modulating placental glucocorticoid passage and local steroid action. Biol Reprod 1999; 60: 234 240 [DOI] [PubMed] [Google Scholar]
- Blaschitz A, Gauster M, Fuchs D, Lang I, Maschke P, Ulrich D, Karpf E, Takikawa O, Schimek MG, Dohr G, Sedlmayr P. Vascular endothelial expression of indoleamine 2,3-dioxygenase 1 forms a positive gradient towards the feto-maternal interface. PLoS One 2011; 6: e21774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautzky-Willer A, Handisurya A. Metabolic diseases and associated complications: sex and gender matter! Eur J Clin Invest 2009; 39: 631 648 [DOI] [PubMed] [Google Scholar]
- Gheorghe CP, Goyal R, Holweger JD, Longo LD. Placental gene expression responses to maternal protein restriction in the mouse. Placenta 2009; 30: 411 417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol 2006; 576: 935 946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario FJ, Jansson N, Kanai Y, Prasad PD, Powell TL, Jansson T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology 2011; 152: 1119 1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Yallampalli U, Yallampalli C. Maternal protein restriction reduces expression of angiotensin I-converting enzyme 2 in rat placental labyrinth zone in late pregnancy. Biol Reprod 2012; 86: 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Yallampalli U, Yallampalli C. Protein restriction to pregnant rats increases the plasma levels of angiotensin II and expression of angiotensin II receptors in uterine arteries. Biol Reprod 2012; 86 (3): 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz KM, Cuffe JS, Wilson LB, Dickinson H, Wlodek ME, Simmons DG, Denton KM. Review: sex specific programming: a critical role for the renal renin-angiotensin system. Placenta 2010; 31 (suppl): S40 S46 [DOI] [PubMed] [Google Scholar]
- Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol 2005; 566: 225 236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricks DM, Bailey LB. Effect of dietary protein restriction on hormone status and embryo survival in the pregnant rat. Biol Reprod 1976; 14: 143 150 [DOI] [PubMed] [Google Scholar]
- Mulay S, Varma DR, Solomon S. Influence of protein deficiency in rats on hormonal status and cytoplasmic glucocorticoid receptors in maternal and fetal tissues. J Endocrinol 1982; 95: 49 58 [DOI] [PubMed] [Google Scholar]
- Acromite MT, Mantzoros CS, Leach RE, Hurwitz J, Dorey LG. Androgens in preeclampsia. Am J Obstet Gynecol 1999; 180: 60 63 [DOI] [PubMed] [Google Scholar]
- Ficicioglu C, Kutlu T. The role of androgens in the aetiology and pathology of pre-eclampsia. J Obstet Gynaecol 2003; 23: 134 137 [DOI] [PubMed] [Google Scholar]
- Ghorashi V, Sheikhvatan M. The relationship between serum concentration of free testosterone and pre-eclampsia. Endokrynol Pol 2008; 59: 390 392 [PubMed] [Google Scholar]
- Katsikis I, Kita M, Karkanaki A, Prapas N, Panidis D. Late pregnancy complications in polycystic ovarian syndrome. Hippokratia 2006; 10: 105 111 [PMC free article] [PubMed] [Google Scholar]
- Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med 2008; 5 (suppl A): S121 S132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar K, Elkins R, Yallampalli U, Balakrishnan M, Yallampalli C. Fetal programming of adult hypertension in female rat offspring exposed to androgens in utero. Early Hum Dev 2011; 87: 407 414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ain R, Konno T, Canham LN, Soares MJ. Phenotypic analysis of the rat placenta. : Soares MJ, Hunt JS. (eds.), Placenta and Trophoblast: Methods and Protocols, vol. 1. Totowa, NJ: Humana Press Inc.; 2008: 295 313 [Google Scholar]
- Gao H, Sathishkumar KR, Yallampalli U, Balakrishnan M, Li X, Wu G, Yallampalli C. Maternal protein restriction regulates IGF2 system in placental labyrinth. Front Biosci (Elite Ed) 2012; 4: 1434 1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinola LA, Poutanen M, Vihko R. Cloning of rat 17 beta-hydroxysteroid dehydrogenase type 2 and characterization of tissue distribution and catalytic activity of rat type 1 and type 2 enzymes. Endocrinology 1996; 137: 1572 1579 [DOI] [PubMed] [Google Scholar]
- Akinola LA, Poutanen M, Vihko R, Vihko P. Expression of 17beta-hydroxysteroid dehydrogenase type 1 and type 2, P450 aromatase, and 20alpha-hydroxysteroid dehydrogenase enzymes in immature, mature, and pregnant rats. Endocrinology 1997; 138: 2886 2892 [DOI] [PubMed] [Google Scholar]
- Rantakari P, Strauss L, Kiviranta R, Lagerbohm H, Paviala J, Holopainen I, Vainio S, Pakarinen P, Poutanen M. Placenta defects and embryonic lethality resulting from disruption of mouse hydroxysteroid (17-beta) dehydrogenase 2 gene. Mol Endocrinol 2008; 22: 665 675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty CB, Lewis RM, Sharkey A, Burton GJ. Placental composition and surface area but not vascularization are altered by maternal protein restriction in the rat. Placenta 2003; 24: 34 38 [DOI] [PubMed] [Google Scholar]