ABSTRACT
The sperm connecting piece is a complex structure that, from a mechanical perspective, appears to play a role in stabilizing the proximal part of the sperm tail. We report the three-dimensional structure of the intact bovine sperm connecting piece, revealing an intricate, asymmetrical architecture with the segmented columns held together by filamentous linkages. The columns fuse, at the proximal end, with each other into structures that form the centriolar vault, and at the distal end, with the outer dense fibers (ODFs). The grouping of the fibers into these structures is consistent with bending only in the plane of the head. Structures reminiscent of the proximal centriole were observed in the vault, while the association of a novel bar structure with ODFs 3 and 8 organizes the distal centriolar vault. It has been proposed that the elastic compliance of the connecting piece provides the underlying mechanism behind initiation of the sperm beat cycle and bend propagation. According to the basal sliding theory of sperm movement, distortion of the connecting piece may store energy that initiates a new beat. The intersegment linkers could serve as mechanosensitive elements that regulate alternation of the sperm tail's bending direction in the beat cycle in addition to providing structural stabilization for the connecting piece segmented structures. On the other hand, our video recordings of the bull sperm movement show little bending of the head with respect to the tail, so it appears that there may be normally little strain within the connecting piece.
Keywords: axoneme, centriole, electron cryotomography, flagella, outer dense fiber, sperm neck
Electron cryotomography of the structure of the sperm connecting piece yields novel insights into the motility of spermatozoa.
INTRODUCTION
To reach and fertilize the egg, mammalian sperm have to travel a long distance in the female reproductive tract. Mammalian spermatozoa swim in extremely viscoelastic fluid by beating their flagella at high frequency, and in case of bovine and human spermatozoa, by propelling their way forward in a rotational, drill-like motion. A complete picture of the molecular mechanisms of mammalian fertilization is impossible without clear understanding of the mechanics involved in sperm motility, which is fundamental for sperm migration. Because of the significance of sperm migration in reproduction as well as the fascination with the mechanics involved in this type of motility, sperm movement has been the subject of extensive studies, including mathematical and biophysical modeling. The beating motion of the tail is reasonably well understood to be a result of the activity of dynein motors sliding adjacent microtubule doublets of the axoneme against each other [1, 2]. However, the initiation and regulation of the beat are much less clearly understood.
One model of regulation suggests that these functions are localized in the supramolecular structure called the connecting piece (CP) [3, 4], which is part of the linkage between the sperm head and the midpiece of the tail (Fig. 1A). Observation of the movement of chinchilla sperm suggests that the sliding of microtubule doublets in the more distal axoneme propagates through the CP, causing the head to wobble back and forth. It has been suggested that strain resulting from sliding within the CP is released when the dynein motors stall, resulting in reversal of the beat direction. It is not clear that wobbling of the head is a universal feature of sperm, so the degree to which CP strain plays a role is unknown. In any case, understanding the structure of the CP is essential for creating useful and testable models of how strain within the CP and tail might be distributed and how it would then initiate motion.
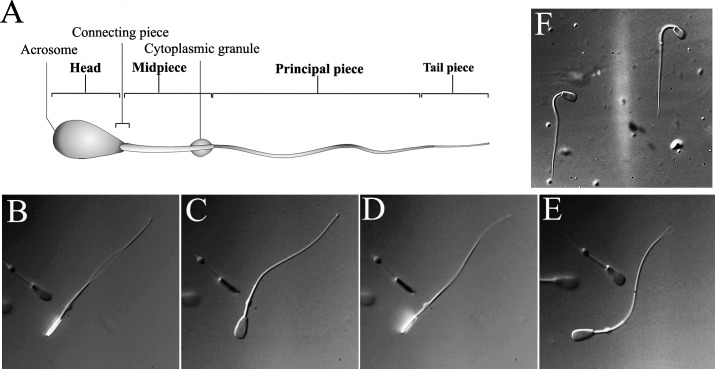
FIG. 1. .
Bovine sperm structure and movement. A) Schematic representation of mammalian sperm morphology showing the location of the CP. B–E) Still images from Supplemental Movie S1 showing the rotation of the sperm head through one full rotation and direction of the tail movement in the sperm beating cycle. F) Image of sperm in a kinked conformation. Motion of such sperm is shown in Supplemental Movie S2. Original magnification ×1200.
Numerous investigations of the CP organization have used electron microscopy of resin-embedded samples to approach a structural description [4–7]. Unfortunately, the size and the intricate nature of the CP have hindered progress in developing a complete model of the complex [4]. Previous electron microscopic imaging of sectioned samples has shown that the CP is very complicated, containing an asymmetrical set of electron-dense, segmented strands or columns. The segmentation is most clear near the head and decreases distally, where these strands fuse with the outer dense fibers (ODFs) that continue along and attach to microtubule doublets along the microtubule-based axoneme [2, 8].
It is well known that centrioles are involved in formation of the axoneme. However, because of the deformation and general disappearance of spermatozoon centrioles during late stages of spermatogenesis, it is difficult to locate the exact position of the centriole in the context of the sperm flagellum. Indeed, early in spermatogenesis, the cell contains two centrioles. The more distal one becomes the basal body that serves as a template for assembling the axoneme, while the proximal one may also serve as a template for an axoneme-like accessory appendage that soon disappears [9]. It has been shown that despite serving as a template for the axoneme, the distal centriole is usually absent in the mature sperm [10, 11]. In some mammals, disintegration of the proximal centriole is also observed during sperm maturation, leaving a hollow pocket [10]. Thus, it is quite unclear how the centriolar proteins are preserved in the sperm to allow formation of a new centriole in the fertilized egg.
To obtain a more complete structural depiction of the connecting piece, in this study we have used electron cryotomography (cryo-ET) to visualize the intact bovine sperm CP. The results reveal the sophisticated asymmetrical organization of the neck region of the sperm, comprising a segmented architecture that forms the proximal centriolar vault (PCV). Several interesting features were observed, including a bar structure that is associated with the segmented columns to form part of the distal centriolar vault and linkages connecting the segment regions. Based on the structure presented here, we hypothesize that the CP may serve mainly as a static structural support for the sperm head that may also have the capacity to use distortion in its regulation of movement.
MATERIALS AND METHODS
Sperm and Axoneme Preparation
Frozen bull semen was purchased from a local source (A.I. Services, Galt, CA). Five hundred microliters of semen was centrifuged at 3000 × g for 5 min to pellet the cells. The pellet was washed twice in 10 mM Tris, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.01% β-mercaptoethanol, and 4 mM MgSO4. The sperm were demembranated by resuspending in hypertonic lysis buffer (1 M sucrose, 10 mM Tris, 0.1 mM EDTA, and 0.01% β-mercaptoethanol, pH 8.0). The membrane was subsequently removed by adding Triton-X100 to a concentration of 2%. Demembranated sperm were pelleted at 16 000 × g and washed twice in 10 mM Tris, 0.1 mM EDTA, and 0.01% β-mercaptoethanol buffer. To remove the sperm heads, cells were vigorously vortexed with 10% (vol/vol) 0.5 mm glass beads. Samples were then incubated in 100 mM dithiothreitol (DTT) for 1 h to remove the mitochondrial sheath [12]. Extracted specimens were then subjected to extensive washing in 10 mM Tris, 0.1 mM EDTA, and 0.01% β-mercaptoethanol buffer. Purified samples were pelleted at 16 000 × g and finally resuspended in 10 mM Tris, 0.1 mM EDTA, and 0.01% β-mercaptoethanol.
Electron Cryotomography
The sample was mixed with 20 nm colloidal gold particles (Ted Pella, Inc., Redding, CA) at a ratio of 10:1 prior to deposition onto glow-discharged, homemade lacey carbon films. Grids were plunge-frozen in liquid ethane using an automatic plunge freezing machine (Vitrobot; FEI, Eindhoven, The Netherlands) prior to being transferred under liquid nitrogen to the cryo-tilt holder of a JEOL 3100FEF electron microscope (JEOL Ltd., Tokyo, Japan). The microscope was operated at an acceleration voltage of 300 kV and was equipped with an Omega energy filter used in the zero-energy-loss mode with a slit width of 25 eV. Tilt series were collected using SerialEM control [13] under low-dose conditions with an accumulated exposure of about 6500 electrons per nm2. Single-axis tilt series included 55–65 images with a tilt increment of 2 degrees. Images were recorded with defocus of 18 μm on a 2048 × 2048 charge coupled device (Gatan, Inc., Pleasanton, CA). The pixel size of the images was about 0.91 nm at the specimen level.
Image Processing and Reconstruction
The tilt series images were aligned with the IMOD software package [14], and final reconstructions were computed using the simultaneous iterative reconstruction technique algorithm in tomo3d [15]. To analyze the periodicity of the pale and dense bands, five images of the segmented column regions containing clear straight band patterns were excised from several regions corresponding to different parts of the CPs in reconstructed tomograms. Images of the extracted bands were manually aligned and averaged. The periodicity plots were obtained using imageJ [16].
Video Recording of Sperm Movement
Bovine semen was allowed to thaw at room temperature for 10 min and gently resuspended in HS solution (130 mM NaCl, 5 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 5 mM glucose, 1 mM sodium pyruvate, 10 mM lactic acid, 20 mM HEPES, pH 7.4 adjusted with NaOH). The resulting suspension was incubated at 24°C for an additional 5 min to precipitate any debris. The supernatant was collected and plated onto 5-mm cover slips in HS solution. Sperm movement was recorded within 3 h after sperm preparation. The recordings were made with a high-speed GX-1 Memrecam camera (NAC Image Technology, Simi Valley, CA) attached to an Olympus IX71 microscope (Olympus America, Center Valley, PA). The speed of recording was 1000 frames/sec, and adjacent frames were averaged for playback at various speeds.
RESULTS
Movement of the Sperm
High-speed video recordings clearly reveals that the sperm head, which is highly flattened, rotates as the cell is propelled by movement of the tail (Supplemental Movie S1; all the supplemental data are available online at www.biolreprod.org). Such motion was observed many years ago [17] but modern high-speed recordings allow a much more detailed view of the process. For example, coordinated rotation of the cytoplasmic granule is also well resolved. As can be seen in the still images in Figure 1, B–E, the beating of the tail occurs mainly in the plane of the head. In most cells, the proximal part of the midpiece remains collinear with the axis of the head, suggesting that there is minimal motion of the head with respect to the CP or within the CP itself. A few cells were found to have a sharp kink near the head that persisted even as the cell rotated (Fig. 1F; Supplemental Movie S2). In these cells the CP exits from the head at an angle of around 30 degrees from the head axis.
Asymmetric Arrangement of the Sperm CP
Cryo-electron microscopy and tomography are well suited for the study of macromolecular complexes in their intact and native state. There is a limit, though, on the thickness of samples that can be studied by cryo-ET because it becomes difficult to obtain high resolution with specimens of the thickness of the intact sperm tail. To reduce the thickness of the sample and make it more suitable for determining the three-dimensional (3-D) structure of the connecting piece using cryo-ET, the membrane and mitochondrial sheath of the active sperm were removed by treatment with detergent (i.e., 2% Triton X-100) and DTT. This treatment may have also removed some components. However, projection images of such samples in negative stain (Fig. 2A) show features similar to those seen earlier in sections of the embedded sperm [5, 7–8]. Samples were subsequently vitrified by plunge freezing into liquid ethane and imaged with no artificial contrast enhancement. We were able to obtain 3-D tomographic reconstructions of intact, unstained CPs, both with (Fig. 2B) and without (Fig. 2C) attached heads, that provide a clear depiction of these structures. In the absence of the head, thinner ice allowed us to more clearly distinguish detailed features within the complex. With or without the head, the natural curvature of the tail ensures that the cells lie on the grid parallel to the head plane.
FIG. 2. .
The bull sperm connecting piece. A) Image of a negatively stained sperm CP showing asymmetrical segmented organization. Projections of approximately 23 nm-thick sections from 3-D cryotomogram reconstructions of (B) CP of sperm with head (inset shows lower magnification view that includes the base of the head) and of (C) CP of headless sperm show similar architecture, indicating the absence of any dramatic effect of decapitation on the overall structure of the CP; c, capitulum; db, dense band; pb, pale band. Three of the segmented columns appear to be folded in order to form the proximal centriolar vault (PCV), suggesting a possible association of the segment peripheries with centrioles (arrowheads in B and C). Bar = 250 nm (A–C). D) Images of the banding pattern exhibiting clear alternating repeats of pale/dark segment bands were extracted from sections of tomographic reconstructions of both intact and headless sperm tails, n = 3 for each. The independent excised images containing an area covering six linear segment repeats were manually aligned and averaged to obtain the average periodic unit (68 nm). Scale bar = 250 nm. Density profiles of the aligned images are plotted in E. The results show no significant variation in the size of the pale bands observed in the tomograms (F), indicating that the structure determined in this work are likely of a quiescent state (n = 10).
In negatively stained samples, the intact CP shows the familiar segmented column structures containing what have been referred to as the dense bands and the pale bands, albeit with inverted contrast due to stain accumulation within the narrow, pale bands (Fig. 2A). The overall structures of the CP, visualized in negative stain (Fig. 2A) and in vitreous ice in complex with the head and in the headless axonemes (Fig. 2, B and C), not only are in good agreement with one another but also are consistent with the previously described ultrastructure [4–6]. The consistency between 3-D renditions of demembranated but otherwise intact, decapitated bovine sperm by cryo-ET and the organization of the CP provided in previous resin-embedded specimens [5, 7–8] revealed that removal of the membrane did not disrupt the connection between the head and the tail or alter the internal morphology (Fig. 2, B and C). The sperm head is firmly associated with a sheet-like structure called the capitulum, which is resistant to our detergent treatment. The complex organization of the capitulum suggests that the sheet is likely to be proteinaceous. The edge of the capitulum appears tightly associated with the segmented structures of the CP (Figs. 2B and 5A) [6]. Just below the capitulum is a thin, continuous density that appears to cap the segmented structures. Moving away from the head, this continuous density separates into a number of distinct structures that become the segmented columns and eventually evolve into the nine ODFs. Within the segmented columns, the majority of the alternating dark and pale bands are aligned perpendicular to the axonemal axis [4, 6].
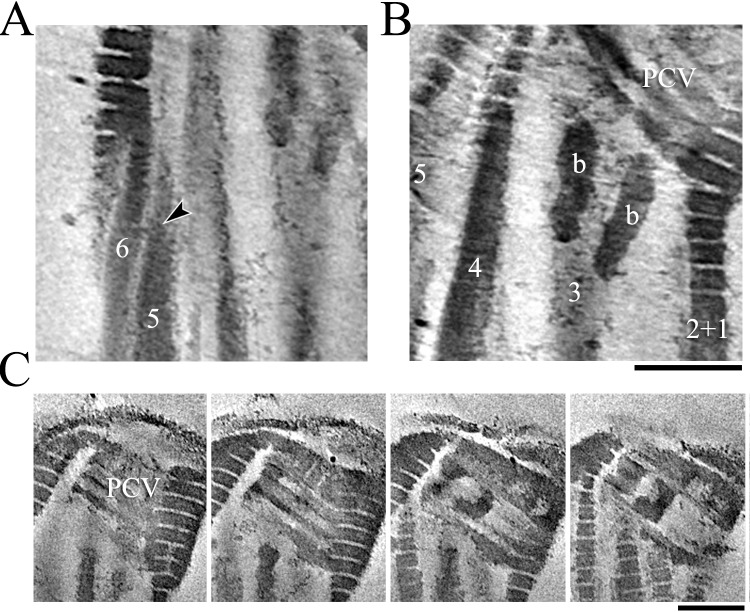
FIG. 5. .
Structural organization of the sperm connecting piece. A) Reconstruction of the sperm connecting piece from electron cryotomography; tomographic longitudinal section (left), and isosurface view (right), showing different parts of the complex. Bar = 250 nm. B–C) Expanded views of segments showing distribution of fibrous linkers (arrowheads) that connect the dark bands. Bar = 50 nm.
Although we see the capitulum tilted through a range of angles, there is remarkably little variation in the spacing of the segments. Figure 2, D–F, present measurements of the spacing along the segmented columns in the intact and headless cells, which are seen to be the same in both cases.
The Organization of the ODFs
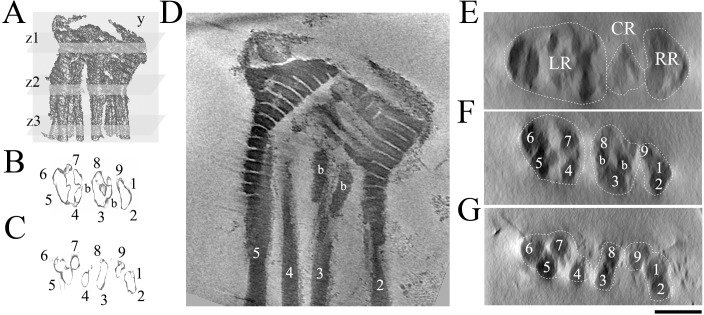
Figure 3 shows the 3-D tomographic CP structure. Figure 3A is a surface view seen from the front, while Figure 3, B and C, show isosurface renditions of thin regions in cross-section. Figure 3D is a longitudinal section through the 3-D volume, and Figure 3, E–G, are densities on three cross-section planes. Supplementary Movie S3 shows the full tomogram density. The proximal part of the CP can be divided roughly into three regions: left, central and right, as seen in Figure 3E. The left region expands into a large segmented area that distally is seen to be the origin of a set of four ODFs. On the right side, the segmented columns are folded near the top to form the PCV and a structure that becomes the origin of a set of three ODFs. Segmented columns of the central region emerge at the location expected for the distal centriole, which has disappeared during maturation. A pair of ODFs that originate in this region is linked tightly by two short, bar-like densities. The ODFs can thus be categorized into three different groups based on their regions of origin.
FIG. 3. .
Three-dimensional reconstruction of the bovine sperm connecting piece. A) Three-dimensional volume rendition of the bovine connecting piece from electron cryotomography, showing segments used in cross-section views. B–C) Cross-sections of isosurface rendering of the reconstructed volume representing the densities at the sampling segments z2 and z3, respectively. Numbers of each ODF were assigned according to [7]; b, bar structure. D) Longitudinal section of a sperm CP corresponding to the sampling plane y in A. E–G) Cross-sections of the corresponding cut planes z1–z3 in A, respectively. Supplemental Movie S3 displays all the longitudinal sections through the whole density. Bar = 250 nm (D–G). LR, left region; CR, central region; and RR, right region.
The origin of the ODFs lies within the distal part of the segmented columns where the segmented parts appear to face outward. As the segmented columns end, the fibers untwist and separate to become the ODFs (Figs. 3 and 4A). To help with the analysis of the structure, we numbered the segmented columns and associated ODFs in a way that best matches the numbering of features in the CP [7]. Although the structures from different bovine sperm cells superimpose very well, there are significant differences in column positioning and association from the 3-D structure of the chinchilla CP presented earlier [7]. However our numbering scheme matches the most significant features of the previous one and is also consistent with the known associations between particular ODFs and microtubule doublets in the more distal regions [18, 19]. The set of the ODFs that originate from the merged segmented columns of the left region are ODFs 4–7, while the density of the right region extends to become ODFs 9, 1, and 2. This numbering aligns the central region ODFs 3 and 8 in the plane perpendicular to the plane of the head, which is the plane of the primary bending motion. It also preserves the notion that the sliding among doublets that drives the alternating phases of the bend is mainly restricted to two sets of doublets, corresponding to the two different sets of ODFs from the right side (9,1, and 2) and the left side (4–7) [7, 18, 20]. Each set of ODFs arises from a discrete density structure that suggests limited ability of the ODFs within a set to move with respect to each other [21].
FIG. 4. .
Distinct features of the connecting piece. A) The twisting nature of segmented column/ODF transition (arrowhead); 5, ODF5; 6, ODF6. B) The position of the bar structure of the CP; b, bar structure; PCV, proximal centriolar vault; 2+1, merged structure of ODF1 and ODF2; 3, ODF3; 4, ODF4; 5, ODF5. Bar in B = 250 nm for A–B. C) Series of slices of an area from a reconstruction of the CP containing centriolar remnant in the PCV. Bar = 250 nm.
Columns 3 and 8 lie parallel to each other and are connected together proximally by two short bar structures at the transitional region between the segmented regions and ODFs, right below the PCV (Figs. 3, B, D, and F, and 4B). This connection, along with the fact that we see no change in its arrangement with variations in the angle of the capitulum, is consistent with the idea that there is minimal relative motion between the corresponding two doublets farther out in the tail.
In the midpiece and principal piece, the ODFs bind abaxially to the microtubule doublets in the axonemes [1, 7, 22], not only providing structural reinforcement for the axoneme but also functioning in the regulation of sperm motility [2, 8]. ODFs 3 and 8 define a plane that is parallel to that of the central pair microtubules, and they also associate with the fibrous sheath (FS) [18, 19], further strengthening the notion that they are involved in forming a structure that limits relative motion of the associated doublets and helps restrict bending to within the plane of the head.
Segment Peripheries Are Specifically Organized to Form the Centriolar Vault
As has been seen in previous work, there is no obvious centriolar structure in our reconstruction at either the proximal or distal position. However, the PCV is a well-defined feature of our map, open at the site of attachment of the transient centriolar adjunct [6]. It is likely that this hollow pocket represents a remnant from the detachment of the centriolar adjunct and disintegration of the proximal centriole. There also remains a basket-like, nonmicrotubular structure attached to the segmented area corresponding to the site of the minus end of the centriole (Fig. 4C). It should be noted that the diameter of the basket matches well with the centriole diameter. This is in good agreement with a finger-like architecture seen in the rodent proximal centriolar remnant [10]. Furthermore, the positions of the basket filaments or fingers are consistent with the positions of the triplet microtubules. These filaments are linked together at both ends by bulky, electron-dense structures (Fig. 5A). The correlation between the positions of the fingers and the anticipated location of the centriolar triplets suggests that the proximal centriole of bovine sperm may not undergo total degeneration [10, 11]. Although, several lines of evidence have suggested the complete disintegration of the distal centriole, unexpectedly, we clearly observed the distal centriolar pocket. The formation of this pocket involves the novel bar structure and the segmented regions corresponding to ODF3 and 8 that form just at the region expected to be the base of the distal centriole. It should also be noted that the transition from segmented peripheries to ODFs takes place at the end of the bar structure where the distal centriole is anticipated to end.
Novel Linkers in the Pale Band Connect Dark Segments
Cryo-ET on unfixed and unstained sperm reveals the existence of novel molecular linkers of filamentous shape within the pale band regions that connect adjacent dark bands of the segmented regions (Fig. 5, B and C). These linkers are about 2–4 nm thick and 14–16 nm long, and do not appear to continue through the dark layers. They vary in number and position from one pale band to the next. Despite their semirandom distribution, a specific organization of the linkers was observed with a denser population toward the axonemal center and lower density toward the outer periphery of the tail. The number of these linkers appears to diminish in the proximal regions where the columns have fused and the segments are tilted.
DISCUSSION
The 3-D Architecture of the Sperm CP
The driving force underlying sperm flagellum beating is the sliding of microtubule doublets driven by axonemal dyneins. It has been long understood that regulated and coordinated movement of dyneins between specific sets of doublets produces the characteristic waveform of the beat [23]. Although various mathematical and theoretical models have been derived to elucidate bend formation and propagation in sperm [1–3, 24, 25], the molecular mechanisms underlying regulation of these vital processes still currently remain unclear [1].
Advances in sample preparation techniques for electron microscopy, for example, rapid freezing and freeze substitution, have brought substantive improvements in the quality and interpretability of images of subcellular structures such as the CP. Electron tomography of unfixed, frozen-hydrated samples provides a highly informative complement to conventional electron microscopy, giving clear 3-D images to elucidate the overall CP structure and details such as the molecular linkages within the pale band regions. The consistency of the structures we see in our demembranated cells with those in previous images [6, 7, 19, 26] gives us confidence that the samples are well preserved in a near-native-state, although the removal of the membrane and mitochondria may have caused loss of components that were not clearly visible in the conventional preparations.
Because the sperm is immobilized after being demembranated and the banding periods are relatively constant along the columns (Fig. 2, D–F), it is likely that the structure of the connecting piece determined in this work is in a quiescent state. We do, though, observe a range of orientations of the end of the CP, best seen as variations in the shape of the left-most segmented column in our figures. For example, the cells in Figures 2C and 3D show a kink of about 40–50 degrees along the edge of this column, while that shown in Figure 2B is nearly straight. In Figure 5A the column has an intermediate shape. This change in shape is not accompanied by any noticeable stretching or change in the spacing along the segments, as seen in Figure 2, D–F. Rather the densities of the left and right regions slide with respect to each other. The kinked structures seen in Figure 1F may represent an extreme case of such sliding, or it may result from permanent structural change, for example, stretching of the interband linkers beyond their elastic limit. Imaging these cells may provide useful constraints for models of movement within the CP.
It has long been observed that flagella bends initiate near the base and propagate to the tip of the tail [1, 2, 7], although it is not clear where in the base initiation occurs. In order to understand the beating mechanism and reveal the principles controlling sperm motility, understanding the basal structure of the sperm flagella is essential. Several models have suggested the involvement of periaxonemal structures in the sperm tail, including the CP, ODFs, and the FS, in the regulation of the oscillation in addition to being the structural reinforcements of the axoneme [2, 3]. Although elements of the CP have been visualized for several decades [6], because of the limitations of traditional electron microscopy techniques, it has been difficult to gain much insight on the material differences within the structure. For example, it has been unclear to what extent the observed periodicities in the pale and dark bands are due to heterogeneous staining or actually arise from substances of totally different densities. Our near-native cryo-electron microscopic structure clearly demonstrates that the pale bands indeed contain much lower density than the dark bands and are not just a stain artifact.
The overall structure appears to be flattened within the plane of the flagellar beat, causing the final rendition to lack the clear 9-fold symmetry of the more distal axoneme. We presume that this flattening is mainly related to the highly flattened shape of the head. It may also be that, because of the thickness of the CP and forces experienced by the sample during grid preparation, there is some flattening and structural deformation in our structure. However, it should be noted that the same flattening was seen in the corresponding region of reconstructions from serial sections of resin-embedded chinchilla sperm [7]. The presence of the bar structures that connect columns 3 and 8 further confirms the tight ODF 3–8 interaction that appears to stabilize the compressed structure. It is likely that more distal regions of our specimens would show much better 9-fold symmetry.
The Organization of the ODFs and Its Influence on Sperm Movement
Our video recordings of bull sperm movement confirm that the sperm tail beats with a strongly asymmetric waveform that lies mainly within a plane corresponding to the orientation of the head. It is clear that the structural constraints among the ODFs, which are fused into distinct groups at the proximal end, are consistent with this planar movement. Whether the asymmetry of the CP structure is directly related to the asymmetry of the waveform remains to be explored, but we can expect that they are strongly connected in some way. A recent cryo-ET reconstruction of the Trypanosoma brucei flagellum suggested a role of the accessory axonemal structure called the paraflagellar rod in governing the asymmetric, bihelical movement characteristic of the parasite flagella [27, 28]. This flexible structure is attached to one side of the axoneme in a way that appears to induce spiral movement in response to the sliding of the axonemal doublets. It is possible that the underlying mechanism behind the motion of the sperm may similarly be hidden within the complex asymmetrical organization of the sperm accessory ODFs and the ways they interact with the doublets [29]. The fact that the bar structures connect columns 3 and 8 together to form the distal centriolar vault suggests that these two particular ODFs are likely to be restrained [18]. It is interesting that these ODFs continue in the sperm principal piece as part of the FS, which also suggests an intrinsic structural constraint on the waveform. It should also be noted that ODFs 3 and 8 are not only arranged in a plane with the central pair microtubules but are also associated with doublets 1 and 6 located on opposite sides of the axoneme. Notably, the same doublets in Chlamydomonas reinhardtii were reported to contain unique beak features that may affect the doublets' elasticity and favor axoneme bending in one plane. Moreover, the algal doublet 1 lacks certain types of dyneins [30]. This observation is consistent with our hypothesis that doublet 1 is likely to be immobilized. Together with doublets 5 and 6 and the immobilized ODFs, this molecular association defines the bending plane of the sperm tail. As mentioned earlier, the other ODFs originate from the CP in two groups such that each member of a group is constrained against movement with respect to the others at its base, providing a firm anchor against which the dynein-driven doublet motion can push to effect sliding. It appears likely that the ODFs from the same groups will slide in a coordinated fashion that constrains the sperm beat characteristics.
Possible Mechanism for Bend Initiation and Oscillation Control
There is no doubt that the complex structure of the CP plays a pivotal role as a structural anchorage of the head to the flagellum in sperm. However, the obscurity in the structure has left considerable ambiguity about the function and mechanism of action of this intricate complex. There is nevertheless ample evidence suggesting that the structural flexibility of the CP plays a role in controlling the bend direction of the sperm beat cycle. As shown by the work of Vernon and Woolley [7], even with the head detached, the tail can still perform repetitive oscillation with its normal, highly asymmetric beat cycle. We have observed the same with headless bovine and human spermatozoa (data not shown). Recent high-pressure freezing/freeze substitution experiments with guinea pig sperm suggest that the elastic compliance of the CP could be responsible for beat initiation and bend propagation [4]. Significant variation in the width of the pale band regions (∼33.3%) was observed while the dense band is much more rigid (∼6.6% width variation) [4]. Thin strands connecting the dark bands were also seen in this work. The elastic compliance in this region is proposed to be sufficient for initiating the bend propagation of the sperm tail and for reversing the direction of the bend [3, 4, 7]. The variation of the widths of both pale and dark bands suggests that the overall flexural compliance of the CP arises mainly in the elasticity of the pale bands, suggesting that the key players controlling the elasticity of the CP are likely to reside in the pale band region of the strands.
Our 3-D rendition of the connecting piece has revealed markedly improved details of the complex organization, including the molecular linkers in the pale bands that appear to connect the segments and stabilize the CP structure. The low pale band density in the reconstructions from the cryo-based data suggests that these linkers are the only moiety found in the flexible pale band region, although it is possible that other, more labile components were removed by the detergent and DTT treatment. According to the model proposed by Woolley et al. [4], these linkers are obvious candidates for playing some role in regulating the oscillation of the flagella rather than being just passive structural elements. In fact, in mechanosensation, various kinds of filamentous protein linkers have been identified as tension sensors, such as the tip links of hair cell stereocilia [31]. It is reasonable to hypothesize that such linkers could act cooperatively both as tensile molecular springs contracting and expanding with the tail motion similar to the springs in a double spring seesaw, and as stretch sensors to control the bend direction during the beat cycle (Fig. 5). However, in our structural snapshot, we did not see the stretching of the linkers.
As shown in Figure 2, D–F, we see essentially no variation in band spacing, even with various orientations of the head connecting surface. It remains to be seen whether this lack of distortion represents a difference between rodent and bovine sperm or might be a result of the removal of the cell membrane. The fact that we see changes in the CP shape corresponding to the head wobbling back and forth, as is seen in swimming chinchilla sperm, suggests the former, but the sensing of distortions within the CP to initiate bending is still an appealing hypothesis. It would indeed be very interesting to investigate whether the force generated by elastic deformation of these linkers would be sufficient to directly initiate the new bend. The basal sliding of the sperm flagella could also induce significant deformation of the banding period of the CP both with the fused regions as well as with columns. These distortions could store energy that forces motion in the reversed direction once the maximal sliding has been reached and the dynein motors stall [7].
The Centriolar Remnant
During spermatid maturation, centrioles migrate toward the cell membrane to initiate formation of the sperm flagellum [9]. Upon maturation of the tail, in some mammals, centrioles undergo significant differentiation and often disappear [10, 11]. In most cases, centriole reduction does not occur until the very late stage of spermatogenesis. Previous ultrastructural characterization suggested that during spermatogenesis, docking of centrioles to the cell membrane initiates the early stage of axonemal development. Segmented structures subsequently form around the centrioles before developing to form the distinct shape of the CP with all the segmented pheripheries pointed toward the triplet microtubules to create a PCV. It is clear that three of the proximal segments fuse and organize perpendicularly to the rest of the columns, retaining the segmented structure, conceivably to facilitate the formation of the proximal vault (Fig. 2, B and C). For this structure to be directionally well organized, there must be a set of centriolar proteins that facilitate the initial recruitment of segment components and help instigate formation of the CP. A recent report suggested the existence of a centriolar protein, SPERIOLIN, that is highly localized to the rim of the CP segments and is thus a candidate for such a protein [5]. It is possible that this protein is involved in the recruitment of molecular components making up the CP. Furthermore, the association between the bar structure and ODFs 3 and 8 enables the formation of the distal centriolar vault. Our results demonstrate the role of the segmental organization as a supportive structure for the axonemal centrioles (Fig. 2, B and C), forming a physical support for the centriole and providing a tether point for the growing axoneme.
Upon maturation of the spermatozoon, centrioles disappear in many organisms. Despite the absence of well-defined centrioles, several lines of evidence indicate that centriolar proteins remain in the CP [5, 10, 11, 32], not only in the remnants of the centrioles but also in the segment peripheries [5]. It should also be noted that the mitotic aster-like, structure-forming protein SPAG5/ASTRIN/DEEPEST is also found associated with the ODFs in rat [33]. The localization of centriolar proteins in the CP and the ODFs demonstrates the importance of the CP not only in providing a structural support, but also in retaining the centriolar proteins.
In conclusion, our structure offers novel insights into the architecture of the connecting piece, revealing the molecular linkers that not only could play a role in stabilizing the segmented column structure of the connecting piece, but could also be important for the oscillation of sperm flagella. The structure also reveals the origin of the ODFs, which allows us to classify them in different sets that are anchored together. The asymmetry of this organization may provide an interesting structural insight into the underlying mechanism of the asymmetrical waveform of sperm movement. Additionally, the structural description provided in this work should enable the generation of more detailed and realistic mathematical models to better understand the physics of sperm motility.
Footnotes
Supported by National Institute of Health Grant No. GM051487.
REFERENCES
- Gaffney EA, Gadelha H, Smith DJ, Blake JR, Kirkman-Brown JC. Mammalian sperm motility: observation and theory. Annu Rev Fluid Mech 2011; 43: 501 528 [Google Scholar]
- Inaba K. Sperm flagella: comparative and phylogenetic perspectives of protein components. Mol Hum Reprod 2011; 17: 524 538 [DOI] [PubMed] [Google Scholar]
- Woolley DM. Flagellar oscillation: a commentary on proposed mechanisms. Biol Rev Camb Philos Soc 2010; 85: 453 470 [DOI] [PubMed] [Google Scholar]
- Woolley DM, Carter DA, Tilly GN. Compliance in the neck structures of the guinea pig spermatozoon, as indicated by rapid freezing and electron microscopy. J Anat 2008; 213: 336 341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, O'Brien DA, Eddy EM. Speriolin is a novel human and mouse sperm centrosome protein. Hum Reprod 2010; 25: 1884 1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW, Phillips DM. The fine structure and development of the neck region of the mammalian spermatozoon. Anat Rec 1969; 165: 153 164 [DOI] [PubMed] [Google Scholar]
- Vernon GG, Woolley DM. Basal sliding and the mechanics of oscillation in a mammalian sperm flagellum. Biophys J 2004; 87: 3934 3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW. The mammalian spermatozoon. Dev Biol 1975; 44: 394 436 [DOI] [PubMed] [Google Scholar]
- Fawcett DW. The Cell. 2nd ed. Philadelphia: W.B. Saunders Company; 1981: 551 574 [Google Scholar]
- Manandhar G, Simerly C, Salisbury JL, Schatten G. Centriole and centrin degeneration during mouse spermiogenesis. Cell Motil Cytoskeleton 1999; 43: 137 144 [DOI] [PubMed] [Google Scholar]
- Manandhar G, Simerly C, Schatten G. Centrosome reduction during mammalian spermiogenesis In: Palazzo RE, Schatten GP. (eds.), Current Topics in Developmental Biology, vol 49, New York: Academic Press; 1999: 343 363 [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Tengowski MW, Navara CS, Zoran SS, Schatten G. Mitochondrial sheath movement and detachment in mammalian, but not nonmammalian, sperm induced by disulfide bond reduction. Mol Reprod Dev 1997; 47: 79 86 [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol 2005; 152: 36 51 [DOI] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol 1996; 116: 71 76 [DOI] [PubMed] [Google Scholar]
- Agulleiro JI, Fernandez JJ. Fast tomographic reconstruction on multicore computers. Bioinformatics 2011; 27: 582 583 [DOI] [PubMed] [Google Scholar]
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int 2004; 11: 36 42 [Google Scholar]
- Gray J. The movement of spermatozoa of the bull. J Exp Biol 1958; 35: 96 108 [Google Scholar]
- Lindemann CB, Orlando A, Kanous KS. The flagellar beat of rat sperm is organized by the interaction of two functionally distinct populations of dynein bridges with a stable central axonemal partition. J Cell Sci 1992; 102: 249 260 [DOI] [PubMed] [Google Scholar]
- Fawcett DW. The anatomy of the mammalian spermatozoon with particular reference to the guinea pig. Z Zellforsch Mikrosk Anat 1965; 67: 279 296 [DOI] [PubMed] [Google Scholar]
- Sale WS. The axonemal axis and Ca2+-induced asymmetry of active microtubule sliding in sea urchin sperm tails. J Cell Biol 1986; 102: 2042 2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanous KS, Casey C, Lindemann CB. Inhibition of microtubule sliding by Ni2+ and Cd2+: evidence for a differential response of certain microtubule pairs within the bovine sperm axoneme. Cell Motil Cytoskeleton 1993; 26: 66 76 [DOI] [PubMed] [Google Scholar]
- Lindemann CB, Gibbons IR. Adenosine triphosphate-induced motility and sliding of filaments in mammalian sperm extracted with Triton X-100. J Cell Biol 1975; 65: 147 162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingyoji C, Murakami A, Takahashi K. Local reactivation of Triton-extracted flagella by iontophoretic application of ATP. Nature 1977; 265: 269 270 [DOI] [PubMed] [Google Scholar]
- Lindemann CB. Testing the geometric clutch hypothesis. Biol Cell 2004; 96: 681 690 [DOI] [PubMed] [Google Scholar]
- Brokaw CJ. Thinking about flagellar oscillation. Cell Motil Cytoskeleton 2009; 66: 425 436 [DOI] [PubMed] [Google Scholar]
- Zamboni L, Stefanini M. The fine structure of the neck of mammalian spermatozoa. Anat Rec 1971; 169: 155 172 [DOI] [PubMed] [Google Scholar]
- Koyfman AY, Schmid MF, Gheiratmand L, Fu CJ, Khant HA, Huang D, He CY, Chiu W. Structure of Trypanosoma brucei flagellum accounts for its bihelical motion. Proc Natl Acad Sci U S A 2011; 108: 11105 11108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes LC, Ralston KS, Hill KL, Zhou ZH. Three-dimensional structure of the Trypanosome flagellum suggests that the paraflagellar rod functions as a biomechanical spring. PLoS One 2012; 7: e25700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann CB. Functional significance of the outer dense fibers of mammalian sperm examined by computer simulations with the geometric clutch model. Cell Motil Cytoskeleton 1996; 34: 258 270 [DOI] [PubMed] [Google Scholar]
- Bui KH, Sakakibara H, Movassagh T, Oiwa K, Ishikawa T. Asymmetry of inner dynein arms and inter-doublet links in Chlamydomonas flagella. J Cell Biol 2009; 186: 437 446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer M, Koster AJ, Ziese U, Bajaj C, Volkmann N, Wang D, Hudspeth AJ. Three-dimensional architecture of hair-bundle linkages revealed by electron-microscopic tomography. J Assoc Res Otolaryngol 2008; 9: 215 224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Eddy EM. Speriolin is a novel spermatogenic cell-specific centrosomal protein associated with the seventh WD motif of Cdc20. J Biol Chem 2004; 279: 42128 42138 [DOI] [PubMed] [Google Scholar]
- Shao X, Xue J, van der Hoorn FA. Testicular protein Spag5 has similarity to mitotic spindle protein Deepest and binds outer dense fiber protein Odf1. Mol Reprod Dev 2001; 59: 410 416 [DOI] [PubMed] [Google Scholar]