CASE SUMMARY
History
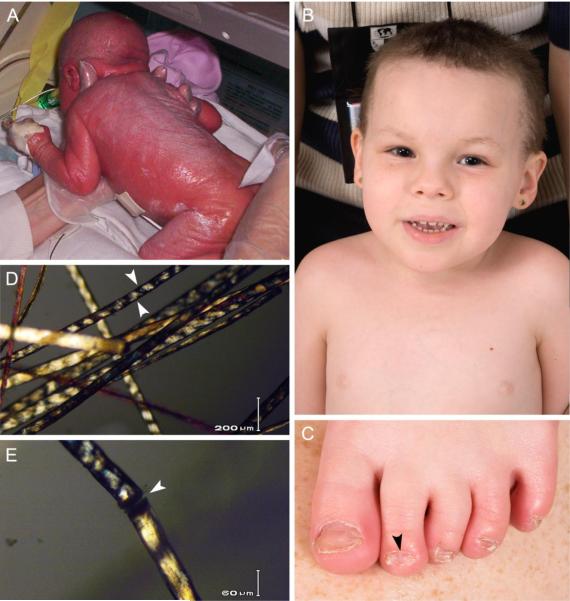
A 4 year old Caucasian girl presented to the National Institutes of Health (NIH) for evaluation of sparse, brittle hair, developmental delay, poor growth, recurrent infections, and photosensitivity. The patient was born preterm at 35 weeks after a pregnancy complicated by elevated maternal alpha-fetoprotein at 16 weeks and pregnancy-induced hypertension beginning at 26 weeks. At birth, her skin showed generalized erythroderma and she was described as having a collodion membrane (Fig 1, A) that resolved over 2.5 weeks. By one month of age, the patient was reported to have congenital ichthyosis and mild scaling on her scalp, trunk, and lower extremities. By 23 months, she was reported to sunburn with minimal sun exposure and sweat very little in hot environments. Short brittle hair, nail spooning, head tremor (titubation), and asymmetrical horizontal nystagmus were also noted. The patient did not sit unassisted until 12 months and did not crawl until 22 months. A magnetic resonance imaging (MRI) scan of her brain at 19 months was read as normal. By age 3 years she had ataxia and ambulated only with a reverse walker. The patient experienced multiple ear infections and pneumonias beginning at 6 months of age that did not require hospitalization, were not cultured, and responded to empiric antibiotic treatment. Her parents reported that when she suffered severe respiratory infections the patient had temporary developmental regression that improved when the infection resolved. Complement and immunoglobulin levels were within normal limits at 16 months.
Fig 1.
Clinical features of patient TTD421BE with trichothiodystrophy. A. Shiny, collodion membrane appearance at birth. B. Facial appearance showing short, sparse, brittle hair on scalp and eyebrows but long eyelashes. C. Thin, peeling, spoon-shaped nails (koilonychia) (arrowhead). D. Tiger tail banding of cut hair under polarized microscopy (4×) (arrowheads point to the alternating dark and light bands on the hair shafts). E. Trichoschisis (10×) (arrowhead).
There was no family history of individuals with similar features. The parents denied consanguinity. The patient had an 8 year old unaffected brother who was delivered following an uneventful pregnancy.
Physical examination
At NIH, the patient was a cheerful, engaging girl with weight and height in the 3rd centile and head circumference in the 10th centile. Examination revealed short brittle hair on her scalp and eyebrows but long eyelashes (Fig 1, B). She had mild micrognathia and a high-arched palate. The patient also had hyperlinear palms and soles and thin, peeling, spoon-shaped toe nails (Fig 1, C). Mild hyperkeratosis was present on the lateral heels of both feet and fine scales were observed on the lateral trunk and ankles. The patient walked with an uneven stiff gait. Ophthalmologic examination revealed astigmatism in both eyes with no signs of cataracts or nystagmus.
Diagnostic studies
Clinical laboratory tests at NIH were significant for leukopenia (white blood cell count, 4.78K/μl [reference: 4.86–13.18K]), neutropenia (absolute neutrophil count, 1.10K/μl) [reference range: 1.60–8.29K]), elevated hemoglobin A2, 4.3% [reference: 2.2–3.2], IgM deficiency, <21 mg/dl [reference: 24–210], and elevated creatine kinase 245 units/l [reference: 0–143]. She had a normal mean corpuscular volume (MCV), 73.3 fL [reference: 72.3–85.0], 25-hydroxyvitamin D, 38 ng/ml [reference: 10–80] and 1,25-dihydroxyvitamin D, 69 pg/ml [reference: 24–86].
Cut hair was examined under polarization with a light microscope and revealed consistent light and dark transverse areas spaced at regular intervals along the entire hair shaft [“tiger tail” banding (Fig 1, D–E)].1 The appearance of tiger tail banding depended on the hair's orientation to the polarizing lenses, such that rotation of the microscope stage and polarizing filters were sometimes necessary to elicit a banded appearance.2 Light microscopy at 4× and 10× magnification demonstrated hair shaft abnormalities including trichoschisis (a clean transverse break through the hair) (Fig 1, E), and trichorrhexis nodosa-like defects, which differed from typical trichorrhexis nodosa in that the release of individual cortical cells was not present. Amino acid analysis of the hair performed by the Hair Research Laboratory, University of California, San Francisco, revealed a sulfur-containing component of 2.3% (normal: ~5%).
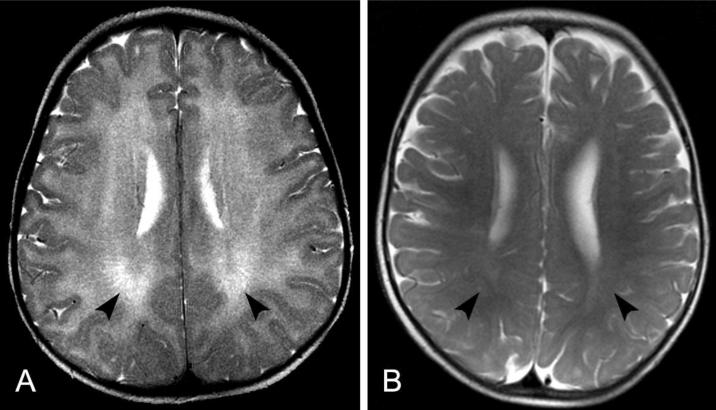
At NIH, speech audiometry was within normal limits and diagnostic bone series showed no evidence of skeletal dysplasia. The MRI performed at 19 months was reviewed by neuroradiology (NJP) and interpreted to be abnormal with extensive white matter abnormalities consistent with dysmyelination or hypomyelination (Fig 2, A). A normal MRI of a 13 month old child (shown for comparison in Fig 2, B) reveals more fully developed myelination.
Fig 2.
Axial T2-weighted MRI scan of the brain of patient TTD421BE at age 19 months (A) compared to a normal MRI of a child at the age 13 months (B) using the same technique. A. The entire white matter of both cerebral hemispheres appears bright (arrowheads) compared to the darker cortical gray matter, indicating lack of normal myelin formation. B. In children with normal myelination, the signal intensity is reversed with the white matter being darker (arrowheads) than the gray matter in this type of scan. These images show that the myelin in the 13 month old child (B) is even more developed than that of our patient at 19 months (A).
Genomic DNA was isolated from the blood of the patient and her mother and sequencing for mutations in the XPD (ERCC2) DNA repair gene was performed using standard techniques.3 A mutation in exon 18 (c.1703-1704delTT, GenBank Accession NM_000400.3) was identified in DNA from the patient and her mother. The TT deletion resulted in an amino acid change at codon 568 from phenylalanine (UUU) to tyrosine (UAU) and created a premature stop codon (p.F568Yfs*2). A second mutation in exon 22 (c.2173G>A) which resulted in a novel amino acid substitution (p.A725T) was also identified in the patient. Thus, the patient (TTD421BE) was a compound heterozygote for these two previously unreported mutations in the XPD gene.
A diagnosis of trichothiodystrophy (TTD) was made and local follow-up was recommended. The patient's parents were advised regarding the necessity for sun protection to avoid sunburns, to implement a physical therapy program to improve range of motion and balance, and to increase caloric density of foods to promote weight gain. They were also urged to closely monitor for infections, making sure to treat any illness early on, and to keep her immunizations up-to-date.
Diagnosis
Trichothiodystrophy (TTD).
DISCUSSION
TTD is a rare (frequency about 1 per million in Western Europe),4 autosomal recessive disease characterized by multisystem abnormalities and sulfur-deficient hair that breaks easily.5–7 The essential diagnostic feature of TTD is a pattern of light and dark bands in the hair shafts, termed “tiger tail banding,” when viewed with polarized light microscopy.1 In conjunction with certain hair shaft abnormalities, tiger tail banding provides a reliable test for TTD.1, 8
TTD arises when both alleles are affected by mutations in one of three DNA repair genes (XPB, XPD or TTDA),7 or in TTDN1,9 a gene of unknown function. Interestingly, XPB and XPD defects are also seen in xeroderma pigmentosum (XP),5, 10, 11 a disease with a 1000-fold increase in skin cancer. In contrast, TTD patients do not have this greatly increased skin cancer risk as confirmed by a recent systematic literature review.12 Nevertheless, rare patients with combined XP and TTD do appear to carry this increased risk.13XPD, XPB and TTDA are involved in both nucleotide excision repair (NER) and basal transcription as part of the basal transcription factor TFIIH. XP and TTD patients have different mutations in the XPD gene. It has been hypothesized that the specific mutation in TTD patients predominantly affects the transcription activities of these genes rather than their repair activities.14
A recent systematic review found only 112 documentable cases of TTD in the literature.12 Patients were reported worldwide with males and females similarly affected. The clinical features were remarkably varied and ranged from solely hair involvement to severe neurological and developmental abnormalities. Important findings included sparse, dry and easily broken scalp hair associated with low sulfur and cysteine content.8 Hair anomalies could be found in other body locations including the eyebrows. However, the eyelashes were usually long. Mothers of TTD patients experience abnormalities during pregnancy including a 4-fold increase in preeclampsia and a 36-fold increase in HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome.15 Many patients are born prematurely or are small for gestational age.12, 15
Other clinical signs associated with organs of ectodermal and neuroectodermal origin include mental and growth retardation, micrognathia, small nose, large ears, microcephaly, nail dysplasia, teeth abnormalities, and ichthyosis.12 Because of this involvement of several ectodermal structures, including the skin, hair, nails and teeth, TTD has been considered an ectodermal dysplasia. At birth, the skin often has a collodion membrane (Fig 1, A), which is often mild and resolves leaving a mild ichthyosis or even normal appearing skin. Many, but not all, TTD patients show persistent erythema after minimal sun exposure.5 In the past, the acronyms BIDS,16 IBIDS17 and PIBIDS18 have been used to describe TTD patients based on the presence or absence of the following signs: Brittle hair, Ichthyosis, Impaired intelligence, Decreased fertility, Short stature, and Photosensitivity. Importantly, these acronyms do not adequately describe the spectrum of clinical involvement for most patients and fail to include many important clinical findings including severe eye involvement, and infections commonly reported in many TTD patients.12
Patients often develop repeated and severe infectious illnesses, commonly of the respiratory tract. Review of the literature revealed a 20-fold increased mortality for TTD patients under the age of 10 years, predominately related to infections.12 Complete immunological characterization of these patients has not yet been reported, but abnormal hematologic findings include neutropenia, elevated hemoglobin A2, low MCV, and anemia. Faghri et al12 described a high frequency of ocular abnormalities (51%), including congenital cataracts (7%). Radiographic bone abnormalities (38%), such as peripheral osteopenia (9%) and central osteosclerosis (14%) were also reported.12
Although TTD patients may have intellectual impairment, they are commonly very social and have outgoing, engaging, and friendly personalities. Standard intelligence tests may underestimate their capacity for social interactions. These patients may also have ataxia and impaired motor control.12 They can develop contractures and may require braces, walkers, and/or tendon release surgery to ambulate. Brain MRI shows predominantly hypomyelination of the white matter while atrophy is not a major feature.5 It has been suggested that the myelin in these patients fails to form properly (dysmyelination). Enlarged ventricles may also be seen in some patients.12
The hallmark of TTD is cut hair that has transverse “tiger tail” banding at regular intervals when visualized under polarized light microscopy. Light microscopy can also detect trichoschisis and trichorrhexis nodosa-like hair shaft abnormalities that may occur anywhere along the hair shaft.2 Some hair shafts may have a flat shape, simulating the appearance of a ribbon, which could lead to folding and twisting when mounted on slides. The proportion of hairs with these findings varies among patients, but the surface cuticle of TTD hairs nearly always show marked disruption ranging from abrasion and lifting of individual cuticle scales to complete absence of cuticle scale pattern. The hair surface may display longitudinal ridging and fluting in some areas.2 A strong inverse correlation between the percentage of abnormal hair shafts and sulfur content of the hair has been reported.8
Effective management of TTD requires a multidisciplinary approach involving numerous medical specialties. Because the disease involves many organ systems, specialties including neonatalogy, pediatrics, ophthalmology, neurology, orthopedics, internal medicine, dentistry, rehabilitation medicine, immunology, infectious disease, hematology, genetics, radiology and dermatology may become involved in patient care. Better recognition of the disease by dermatologists and other physicians can facilitate early diagnosis and treatment, and identify families that will benefit from genetic counseling.
KEY TEACHING POINTS
Trichothiodystrophy (TTD) is an autosomal recessive disease reported in many countries around the world. Patients with TTD have defects in one of three DNA repair genes (XPB, XPD, TTDA) or a gene of unknown function (TTDN1), and do not have the increased risk of skin cancer seen in patients with xeroderma pigmentosum (XP).
The diagnosis of TTD can be made on the basis of clinical features. The defining characteristic is brittle hair that exhibits tiger tail banding on polarized microscopy that is associated with hair shaft abnormalities such as trichoschisis, trichorrhexis nodosa-like abnormalities, and ribboning. Care providers who suspect TTD should obtain a cut sample of hair and view it under a polarized light microscope.
A spectrum of clinical features are associated with TTD. Many patients have abnormalities of ectodermal and neuroectodermal origin and are often born after complicated pregnancies, with pregnancy-induced hypertension as a common feature.
Early diagnosis of TTD is important for effective management since there is increased mortality from infections.
Table I.
CLINICAL FEATURES OF TTD PATIENT TTD421BE*
| Hair Abnormalities | Neurological Abnormalities |
| Tiger tail banding | Developmental Delay |
| Brittle hair | Intellectual impairment |
| Sparse hair | Abnormal gait/ataxia |
| Hair shaft abnormalities | Abnormal MRI (diffuse dysmyelination) |
| Nail Abnormalities | |
| Brittle nails | Personality Characteristics |
| Koilonychia | Sociable/Engaging/Cheerful |
| Soft nails | Hematological/Immunological Abnormalities |
| Skin Abnormalities | Recurrent infections (particularly respiratory) |
| Ichthyosis | |
| Photosensitivity | Neutropenia |
| Hypohidrosis | Elevated hemoglobin A2 |
| Oral Abnormalities | Low serum IgM |
| High-arched palate | Maternal Pregnancy and Fetal Abnormalities |
| Abnormal teeth | Pregnancy-induced hypertension |
| Frequent caries | Spotting during pregnancy |
| Growth Abnormalities | Abnormal prenatal screening (elevated maternal alpha-fetoprotein) |
| Short stature (~3rd percentile) | |
| Poor weight gain (~3rd percentile) | Premature birth |
| Microcephaly (~10th percentile) | Intrauterine growth restriction (IUGR) |
| Neonatal skin abnormalities | Birth weight low for gestational age |
| Collodion membrane | Placental abnormalities |
| Erythroderma |
Selected from clinical features reviewed in Faehri et al 2008.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We thank Mary King and John Crawford, NIH Clinical Center patient photographers, for their excellent images, Dr. Carmen Brewer of the National Institute of Deafness and Other Communication Disorders, NIH, for audiology evaluation, Drs. Vera Price and Emory Menefee, Hair Research Laboratory, University of California, San Francisco, for hair amino acid analysis and interpretation and Dr. Raphaela Goldbach-Mansky, NIAMS, NIH for permission to use the normal MRI image.
This research was supported by the Intramural Research Programs of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (NIH). Support for author Zhou was made possible through the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for NIH from Pfizer Inc).
Abbreviations
- TTD
trichothiodystrophy
- XP
xeroderma pigmentosum
- MRI
magnetic resonance imaging
- NER
nucleotide excision repair
- NIH
National Institutes of Health
- TFIIH
transcription factor IIH
- UV
ultraviolet radiation
- AFP
alpha-fetoprotein
- MCV
mean corpuscular volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None declared.
REFERENCES
- 1.Price VH, Odom RB, Ward WH, Jones FT. Trichothiodystrophy: sulfur-deficient brittle hair as a marker for a neuroectodermal symptom complex. Arch Dermatol. 1980;116:1375–84. doi: 10.1001/archderm.116.12.1375. [DOI] [PubMed] [Google Scholar]
- 2.Liang C, Kraemer KH, Morris A, Schiffmann R, Price VH, Menefee E, et al. Characterization of tiger-tail banding and hair shaft abnormalities in trichothiodystrophy. J Am Acad Dermatol. 2005;52:224–32. doi: 10.1016/j.jaad.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Boyle J, Ueda T, Oh KS, Imoto K, Tamura D, Jagdeo J, et al. Persistence of repair proteins at unrepaired DNA damage distinguishes diseases with ERCC2 (XPD) mutations: cancer-prone xeroderma pigmentosum vs. non-cancer-prone trichothiodystrophy. Hum Mutat. 2008;29:1194–208. doi: 10.1002/humu.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleijer WJ, Laugel V, Berneburg M, Nardo T, Fawcett H, Gratchev A, et al. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair (Amst) 2008;7:744–50. doi: 10.1016/j.dnarep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Kraemer KH, Patronas NJ, Schiffmann R, Brooks BP, Tamura D, DiGiovanna JJ. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: a complex genotype-phenotype relationship. Neuroscience. 2007;145:1388–96. doi: 10.1016/j.neuroscience.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefanini M, Ruggieri M. Trichothiodystrophy. In: Ruggieri M, Pascual-Castroviejo I, Di Rocco C, editors. Neurocutaneous Disorders, Phakomatoses and Hamartoneoplastic Syndromes. Springer Vienna; New York: 2008. pp. 821–45. [Google Scholar]
- 7.Ruenger TM, DiGiovanna JJ, Kraemer KH. Hereditary Diseases of genome instability and DNA repair. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7 ed. Vol. 1311. McGraw-Hill Medical; New York: 2008. 25 pp. [Google Scholar]
- 8.Liang C, Morris A, Schlucker S, Imoto K, Price VH, Menefee E, et al. Structural and molecular hair abnormalities in trichothiodystrophy. J Invest Dermatol. 2006;126:2210–6. doi: 10.1038/sj.jid.5700384. [DOI] [PubMed] [Google Scholar]
- 9.Nakabayashi K, Amann D, Ren Y, Saarialho-Kere U, Avidan N, Gentles S, et al. Identification of C7orf11 (TTDN1) gene mutations and genetic heterogeneity in nonphotosensitive trichothiodystrophy. Am J Hum Genet. 2005;76:510–6. doi: 10.1086/428141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 1994;130:1018–21. [PubMed] [Google Scholar]
- 11.Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987;123:241–50. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- 12.Faghri S, Tamura D, Kraemer KH, DiGiovanna JJ. Trichothiodystrophy: a systematic review of 112 published cases characterises a wide spectrum of clinical manifestations. J Med Genet. 2008;45:609–21. doi: 10.1136/jmg.2008.058743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broughton BC, Berneburg M, Fawcett H, Taylor EM, Arlett CF, Nardo T, et al. Two individuals with features of both xeroderma pigmentosum and trichothiodystrophy highlight the complexity of the clinical outcomes of mutations in the XPD gene. Hum Mol Genet. 2001;10:2539–47. doi: 10.1093/hmg/10.22.2539. [DOI] [PubMed] [Google Scholar]
- 14.Dubaele S, Proietti De Santis L, Bienstock RJ, Keriel A, Stefanini M, Van Houten B, et al. Basal transcription defect discriminates between xeroderma pigmentosum and trichothiodystrophy in XPD patients. Mol Cell. 2003;11:1635–46. doi: 10.1016/s1097-2765(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 15.Moslehi R, Signore C, Tamura D, Mills JL, DiGiovanna JJ, Tucker MA, et al. Adverse effects of trichothiodystrophy DNA repair and transcription gene disorder on human fetal development. Clin Genet. 2009 doi: 10.1111/j.1399-0004.2009.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baden HP, Jackson CE, Weiss L, Jimbow K, Lee L, Kubilus J, et al. The physicochemical properties of hair in the BIDS syndrome. Am J Hum Genet. 1976;28:514–21. [PMC free article] [PubMed] [Google Scholar]
- 17.Jorizzo JL, Crounse RG, Wheeler CE., Jr. Lamellar ichthyosis, dwarfism, mental retardation, and hair shaft abnormalities. A link between the ichthyosis-associated and BIDS syndromes. J Am Acad Dermatol. 1980;2:309–17. doi: 10.1016/s0190-9622(80)80043-0. [DOI] [PubMed] [Google Scholar]
- 18.Crovato F, Borrone C, Rebora A. Trichothiodystrophy--BIDS, IBIDS and PIBIDS? Br J Dermatol. 1983;108:247. doi: 10.1111/j.1365-2133.1983.tb00068.x. [DOI] [PubMed] [Google Scholar]