Abstract
J Clin Hypertens (Greenwich). 2012;14:694–700 ©2012 Wiley Periodicals, Inc.
Low medication adherence may explain part of the high prevalence of apparent treatment‐resistant hypertension (aTRH). The authors assessed medication adherence and aTRH among 4026 participants taking ≥3 classes of antihypertensive medication in the population‐based Reasons for Geographic and Racial Differences in Stroke (REGARDS) trial using the 4‐item Morisky Medication Adherence Scale (MMAS). Low adherence was defined as an MMAS score ≥2. Overall, 66% of participants taking ≥3 classes of antihypertensive medication had aTRH. Perfect adherence on the MMAS was reported by 67.8% and 70.9% of participants with and without aTRH, respectively. Low adherence was present among 8.1% of participants with aTRH and 5.0% of those without aTRH (P<.001). Among those with aTRH, female sex, residence outside the US stroke belt or stroke buckle, physical inactivity, elevated depressive symptoms, and a history of coronary heart disease were associated with low adherence. In the current study, a small percentage of participants with aTRH had low adherence.
Apparent treatment‐resistant hypertension (aTRH) is defined by the use of 3 classes of antihypertensive medication with uncontrolled hypertension or the use of ≥4 classes regardless of blood pressure (BP) control. 1 Using data from the National Health and Nutrition Examination Survey (NHANES) 2005–2008, Egan and colleagues 2 estimated that 11.8% of all hypertensive US adults have aTRH. This represents an almost 100% increase since 1988 to 1994 when the prevalence of aTRH among US adults with hypertension was 5.5%. The diagnosis of TRH is complex and requires the exclusion of secondary causes of uncontrolled hypertension. 1
Many individuals with aTRH may not undergo a comprehensive assessment of the potential causes of their condition. Of particular relevance is low adherence to antihypertensive medication. Low medication adherence is common among persons with hypertension, and many studies have reported low adherence to antihypertensive treatment to be associated with worse BP control. 3 , 4 , 5 , 6 , 7 , 8 However, few data are available on antihypertensive medication adherence among individuals with aTRH. Documenting the prevalence of low adherence in aTRH may help better explain the high prevalence of this condition. Additionally, identifying factors associated with low medication adherence can help target future interventions to improve adherence in persons with aTRH. Therefore, we examined levels of medication adherence compared with individuals taking 3 antihypertensive medication classes who had controlled BP levels. Additionally, factors associated with low medication adherence among individuals with aTRH were identified. For this investigation, we analyzed data from a large sample of US adults enrolled in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. 9
Methods
Study Population
The REGARDS study has been described previously. 9 Briefly, the REGARDS study included adults 45 years and older from all 48 continental US states and the District of Columbia. Between January 2003 and October 2007, 30,239 individuals were enrolled into the REGARDS study. By design, blacks and residents of the stroke belt and stroke buckle region of the United States were oversampled. The “stroke buckle” was defined as coastal North Carolina, South Carolina, and Georgia and the “stroke belt” as the remainder of North Carolina, South Carolina, and Georgia as well as Alabama, Mississippi, Tennessee, Arkansas, and Louisiana. The current study population was restricted to individuals treated with ≥3 classes of antihypertensive medication (n=4128) in order to make comparisons for those with aTRH with a group treated with a comparable number of antihypertensive medications. Participants missing BP data (n=17) or information on medication adherence (n=85) were excluded, leaving 4026 participants for the analysis. The REGARDS study protocol was approved by the institutional review boards governing research in human patients at the participating centers and all participants provided informed consent.
Data Collection
The current analysis used data from the baseline visit of the REGARDS study. Baseline REGARDS study data were collected via a telephone interview, a self‐administered questionnaire, and in‐home examination. Of relevance to the current analysis, computer‐assisted telephone interviews administered by trained staff were used to collect information on participants’ age, smoking status, education, physical activity, alcohol consumption, symptoms of depression, and self‐reports of prior physician‐diagnosed comorbid conditions (eg, diabetes, hypertension, stroke, and myocardial infarction). Elevated symptoms of depression were defined as being present for participants with scores ≥4 on the 4‐item Centers for Epidemiologic Studies of Depression scale. 10 Trained health professionals conducted in‐home examinations that included weight, height, BP measurements, electrocardiography (ECG), the collection of blood and a spot urine samples, and a review of prescription and over‐the‐counter medications used during the prior 2‐week period. Medication names were recorded and subsequently coded into drug classes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Coronary heart disease (CHD) was defined by a self‐reported history or ECG evidence of myocardial infarction or a self‐reported history of a revascularization procedure. Diabetes was defined as a serum glucose ≥126 mg/dL for participants who had fasted ≥8 hours prior to their blood draw, serum glucose ≥200 mg/dL for those who had not fasted, or self‐report of a prior diagnosis of diabetes while not pregnant with current use of insulin or oral hypoglycemic medications. Using isotope‐dilution mass spectrometry–traceable serum creatinine, estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation. 11 Low eGFR was defined as levels <60 mL/min/1.73 m2. Albuminuria was defined as being present for individuals with a urinary albumin to urinary creatinine ratio ≥30 mg/g. Chronic kidney disease was defined by the presence of low eGFR or albuminuria.
BP Measurement, Medication Use, and aTRH
During the in‐home examination, BP was measured two times using aneroid sphygmomanometers following a standardized protocol by trained examiners. Participants were asked to sit for 3 minutes with both feet on the floor prior to the BP measurement. Based on the average of the 2 measurements, uncontrolled hypertension was defined as a systolic BP (SBP) ≥140 mm Hg or diastolic BP (DBP) ≥90 mm Hg except for in individuals with diabetes or chronic kidney disease, wherein uncontrolled hypertension was defined as SBP ≥130 mm Hg or DBP ≥80 mm Hg, respectively. Antihypertensive medication classes were defined using those listed in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). 12 They included angiotensin‐converting enzyme inhibitors, aldosterone receptor blockers, α‐blockers, angiotensin receptor blockers, β‐blockers, calcium channel blockers, central‐acting agents, diuretics, and direct vasodilators. One‐pill combinations were classified into individual medication classes. Information on medication dose was not recorded. aTRH was defined as being present for participants taking 3 antihypertensive medication classes with uncontrolled hypertension or taking ≥4 classes regardless of hypertension control status.
Adherence
During the telephone interview, medication adherence was assessed using the 4‐item Morisky Medication Adherence Scale (MMAS). 13 The 4 items on the MMAS are (1) Do you ever forget to take medications? (2) Are you ever careless in taking your medications? (3) Do you ever miss taking your medications when you are feeling better? (4) Do you ever miss taking any of your medications because you are feeling sick? Each item has a yes/no response option. One point is assigned to each ‘‘yes’’ response, and points were summed (totaling 0–4), with a higher score indicating worse adherence. 14 MMAS scores of 3 and 4 were pooled together for the current analysis due to a low number of participants in these groups. Additionally, consistent with a previous analysis of REGARDS study data, 15 individuals with MMAS scores ≥2 were categorized as having low medication adherence. In a prior study, the MMAS was reported to have acceptable internal consistency (Cronbach’s α=0.61) and the items maintained a high item‐to‐total correlation (>0.4 for each item). 13 Additionally, scores on the MMAS have previously been correlated with BP control. 13
Statistical Analysis
The prevalence of aTRH was calculated for all REGARDS participants with hypertension and for those with hypertension taking ≥3 antihypertensive medication classes. Characteristics of REGARDS study participants taking ≥3 antihypertensive medication classes were calculated by aTRH status, with the statistical significance of differences for those with controlled BP taking 3 classes of antihypertensive medication vs aTRH determined using chi‐square and t tests, as appropriate. The percentage of participants with controlled BP (SBP/DBP <140/90 mm Hg except for individuals with diabetes or chronic kidney disease wherein <130/80 mm Hg was required) was calculated by MMAS scores. Responses to each MMAS item and the distribution of MMAS scores were calculated by aTRH status and compared using chi‐square tests. Next, for individuals with aTRH, the odds ratios for low medication adherence associated with participant characteristics were calculated using logistic regression. These characteristics included age, race, sex, geographic region of residence, education, income, marital status, smoking status, alcohol consumption, physical activity level, BMI, diabetes, reduced eGFR, albuminuria, history of CHD or stroke, and elevated depressive symptoms. These variables were chosen based on their observed association with low medication adherence in the general population of persons with hypertension. 16 An initial model included adjustment for age, race, sex, and geographic region of residence. A subsequent model included all variables simultaneously. Data analysis was conducted using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Prevalence of aTRH
In the REGARDS study, 16,927 participants had hypertension. For the current analysis, we focused on the 4026 (24% of those with hypertension) who were treated with ≥3 antihypertensive medication classes. Overall, 2654 REGARDS participants had aTRH. This represents 16% of all REGARDS participants with hypertension and 66% of REGARDS participants taking ≥3 antihypertensive classes. Of those with aTRH, 58% were taking 3 classes of antihypertensive medication and had uncontrolled hypertension (n=1547) and 42% were taking ≥4 classes of antihypertensive medication (n=1107).
Participant Characteristics
In comparison to those with controlled BP taking 3 classes of antihypertensive medications, those with aTRH were more likely to be black, men, have less than a high school education, an annual household income <$20,000, participate in less physical activity, and have elevated depressive symptoms (Table I). Each comorbidity (low eGFR, albuminuria, diabetes, and a history of CHD and stroke) was more common among individuals with aTRH. Also, mean BMI was higher for individuals with vs without aTRH.
Table I.
Characteristics of REGARDS Study Participants Taking ≥3 Antihypertensive Medication Classes by Apparent Treatment‐Resistant Hypertension Status
| Controlled on 3 Medicationsa (n=1372) | Apparent‐Treatment Resistant hypertensionb (n=2654) | P Value | |
|---|---|---|---|
| Age, y | 67.3 (8.8) | 67.4 (8.7) | .69 |
| Black, % | 46.9 | 60.0 | <.001 |
| Male, % | 40.7 | 48.2 | <.001 |
| Region of residence, % | |||
| Non‐belt | 42.1 | 45.3 | .08 |
| Stroke belt | 35.1 | 34.6 | |
| Stroke buckle | 22.8 | 20.2 | |
| <High school education, % | 14.1 | 19.9 | <.001 |
| Household Income <$20,000 per year, % | 23.6 | 28.5 | .002 |
| Currently married, % | 55.0 | 53.7 | .41 |
| Current smoking, % | 11.4 | 12.5 | .33 |
| Physical activity, % | |||
| None | 40.4 | 44.4 | .007 |
| 1–3 times per week | 32.7 | 32.9 | |
| >3 times per week | 26.9 | 22.7 | |
| Alcohol consumption, % | |||
| None | 68.1 | 70.0 | .30 |
| Moderate | 28.3 | 27.1 | |
| Heavy | 3.6 | 2.9 | |
| BMI, kg/m2 | 30.8 (6.2) | 32.4 (6.8) | <.001 |
| Depressive symptoms, %c | 11.5 | 14.4 | .01 |
| Low eGFR <60 mL/min/1.73 m2, % | 22.9 | 33.6 | <.001 |
| Albuminuria, % | 12.8 | 35.7 | <.001 |
| Diabetes, % | 27.3 | 49.6 | <.001 |
| History of CHD, % | 29.2 | 34.6 | <.001 |
| History of stroke, % | 11.2 | 13.4 | .05 |
Abbreviations: BMI, body mass index; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; REGARDS, Reasons for Geographic and Racial Differences in Stroke. aControlled blood pressure (BP) was defined as systolic/diastolic blood pressure <140/90 mm Hg except for individuals with diabetes or chronic kidney disease wherein controlled BP was systolic/diastolic blood pressure <130/80 mm Hg. bTreatment‐resistant hypertension was defined as having uncontrolled BP while taking 3 antihypertensive medication classes or taking 4 antihypertensive medication classes regardless of BP control. cScore ≥4 on the 4‐item Centers for Epidemiologic Studies of Depression scale.
Level of Medication Adherence
The distribution of MMAS scores of 0 (best adherence) and 1, 2, and 3 or 4 (worst adherence), was 68.8%, 24.1%, 5.0%, and 2.0%, respectively, among the entire study population (N=4026). Individuals with worse adherence were less likely to have controlled hypertension (Figure 1). The prevalence of controlled hypertension was 46.8%, 46.6%, 37.9%, and 27.2% for MMAS scores of 0, 1, 2, and 3 or 4, respectively.
Figure 1.
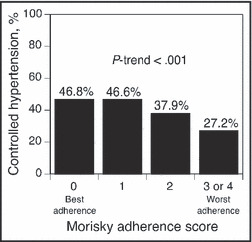
Hypertension control rates associated with level of medication adherence among Reasons for Geographic and Racial Differences in Stroke (REGARDS) study participants taking at least 3 antihypertensive medication classes.
While 28.9% of individuals with aTRH reported sometimes forgetting to take their medication, this was reported by 26.4% of individuals taking 3 BP medications with controlled hypertension (Table II, P=.088). Compared with participants with controlled BP while taking 3 medications, those with aTRH were more likely to report being careless about taking their medication and to stop taking their medication when they were feeling better or when they were feeling worse. However, none of these 3 poor medication‐taking behaviors was reported by more than 6% of individuals with aTRH. Overall MMAS scores were significantly higher, indicating worse adherence, for individuals with aTRH (Figure 2). Overall, 8.1% of individuals with aTRH and 5.0% of individuals with controlled BP while taking 3 antihypertensive medications had low adherence defined by MMAS score ≥2 (P<.001).
Table II.
Responses to Individual 4‐Item MMAS Questions by Apparent Treatment‐Resistant Hypertension Status
| Morisky Question | Controlled on 3 Medicationsa (n=1372) | Apparent Treatment Resistant Hypertensionb (n=2654) | P Value |
|---|---|---|---|
| 1. Sometimes forget to take medication (yes) | 26.4% | 28.9% | .09 |
| 2. Careless about taking medication (yes) | 2.6% | 4.6% | .003 |
| 3. Stop taking medication when feeling better (yes) | 3.5% | 5.9% | <.001 |
| 4. Stop taking medication when feeling worse (yes) | 3.0% | 4.3% | .04 |
Abbreviation: MMAS, Morisky Medication Adherence Scale. aControlled blood pressure was defined as systolic/diastolic blood pressure <140/90 mm Hg except for individuals with diabetes or chronic kidney disease wherein controlled blood pressure was systolic/diastolic blood pressure <130/80 mm Hg. bTreatment‐resistant hypertension was defined as having uncontrolled blood pressure while taking 3 antihypertensive medication classes or taking 4 antihypertensive medication classes regardless of blood pressure control.
Figure 2.
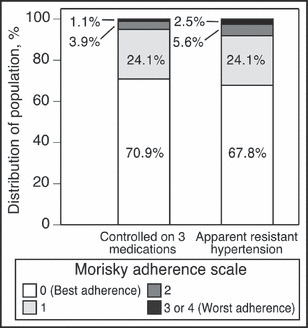
Level of adherence among Reasons for Geographic and Racial Differences in Stroke (REGARDS) study participants by apparent treatment‐resistant hypertension status. P<.001 comparing the distribution of Morisky Medication Adherence Scale scores.
Factors Associated With Low Medication Adherence in aTRH
After adjustment for age, sex, and geographic region of residence, blacks were more likely to have low medication adherence (Table III, Model 1). After age, race, and geographic region of residence adjustment, women were more likely than men to have low medication adherence. After age, race, sex, and geographic region of residence adjustment, individuals with an annual household income <$20,000, elevated depressive symptoms, and a history of CHD were more likely to have low medication adherence. Additionally, participants residing in the stroke belt or stroke buckle vs non‐belt regions and those participating in physical activity 1 to 3 or >3 times per week were less likely to have low medication adherence. The results were similar after full multivariable adjustment (Table III, Model 2), with the exception that the associations of black race and an annual household income <$20,000 with low medication adherence were attenuated and no longer statistically significant.
Table III.
Odds Ratios for Low Medication Adherence Defined as 4‐Item MMAS Score ≥2 Among REGARDS Study Participants With Apparent Treatment‐Resistant Hypertension
| Odds Ratio (95% CI) for Low Adherence | ||
|---|---|---|
| Model 1a | Model 2b | |
| Age, per 10 y | 0.88 (0.75–1.04) | 0.83 (0.68–1.01) |
| Black | 1.47 (1.07–2.02) | 1.33 (0.93–1.91) |
| Male | 0.70 (0.53–0.94) | 0.64 (0.44–0.93) |
| Residence in stroke belt | 0.69 (0.50–0.95) | 0.66 (0.46–0.95) |
| Residence in stroke buckle | 0.50 (0.33–0.76) | 0.49 (0.31–0.78) |
| <High school education | 1.30 (0.93–1.84) | 1.09 (0.73–1.63) |
| Household income <$20,000 per year | 1.56 (1.12–2.16) | 1.32 (0.88–1.97) |
| Currently married | 0.99 (0.72–1.36) | 1.22 (0.84–1.97) |
| Current smoking | 1.36 (0.92–2.00) | 1.18 (0.75–1.85) |
| Body mass index, kg/m2 | ||
| <25 | 1 (ref) | 1 (ref) |
| 25–29 | 0.70 (0.44–1.10) | 0.71 (0.42–1.19) |
| ≥30 | 0.73 (0.48–1.10) | 0.75 (0.47–1.21) |
| Physical activity | ||
| None | 1 (ref) | 1 (ref) |
| 1–3 times per week | 0.58 (0.42–0.81) | 0.60 (0.42–0.87) |
| >3 times per week | 0.61 (0.41–0.89) | 0.66 (0.43–1.00) |
| Alcohol consumption | ||
| None | 1 (ref) | 1 (ref) |
| Moderate | 0.94 (0.67–1.33) | 0.98 (0.67–1.43) |
| Heavy | 0.92 (0.36–2.34) | 0.86 (0.29–2.50) |
| Depressive symptoms | 1.74 (1.23–2.46) | 1.62 (1.09–2.40) |
| Low eGFR <60 mL/min/1.73 m2 | 0.93 (0.68–1.26) | 0.83 (0.58–1.18) |
| Albuminuria | 1.08 (0.79–1.47) | 1.06 (0.76–1.48) |
| Diabetes | 0.88 (0.66–1.18) | 0.87 (0.63–1.20) |
| History of CHD | 1.61 (1.20–2.16) | 1.54 (1.11–2.15) |
| History of stroke | 1.07 (0.72–1.61) | 0.75 (0.47–1.20) |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; eGFR, estimated glomerular filtration rate; MMAS, Morisky Medication Adherence Scale; REGARDS, Reasons for Geographic and Racial Differences in Stroke. aModel 1 included age, race, sex, and geographic region of residence. bModel 2 included all variables simultaneously. cScore ≥4 on the 4‐item Centers for Epidemiologic Studies of Depression scale.
Discussion
Studies have consistently demonstrated that low antihypertensive medication adherence is a barrier for achieving adequate BP control. 8 , 16 Therefore, low medication adherence may be a major cause of aTRH among individuals taking multiple antihypertensive medications. Low adherence to antihypertensive medications should be evaluated and excluded as a cause of uncontrolled BP before a diagnosis of true TRH is made. 1 In the current study, we examined the association between low medication adherence and aTRH in individuals taking antihypertensive medications from ≥3 classes using a validated scale in a large population‐based sample of US adults. While medication adherence was statistically significantly better among individuals without vs those with aTRH, the differences were small. Almost 70% of individuals with and without aTRH reported good medication‐taking behaviors according to all 4 adherence items in the 4‐item MMAS. Furthermore, <10% of REGARDS participants with aTRH had low medication adherence, defined by scores ≥2 on the 4‐item MMAS. Our findings suggest that overall self‐reported low medication adherence in aTRH is quite low.
The prevalence of aTRH in the United States has only recently been reported and is estimated to be 11% to 15% among persons with hypertension. 2 , 17 , 18 The estimate we report in the REGARDS cohort is slightly higher (16%), which may be due to oversampling of black participants in the REGARDS study compared with other studies. For example, approximately 15% of the NHANES 2005–2008 sample was black and that group was 2 times more likely to have aTRH. 2 More recently, using patient data collected over a 4‐year period in the Kaiser Permanente Colorado and Northern California healthcare systems, Daugherty and colleagues 19 identified 205,750 patients who were started on antihypertensive therapy for newly diagnosed hypertension. During follow‐up, close to 21% were eventually prescribed ≥3 medications and 24,499 participants were found eligible for determination of aTRH status. Among that group, the prevalence of aTRH was 16%. In the current study, the prevalence of aTRH among persons treated with ≥3 antihypertensive medications was 66%. Differences between the current study and that of Daugherty and colleagues may be explained by the sampling of incident hypertension cases, low percentage of blacks, and the relatively younger age of the cohort (on average, 7 years younger) reported by Daugherty and colleagues. Among the 24,499 participants found eligible for determination of aTRH status in the Daugherty study, only 587 (2.3%) were excluded from further analysis for low medication adherence based on proportion of days covered <80% determined by pharmacy fill rates. Similar to the current study, the Daugherty study found that a small percentage of persons treated with ≥3 antihypertensive medications had low adherence.
Small studies have previously examined the prevalence of nonadherence to antihypertensive medication in the context of aTRH. One prospective study from Brazil in 44 persons with hypertension resistant to a 3‐drug regimen assessed adherence using the 4‐item MMAS and pill count method. Overall, 36% of participants reported perfect adherence at study initiation, and this figure rose to 68% at 1 year. By pill count, 64% of the population was adherent at the start of the study and 94% at the end, yet 60% of participants did not achieve BP control. 20 In contrast, another study evaluated serum drug levels in persons with difficult‐to‐treat hypertension (N=84) and found only 35% of study participants to be perfectly adherent. 21 Whether low medication adherence is more prevalent in aTRH than in a comparison group with a similar treatment regimen is less studied. While significant differences in low medication adherence were present between the REGARDS study participants with and without aTRH in the current analyses, these differences were small. These results suggest that factors other than low medication adherence may contribute to hard‐to‐control BP in most persons with aTRH.
Despite the low rate of poor adherence in the current study, it is still important to evaluate adherence among all persons with hypertension given the established association of medication adherence with BP control. The strengths and weaknesses of various approaches to assess medication adherence have been previously published. 22 The consequences of uncontrolled BP are serious due to increased risk for poor clinical outcomes and increased health care costs. 23 , 24 , 25 Few large population‐based studies have examined the relationship between adherence and BP control in individuals taking multiple antihypertensive medications. In the current large population‐based sample of US adults taking ≥3 antihypertensive medication classes, we show a graded relationship between better adherence and higher rates of BP control. The evaluation of low medication adherence in the context of aTRH is especially important as some persons may not need to undergo comprehensive clinical tests required for the diagnosis of true TRH. 1
While low medication adherence remains an important consideration when determining the causes of aTRH, other factors including genes, environment, and their interactions may be worthy of exploration in future studies. Recent reviews targeting strategies to uncover the genetic background of complex traits (such as hypertension) have suggested that extreme phenotype samples (such as TRH) may be enriched for variants underlying the disease. 26 , 27 Several small candidate gene studies have reported associations with TRH that warrant follow‐up investigations. 28 , 29 , 30 For instance, one study reported the angiotensinogen M235T polymorphism was associated with increased risk for TRH, especially after age 50, highlighting a potential gene variant by age interaction. 29 Replication of preliminary results from smaller studies should be carried out in larger cohorts. Such studies will continue to shed light on the mechanisms underlying TRH and could translate to better understanding of the genetic background of less extreme forms of hypertension.
Many studies have considered factors that affect medication adherence in the general population of hypertensives. 16 , 31 , 32 , 33 , 34 , 35 However, factors affecting medication adherence in aTRH have not been widely studied. In the current study of individuals with aTRH, women were more likely to have low medication adherence. Previous studies in the general population of individuals with hypertension have reported either no association of sex with adherence or that women newly diagnosed with hypertension were more adherent than men. 33 , 36 , 37 Consistent with reports in other chronic conditions and in hypertension, 34 , 38 , 39 , 40 , 41 , 42 elevated depressive symptoms were associated with low medication adherence. We also found any physical activity to be protective against low medication adherence. This may reflect a healthier lifestyle among people who are adherent. Interestingly, our results suggest that a history of CHD was associated with low medication adherence. Low adherence to antihypertensive and lipid‐lowering medication has been reported after myocardial infarction in prior studies. 43 Whether very complex treatment regimens and/or persistence of behaviors associated with low medication adherence underlie this association in aTRH requires further study.
Strengths and Limitations
There are several strengths and limitations regarding the current analysis that should be mentioned. The adherence scale used in the REGARDS study was not specific to antihypertensive medications and adherence was assessed only at a single time point. Additionally, adherence was assessed using self‐report only. Electronic data on medication adherence (eg, pharmacy fill data) is not available in the REGARDS study. While the 4‐item MMAS has been validated, participants may have provided socially desirable responses. Additionally, we were unable to confirm optimal dosing of antihypertensive medications as a criterion to define aTRH as dose was not recorded in the REGARDS study. Despite these limitations, this study is the largest to characterize medication adherence in aTRH and is strengthened by the population‐based sample in REGARDS and use of an adherence tool validated for studies of BP control. Additionally, the large sample size of the REGARDS study allowed for comparison of aTRH with people who had controlled hypertension while taking 3 antihypertensive medication classes. Finally, the REGARDS study recruited individuals irrespective of socioeconomic or health insurance status and used standardized protocols with stringent quality‐control procedures to collect data.
Conclusions
In this population‐based sample of black and white adults with hypertension treated with at least 3 antihypertensive medication classes, better adherence was associated with higher BP control rates. Among individuals with aTRH, 67.8% reported perfect adherence and only 8.1% had low medication adherence and this was comparable to participants taking 3 antihypertensive medication classes with controlled BP. Further, many factors previously reported to be associated with low medication adherence in persons with hypertension were also associated with low medication adherence when investigated among individuals with aTRH. Taken together, these results help to distinguish TRH as an extreme phenotype within uncontrolled hypertension and emphasize the need for further studies to help better define its etiology. In the meantime, health care providers should continue to assess adherence and assist patients with aTRH in overcoming barriers to taking their medication, similar to other patients with hypertension.
Acknowledgment and disclosure: This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
References
- 1. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. [DOI] [PubMed] [Google Scholar]
- 2. Egan BM, Zhao Y, Axon RN, et al. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elliott WJ. Improving outcomes in hypertensive patients: focus on adherence and persistence with antihypertensive therapy. J Clin Hypertens (Greenwich). 2009;11:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mazzaglia G, Mantovani LG, Sturkenboom MC, et al. Patterns of persistence with antihypertensive medications in newly diagnosed hypertensive patients in Italy: a retrospective cohort study in primary care. J Hypertens. 2005;23:2093–2100. [DOI] [PubMed] [Google Scholar]
- 5. Van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Rate and determinants of 10‐year persistence with antihypertensive drugs. J Hypertens. 2005;23:2101–2107. [DOI] [PubMed] [Google Scholar]
- 6. Yiannakopoulou ECh, Papadopulos JS, Cokkinos DV, Mountokalakis TD. Adherence to antihypertensive treatment: a critical factor for blood pressure control. Eur J Cardiovasc Prev Rehabil. 2005;12:243–249. [DOI] [PubMed] [Google Scholar]
- 7. Romanelli RJ, Schiro TA, Jukes T, et al. Disparities in blood pressure control within a community‐based provider network: an exploratory analysis. Ann Pharmacother. 2011;45:1473–1482. [DOI] [PubMed] [Google Scholar]
- 8. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–209. [DOI] [PubMed] [Google Scholar]
- 9. Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 10. Radloff LS. The CES‐D scale: A self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 13. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 14. Shalansky SJ, Levy AR, Ignaszewski AP. Self‐reported Morisky score for identifying nonadherence with cardiovascular medications. Ann Pharmacother. 2004;38:1363–1368. [DOI] [PubMed] [Google Scholar]
- 15. Muntner P, Halanych JH, Reynolds K, et al. Low medication adherence and the incidence of stroke symptoms among individuals with hypertension: the REGARDS study. J Clin Hypertens (Greenwich). 2011;13:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krousel‐Wood M, Thomas S, Muntner P, Morisky D. Medication adherence: a key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr Opin Cardiol. 2004;19:357–362. [DOI] [PubMed] [Google Scholar]
- 17. Persell SD. Prevalence of resistant hypertension in the United States, 2003‐2008. Hypertension. 2011;57:1076–1080. [DOI] [PubMed] [Google Scholar]
- 18. Pimenta E, Calhoun DA. Resistant hypertension: incidence, prevalence, and prognosis. Circulation. 2012;125:1594–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Souza WA, Sabha M, de Faveri Favero F, et al. Intensive monitoring of adherence to treatment helps to identify “true” resistant hypertension. J Clin Hypertens (Greenwich). 2009;11:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ceral J, Habrdova V, Vorisek V, et al. Difficult‐to‐control arterial hypertension or uncooperative patients? The assessment of serum antihypertensive drug levels to differentiate non‐responsiveness from non‐adherence to recommended therapy Hypertens Res. 2011;34:87–90. [DOI] [PubMed] [Google Scholar]
- 22. Krousel‐Wood M, Hyre A, Muntner P, Morisky D. Methods to improve medication adherence in patients with hypertension: current status and future directions. Curr Opin Cardiol. 2005;20:296–300. [DOI] [PubMed] [Google Scholar]
- 23. Esposti LD, Saragoni S, Benemei S, et al. Adherence to antihypertensive medications and health outcomes among newly treated hypertensive patients. Clinicoecon Outcomes Res. 2011;3:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. [DOI] [PubMed] [Google Scholar]
- 25. Stroupe KT, Teal EY, Tu W, et al. Association of refill adherence and health care use among adults with hypertension in an urban health care system. Pharmacotherapy. 2006;26:779–789. [DOI] [PubMed] [Google Scholar]
- 26. Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi G, Rao DC. Optimum designs for next‐generation sequencing to discover rare variants for common complex disease. Genet Epidemiol. 2011;35:572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hannila‐Handelberg T, Kontula K, Tikkanen I, et al. Common variants of the beta and gamma subunits of the epithelial sodium channel and their relation to plasma renin and aldosterone levels in essential hypertension. BMC Med Genet. 2005;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yugar‐Toledo JC, Martin JF, Krieger JE, et al. Gene variation in resistant hypertension: multilocus analysis of the angiotensin 1‐converting enzyme, angiotensinogen, and endothelial nitric oxide synthase genes. DNA Cell Biol. 2011;30:555–564. [DOI] [PubMed] [Google Scholar]
- 30. Cruz‐Gonzalez I, Corral E, Sanchez‐Ledesma M, et al. An association between resistant hypertension and the null GSTM1 genotype. J Hum Hypertens. 2009;23:556–558. [DOI] [PubMed] [Google Scholar]
- 31. World Health Organization: Hypertension in Adherence to Long‐Term Therapies: Evidence for Action, 2003. http://apps.who.int/medicinedocs/en/d/Js4883e/ Accessed July 10, 2012. [Google Scholar]
- 32. Furberg CD, Black DM. The systolic hypertension in the elderly pilot program: methodological issues. Eur Heart J. 1988;9:223–227. [DOI] [PubMed] [Google Scholar]
- 33. Marentette MA, Gerth WC, Billings DK, Zarnke KB. Antihypertensive persistence and drug class. Can J Cardiol. 2002;18:649–656. [PubMed] [Google Scholar]
- 34. Wang PS, Bohn RL, Knight E, et al. Noncompliance with antihypertensive medications: the impact of depressive symptoms and psychosocial factors. J Gen Intern Med. 2002;17:504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim MT, Han HR, Hill MN, et al. Depression, substance use, adherence behaviors, and blood pressure in urban hypertensive black men. Ann Behav Med. 2003;26:24–31. [DOI] [PubMed] [Google Scholar]
- 36. Caro JJ, Salas M, Speckman JL, et al. Persistence with treatment for hypertension in actual practice. CMAJ. 1999;160:31–37. [PMC free article] [PubMed] [Google Scholar]
- 37. Krousel‐Wood MA, Muntner P, Islam T, et al. Barriers to and determinants of medication adherence in hypertension management: perspective of the cohort study of medication adherence among older adults. Med Clin North Am. 2009;93:753–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta‐analysis. J Acquir Immune Defic Syndr. 2011;58:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morris AB, Li J, Kroenke K, et al. Factors associated with drug adherence and blood pressure control in patients with hypertension. Pharmacotherapy. 2006;26:483–492. [DOI] [PubMed] [Google Scholar]
- 40. Maguire LK, Hughes CM, McElnay JC. Exploring the impact of depressive symptoms and medication beliefs on medication adherence in hypertension‐‐a primary care study. Patient Educ Couns. 2008;73:371–376. [DOI] [PubMed] [Google Scholar]
- 41. Wang PS, Avorn J, Brookhart MA, et al. Effects of noncardiovascular comorbidities on antihypertensive use in elderly hypertensives. Hypertension. 2005;46:273–279. [DOI] [PubMed] [Google Scholar]
- 42. Krousel‐Wood M, Islam T, Muntner P, et al. Association of depression with antihypertensive medication adherence in older adults: cross‐sectional and longitudinal findings from CoSMO. Ann Behav Med. 2010;40:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Akincigil A, Bowblis JR, Levin C, et al. Long‐term adherence to evidence based secondary prevention therapies after acute myocardial infarction. J Gen Intern Med. 2008;23:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]


