Abstract
We propose a tripartite biochemical mechanism for memory. Three physiologic components are involved, namely, the neuron (individual and circuit), the surrounding neural extracellular matrix, and the various trace metals distributed within the matrix. The binding of a metal cation affects a corresponding nanostructure (shrinking, twisting, expansion) and dielectric sensibility of the chelating node (address) within the matrix lattice, sensed by the neuron. The neural extracellular matrix serves as an electro-elastic lattice, wherein neurons manipulate multiple trace metals (n > 10) to encode, store, and decode coginive information. The proposed mechanism explains brains low energy requirements and high rates of storage capacity described in multiples of Avogadro number (NA = 6 × 1023). Supportive evidence correlates memory loss to trace metal toxicity or deficiency, or breakdown in the delivery/transport of metals to the matrix, or its degradation. Inherited diseases revolving around dysfunctional trace metal metabolism and memory dysfunction, include Alzheimer's disease (Al, Zn, Fe), Wilson’s disease (Cu), thalassemia (Fe), and autism (metallothionein). The tripartite mechanism points to the electro-elastic interactions of neurons with trace metals distributed within the neural extracellular matrix, as the molecular underpinning of “synaptic plasticity” affecting short-term memory, long-term memory, and forgetting.
Keywords: Memory, information, ionic chip, neuron, extracellular matrix, trace metal
Background
Biologic memory in the brain is a mystery. Various adjectives have been used to describe memory (i.e., active, declarative, passive, associative, short-term (STM), long-term (LTM), super memory), but none in molecular terms. No consensus exists for how cognitive information (cog-info) is encoded or stored in the brain.
Scientists from disparate disciplines have suggested various molecular mechanism, such as DNA/RNA-based processes, to describe memory. Neuroscientists proposed neural firing patterns, neurocircuits, neural-networks, neurotransmittors, and synaptic firing as the basis for encoding sensory perceptions as memory.1−22 The “synaptic plasticity” model is unsatisfactory from the perspective of compactness, kinetics, energy requirements, and lack of an information theory.21 What is missing is a physiologically relevant, molecular mechanism, whereby cog-info derived from the senses can be encoded, stored, and recalled by the neural system.
In computer ionic memory chips,23−38 information is received, processed, and stored by manipulating the distribution of elemental cations (dopants) within the chip matrix, usually solid electrolytes (metal sulfides, Ge-based chalcogenides, or oxides such as TaO3, WO3, SiO2, TiO2). Ionic memory chips are doped with Ag, Cu, and Zn . The information theories and binary value (0, 1) algorithms developed by von Neumann, Turing, Weiner, and Shannon are used to encode digital information in the memory chip.39−49
Neural Memory Traits
The ionic memory chip is a compelling model for how one would like to describe biological memory in the brain. The characteristics and traits that one wishes to describe include the following:
-
a)
A credible mechanism for memory, based on generally accepted biochemical principles, with available physiologic components.
-
b)
Molecular-scale encoding/decoding process, faster than the rate of neural firing (<100 ms).
-
c)
Large storage capacity for physically encoding cog-info.
-
d)
Low energy requirements (<400 cal/day).
-
e)
Capacity for storing cog-info for short and long durations (recall: 1 min to >1 day).
-
f)
Capable of acquiring (learning), storing, and losing (forgetting) cog-info.
-
g)
Applicable to all animals with neural circuitry.
Hypothesis: The Tripartite Mechanism of Memory
We propose that “memory” emerges from the dynamic interaction of three physiologic compartments, consisting of the following:
-
1)
Neurons.
-
2)
Neural extracellular matrix (nECM) around the neuron.
-
3)
Trace metal cations dispersed within the nECM (dopants).
The nECM constitutes a hydrated lattice wherein cog-info is encoded, processed, and stored. The neurons manipulate and electro-elastically sense the surrounding ensembles of [nECM/metal] complexes, to encode and recall memory. More specific descriptions of these compartments follow.
1. Neuron50−84
The neuron and neural circuits, the computational components of the brain, operate by electrical signaling within an aqueous environment. The cells are intimately connected to the external nECM by electrically sensitive surface features, notably integrin receptors, gap junctions, nodes of Ranvier, and synapses. The electro-elastic contact between the neural surface and its external environment (nECM) is the keystone to neural computation.
2. nECM85−135
The nECM surrounding the neurons comprises 20–25% of the total brain volume. It exhibits gross- and microanisotropies in terms of ultrastructure, composition, and dielectric properties. It comprises a block copolymer lattice around the neuron, composed of polymeric glycosaminoglycans (GAG) (hyaluronic acid (HA), lecticans, proteoglycans, chondroitin sulfates (Chon), heparan sulfate (Hep)), which present many anionic/Lewis base moieties for attaching metal cations. Interspersed within the GAG lattice are also many proteins (e.g., collagens, integrins, tenascins, phosphacan, various enzymes) and glycoproteins (e.g., nidogen, reelin, TNC, thrombospondin).
The nECM lattice is an electroelastic hydrogel, characterized by viscoelastic traits, carrying currents on the order of 200 nAmp, with conduction velocities 2–10 m/s, accommodating voltage changes exerted at neuronal synapses and other anatomical sites (i.e., gap junctions, nodes of Ranvier).
We propose that the complexation of a metal cation to specific chelating groups (nodes) within the nECM concomitantly modulates the nanoscale structural, viscoelastic, and dielectric properties of the lattice, sensed by the neuron.
Thus, the nECM serves two functions:
-
•
As the structural scaffolding encasing the neurons, through which gases (oxygen and carbon dioxide), metals, and metabolites diffuse.
-
•
As an electro-elastic lattice used by the neuron to encode and decode cog-info, by modulating or sensing the pattern of metal cations bound to specific nodes (addresses).
3. Trace Metals in the Brain136−153
Brain levels of more than 15 trace metals have been measured within the gross tissue as well as within the individual neurons. Metal levels were highest within the neuron, but were present in the nECM, at levels ranging from 10–6 to 10–9 M (Table 1). Table 1 shows the composition of total human brain tissue, presenting the 15 most prevalent elemental metals. Most (>90%) are sequestered within the neurons, with <10% found in the nECM. The distribution of trace metals is not homogeneous between and within anatomical regions of the brain.
Table 1. Brain Levels of Elements.
| metal | ∼value | unit | + valence |
|---|---|---|---|
| K | 3.4 | M | 1 |
| Na | 2.7 | M | 1 |
| Mg | 0.3 | M | 2 |
| Ca | 60 | mM | 2 |
| Fe | 10 | mM | 2/3 |
| Co | 6 | mM | 2/3 |
| Zn | 6 | mM | 2 |
| Cu | 3 | mM | 2/1 |
| Rb | 3 | mM | 1 |
| Li | ∼ 1 | mM | 1 |
| Sc | 370 | uM | 3 |
| Mn | 211 | uM | 2/3 |
| Cr | 153 | uM | 2/3 |
| Al | 14–20 | uM | 2/3 |
| Cd | 18 | uM | 2 |
| Pb | 3 | uM | 2 |
| Hg | 0.5 | uM | 1 |
nECM/Metal Complexes: Conformations, Kinetics, and Energetics154−165
Parameters, such as metal chelate dissociation constant (Kd), solubility constants of salts (Ksp), or molar binding ratios, reflect the inherent binding proclivities of elemental cations for the various anionic moieties (such as carboxyl, sulfate, amine, hydroxyl, phosphate, and other electron-rich groups), within the nECM lattice encasing the neuron. The anionic and electron rich moieties anchor the metal to specific nodes on the lattice, serving as “addresses” for encoding the cog-info.
The inherent bonding geometries of the elemental metals range from square planar (d2sp3), tetrahedral (sp3), to octahedral (d2sp3), with individual bonding distances for each. Thus, each elemental oxidation state presents a unique bonding “signature”. The polyanionic nECM can flex to achieve the most energetically favorable metal complexes.
The disposition of different metal cations within the nECM is modified by iontophoretic and electro-elastic effects, exerted or sensed by the neurons. The binding/desorption of hydrated metal cations to/from hydrated anionic substrates are among the most rapid biologic reactions, requiring low energy of activation (Eact) and generate little heat (low ΔH). The low levels of various elements (millimolar to nanomolar (10–3 to 10–9 M) concentration) further minimize heat generation.
nECM as “Information Lattice”
Consider that the nECM around each neuron as a three-dimensional lattice capable of binding more than 15 different mobile components (elemental cations) as arrays/clusters/packets/stacks of almost limitless, combinatorial complexity.
The diversity of the nECM/Metal is enormous. It arises from the following:
-
1)
Many elemental metals: various metals are present in the brain, each binding with a unique binding configuration, depending on its electron shell disposition. Some elements can exist in more than one oxidation state (i.e., Cu+1/2, Fe+2/3, Mn+2/4/5).
-
2)
The nECM is a complex lattice of polymeric components, each which present multiple anionic and electron rich moieties. These moieties can entrap metal cations; the lattice flexes to achieve the optimal disposition of groups of anionic moieties (variable size chelate rings) to accommodate the bonding preferences of the various elemental cations. Stable configurations encode cog-info.
Metal binding to a particular nECM locale (address) results in conformational changes (flexing, contraction) of the local lattice geometry, with resultant modifications in the local neural membrane polarizabiltity/resistivity/elasticity sensed by the neuron. The [nECM/metal] complexes comprise configurable molecular switches by which neurons encode/decode cog-info.166−196
The computational possibilities are astronomic. The metal-binding capacity of the nECM around each neuron reflects the molar equivalents of anionic moieties, multiplied by the Avogadro number, NA (6 × 1023). Thus, the molar cog-info storage capacity is very large (multiples or exponentials of Avogadro’s number (NA = 6 × 1023). If only a fraction of the elemental cations in the nECM function in a combinatorial mode, there are enough to serve as mobile components for encoding/decoding and processing large amounts of cog-info, on which memory is based.
It is interesting to compare the operation of computer ionic memory chips with the biological mnemonic system, as in Table 2.
Table 2. Comparing Information Processing.
| item | computer | brain |
|---|---|---|
| unit component | ionic chip | neuron |
| matrix composition | solid state matrix | nECM hydrogel |
| dopant(s) | 1 elemental cation | n elemental cations (n > 10) |
| construction | static hardwiring | synaptic neural network |
| computational format | digital | analogue |
| information unit | bit/word | cuinfo |
| # coding options | n = 2 (0,1) | n > 10 |
| programming mode | serial2 | paralleln |
| groupings | dedicated circuits | sparse neural ensembles |
| underlying physics | electro-optic/magnetic | electro-elastic/chemical |
| read/write driver | voltage differential | iontophoresis, chelation |
| signal speed | c (speed of light) | 1–80 m/s |
| signal frequency | 50–60 Hz | 2–70 kHz |
| energy | external (∼225 W·h) | metabolism (22 W·h) |
Discussion
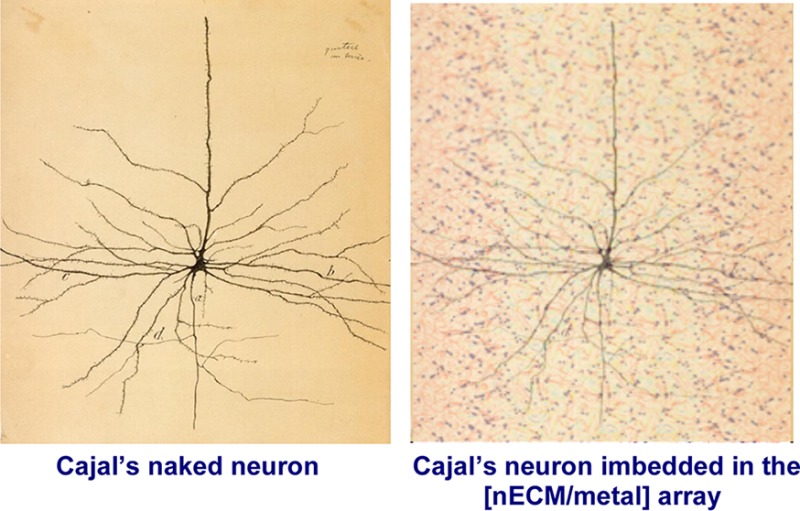
The brain contains 1010 to 1011 neurons, which can each have 104 excitable synapses. The histologic tissue sections by Cajal197 and Golgi198 (Figure 1) more than 100 years ago, which revealed neurons with synaptic contacts, were based on a selective staining process to image the neuron, but ignored the surrounding matrix. Since then, the neuron has been generally represented as a cell suspended in an “invisible” context or “space”, like an interplanetary object. How can one explain the function of a ship without reference to water?
Figure 1.
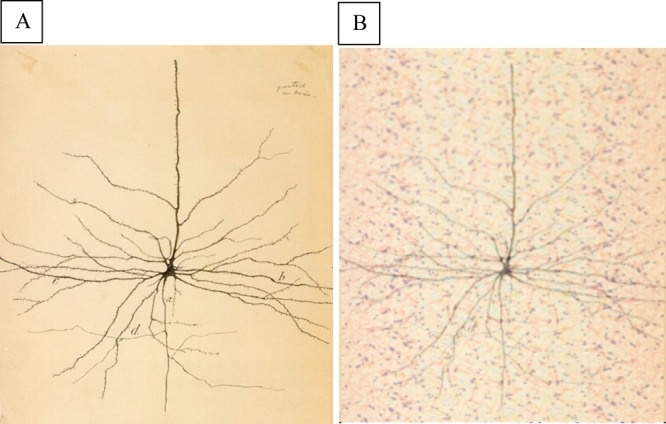
(A) Drawing of a single neuron by Cajal, based on Golgi’s silver stain technique which ignored the nECM and (B) with superimposed image of the [nECM/metal] array, lightly stained for everything . With many histologic stains, one cannot see the neuron for the “trees” of the nECM.
Biochemists know that the “space” around the neuron is a reticulum composed of polyanionic biopolymers called the nECM.85−135 We point out that the neuron is encased in a complex, nonhomogeneous nECM which functions both as a structural environment for the neuron, as well as a computational matrix wherein it encodes and stores cog-info.
Stacks and arrays of [nECM/metal] engulf the neuron with a continuum of stoichiometries, constituting the molecular correlates of cog-info, on which memory is based. In support of the tripartite mechanism of memory, we cite the following observations:
-
1)
The literature describes effects associated with metal toxicity or deficiency, in terms of memory loss, as well as associated behavioral perturbations (confusion, disorientation, poor learning, personality changes) (Table 3).199−210
These all indicate that perturbations of the optimum concentrations of elemental metals, either by excess or low levels, are manifest by clinically observed changes in behavioral processes, ascribed to dysfunctional memory.
-
2)Metabolic disorders related to metals and memory:
-
•Alzheimer disorder (aluminum, zinc).
-
•Wilson’s disease (copper).
-
•Thalassemia (iron).
-
•Treatments: Li salts, zinc salts.
-
•
-
3)Chelation drugs that effects memory (aspirin, EDTA, deferrioxamine, penacillamine):
-
•Binding of zinc by drug disrupts hippocampal-dependent spatial-working memory.
-
•Chelating treatment correlated with performance tests of abstract reasoning... memory.
-
•Iron chelators used to treatage-related memory dysfunction.
-
•Aspirin use associated with greater prospective cognitive decline on select measures.The ameliorative effects of chelation drugs or zinc salts suggest that optimal levels of free (diffusible) metals in the brain underlie the mechanism of memory.
-
•
-
4)Metallothioneins (MT; 4 isotypes) function to transport metals throughout the body, notably the brain:211−224
-
a)Knockout mice (KO) with deletions of MT-1 and MT-2 showed poorer rates of learning, evidence of poor working memory. (224)
-
b)MT dysfunction has been correlated with the following diseases or problems:
-
•Autism (a disorder of neural development characterized by impaired social interaction and communication, and by restricted and repetitive behavior).
-
•Behavior control and development of memory and social skills.
-
•Obsessive compulsive disorders (from Web site: Herb-Discovery.com).
-
•
-
a)
-
5)
Zinc transporters (ZnT; 4 types):225−231
ZnT3 KO mice have complete deficits in contextual discrimination and spatial working memory. Such animals were used to demonstrate that ZnT3 is involved in associative fear memory and extinction.
-
6)
Tenascins C and R114−129,133,135,232−242 are protein components of the nECM which express “fibrinogen-knobs” including haptide epitopes. KO mice, which were incapable of synthesizing tenascins, exhibited behavioral abnormalities associated with memory dysfunction.
-
7)
Chondroitinase (an enzyme which selectively degrades chondroitins) was injected into the mouse brain, resulting in the loss of fear-driven responses. This demonstrated that learned traumatic fear memory, which usually lasts a lifetime, is located in the degradable nECM. (109)
-
8)
Histology revealed that human brain tissue comprises significantly more nECM between neurons than chimpanzees.101 We interpret this as indicating increased memory capacity for superior cognitive ability (e.g., language, memory).
Table 3. Metal Correlations with Memory.
| metal | levels | behavioral changes |
|---|---|---|
| aluminum (Al) | toxic | memory loss, altered behavior, confusion, disorientation (see Alzheimer's disorder) |
| calcium (Ca) | deficiency | severe intellectul changes, mental retardation, poor memory |
| copper (Cu) | tissue overload (inherited) | Wilson’s disease; psychiatric manifestations |
| iron (Fe) | deficiency | poor memory; dietary iron supplemetntation correlated with improvement of memory |
| iron (Fe) | tissue overload (inherited) | thalassemia; anxiety, depression, psychiatric disfunctions |
| lead (Pb) | toxic | mental deterioration, aggressive, poor memory, lower IQ |
| lithium (Li) | therapy (high dose) | memory improvement, mental slowness (variable reports) |
| magnesium (Mg) | toxic | mental confusion, impaired memory |
| mercury (Hg) | toxic | loss of memory, behavioral changes |
| thorium (Th) | toxic | mental confusion |
| zinc (Zn) | deficiency | loss of memory |
Conclusion
We propose that the nECM, in combination with diffusible metal cations, is the locus wherein the neurons encode basic, molecular correlates of cog-info. The minimal cognitive unit of information (cuinfo) corresponds to the formation of a single metal-complex, with one or more metal cations trapped at specific chelating nodes of the nECM (presented in Figure 2).
Figure 2.
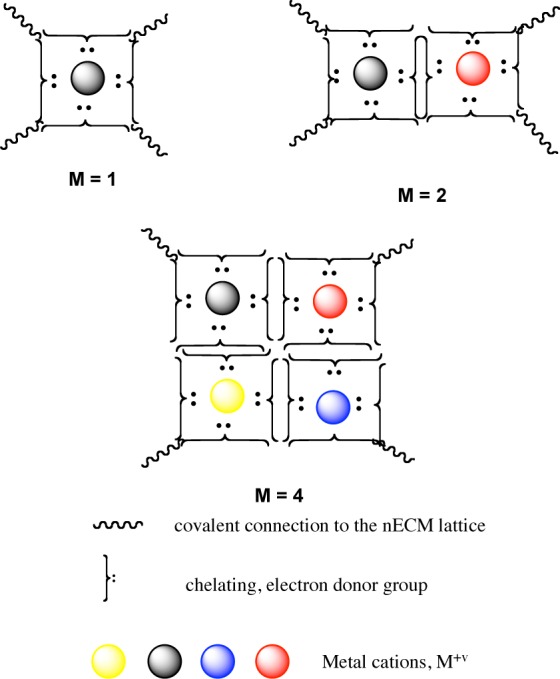
Schematic representation of cuinfo* formed with M = 1, 2, or 4 metal cations per unit. Such catenary metal complex units are formed within the chelating node of the nECM, as molecular correlates of cog-info. At least one metal atom is required, though more could be involved in forming the minimal cuinfo ensemble.
The cuinfo is equivalent to the “bit” of the computer chip. Instead of representing information linearly with only two parameters (0 or 1), the neuron/[ECM/metal] complex operates with many ionic mobile components (n > 10) constrained within a flexible lattice, providing very large information storage and parallel processing capabilities.
Short-term storage of cog-info can employ trace monovalent elemental cations Li+, Rb+, and Cs+ (excluding Na+ and K+ which are present in much higher levels to generate the intraneural high voltage potentials). The monovalent [nECM/M+1] complexes are not very stable, and could be expected to decay rapidly, manifest as short-term memory (STM).
Longer term storage of cognitive information units (derivative cuinfo) would employ polyvalent cations [nECM/M+2/3/4/5] to form more stable complexes. Cross-linking, by enzymes (transglutaminases) or free radical reactions, stabilizes the cuinfo (metal complexes), appropriate for long-term memory (LTM).
When the [nECM/cation] arrays become degraded, the cog-info encoded therein also decays, manifesting as memory loss (storage failure). Of course, breakdowns at any critical point of the neural network chain (circuit failure) are also manifest as memory loss.
To conclude, we posit that:
-
•
Memory is based on tripartite interaction of neurons, nECM, and trace elements.
-
•
The tripartite mechanism involves low energetics with high speed/computational capabilities.
-
•
Cog-info is encoded by the neuron as cuinfo, like bits in memory chips
-
•
Degradation of nECM or metals excess/deficiency correlates with memory loss.
-
•
Cited experimental observations support the proposed tripartite mechanism.
Just like other metabolic processes, man and animals share the biochemical basis of memory. The tripartite mechanism operates in all animals with brains, albeit at increasing degrees of complexity, coincident with the increasing complexity of the anatomical subunits arising from evolutionary development.
Of course, much clarification is required to discern the workings of this tripartite mechanism. A formalism is lacking which elaborates on how sensory input (cog-info) is encoded via the distribution of n-metals within the nECM enveloping the neurons. Detailed metabolic, viscoelastic, and dielectric characterizations of the nECM with various elemental cations would clarify the nanoscale modifications employed by neurons to encode cog-info. Nonetheless, we identify the key physiologic compartments and suggest a credible biochemical mechanism for the phenomenon of recall.
Acknowledgments
Dedicated by G.M. to his late wife, the artist Georgette Batlle, who provided a sympathetic ear and emotional support for many years. Special thanks are due to Prof. Randy Gallistel (Rutgers University) and Prof. Tamar Zelniker (Tel Aviv University) whose critical comments guided us to this condensed exposition. We would also like to thank Prof. Horst Kessler (Technical University Munchen), Prof. Sason Shaik (Hebrew University), Mr. Ron Moussafi, and our families and friends for their criticism, suggestions and support.
The authors declare no competing financial interest.
This paper was published on the web on August 15, 2012, with the August 2012 issue. The blank reference 189 has been deleted (and subsequent references renumbered) in this online version published August 31, 2012, and an Addition and Correction appears on the Web on August 31, 2012, and in the September 2012 issue.
References
- Talland G. A., and Waugh N. C. (1969) The Pathology of Memory, Academic Press, New York. [Google Scholar]
- Roberts R. B.; Flexner L. B. (1969) The biochemical basis of long-term memory. Q. Rev. Biophys. 135–173. [DOI] [PubMed] [Google Scholar]
- Hyden H. (1970) The questions of a molecular basis for the memory trace. In Biology of Memory (Pribram K. H., and Broadbent D. E., Eds), Academic Press, New York, NY. [Google Scholar]
- Joynt R. J. (1975) In The Nervous System (Tower D. B., Ed.), Clinical Neurosciences Vol. 2, pp 441–447, Raven Press, New York, NY. [Google Scholar]
- Cofer C.N ., Ed. (1976) Symposium on the Structure of Human Memory, WH Freeman, New York. [Google Scholar]
- Kandel E. R.; Schwartz J. H. (1982) Molecular Biology of Memory: Modulation of Transmitter Release. Science 218, 433–442. [DOI] [PubMed] [Google Scholar]
- Lynch G; Baudry M. (1984) The biochemistry of memory: A new and specific hypothesis. Science 224, 1057–1063. [DOI] [PubMed] [Google Scholar]
- Squire L. R. (1986) Mechanisms of memory. Science 232, 1612–1619. [DOI] [PubMed] [Google Scholar]
- Mishkin M.; Appendzeller T. (1987) The anatomy of memory. Sci. Am. Spec. Rep. 256(6), 80–89. [DOI] [PubMed] [Google Scholar]
- Matzel L. D.; Collin C.; Alkon D. L. (1992) Biophysical and behavioral correlates of memory storage, degradation, and reactivation. Behav. Neurosci. 106, 954–963. [DOI] [PubMed] [Google Scholar]
- Taubenfeld S. M.; Wiig K. A.; Bear M. F.; Alberini C. M. (1999) A molecular correlate of memory and amnesia in the hippocampus. Nat. Neurosci. 2, 309–310. [DOI] [PubMed] [Google Scholar]
- McGaugh J. L. (2000) Memory--a Century of Consolidation. Science 287, 248–251. [DOI] [PubMed] [Google Scholar]
- Dudai Y. (2004) The neurobiology of consolidations, or how stable is the engram?. Science 333, 108–111. [DOI] [PubMed] [Google Scholar]
- Kandel E. R. (2006) In Search of Memory, Norton & Co, New York. [Google Scholar]
- Miller P.; Wang X. J. (2006) Stability of discrete memory states to stochastic fluctuations in neuronal systems. Chaos 16, 026109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien J. Z. (2007) Real-time neural coding of memory. Prog. Brain. Res. 165, 105–22. [DOI] [PubMed] [Google Scholar]
- Routtenberg A. (2008) Long-lasting memory from evanescent networks. Eur. J. Pharmacol. 585, 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S.; Frey J. U. (2008) ’Synaptic tagging’ and ’cross-tagging’ and related associative reinforcement processes of functional plasticity as the cellular basis for memory formation. Prog. Brain Res. 169, 117–143. [DOI] [PubMed] [Google Scholar]
- Tzvetanov T.; Womelsdorf T. (2008) Predicting human perceptual decisions by decoding neuronal information profiles. Biol. Cybern. 98, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez P. J.; Abel T. (2008) The role of protein synthesis in memory consolidation: Progress amid decades of debate. Neurobiol. Learn. Mem. 89, 293–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel C. R., King A. P. (2009) Memory and the Computational Brain, Chapter 16, Wiley Blackwell, New York. [Google Scholar]
- Keogh R.; Pearson J. (2011) Mental imagery and visual working memory. PLoS ONE 6(12), e29221. 10.1371/journal.pone.0029221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben M.; Rojo J.; Romero-Salguero F. J.; Uppadine L. H.; Lehn J.,M. (2003) Grid-type metal ion architecture: Functional metallosupramolecular arrays. Ang. Chem., Int. Ed. 43, 3644–3662. [DOI] [PubMed] [Google Scholar]
- Weitz R. T.; Walter A.; Engl R.; Sezi R.; Dehm C. (2006) New charge-transfer salts for reversible resistive memory switching. Nano Lett. 6(12), 2810–2813. [DOI] [PubMed] [Google Scholar]
- Green J. E.; Choi J. W.; Boukai A.; Bunimovich Y.; Johnston-Halperin E.; DeIonno E.; Luo Y.; Sheriff B. A.; Xu K.; Shin Y. S.; Tseng H. R.; Stoddart J. F.; Heath J. R. (2007) A 160-kilobit molecular electronic memory patterned at 10(11) bits per square centimetre. Nature 445, 414–417. [DOI] [PubMed] [Google Scholar]
- Gilbert N. E.; Kozicki M. N. (2007) An embeddable multilevel-cell solid electrolyte memory array. IEEE J. Solid-State Circuits 42, 1383–1391. [Google Scholar]
- Chen A. (2008) Ionic memories: Status and challenges. IEEE 1–5. [Google Scholar]
- Hutchby J. A.; Cavin R.; Zhirnov V.; Brewer J. E.; Bourianoff G. (2008) Emerging Nanoscale Memory and Logic Devices: A Critical Assessment. Computer 41, 28–32. [Google Scholar]
- Valov I.; Waser R.; Jameson J. R.; Kozicki M. N. (2011) Electrochemical metallization memories--fundamentals, applications, prospects. Nanotechnology 22, 254003. [DOI] [PubMed] [Google Scholar]
- Lee D. H.; Gupta J. A. (2010) Tunable field control over the binding energy of single dopants by a charged vacancy in GaAs. Science 330, 1807–10. [DOI] [PubMed] [Google Scholar]
- Lee P. F.; Dai J. Y. (2010) Memory effect of an organic based trilayer structure with Au nanocrystals in an insulating polymer matrix. Nanotechnology 21(29), 295706. [DOI] [PubMed] [Google Scholar]
- Muralidharan G.; Bhat N.; Santhanam V. (2011) Scalable processes for fabricating non-volatile memory devices using self-assembled 2D arrays of gold nanoparticles as charge storage nodes. Nanoscale 3, 4575–4579. [DOI] [PubMed] [Google Scholar]
- Lee M. J.; Lee C. B.; Lee D.; Lee S. R.; Chang M.; Hur J. H.; Kim Y. B.; Kim C. J.; Seo D. H.; Seo S.; Chung U. I.; Yoo I. K.; Kim K. (2011) A fast, high-endurance and scalable non-volatile memory device made from asymmetric Ta2O(5-x)/TaO(2-x) bilayer structures. Nat. Mater. 10, 625–630. [DOI] [PubMed] [Google Scholar]
- Sahu B. S.; Gloux F.; Slaoui A.; Carrada M; Muller D.; Groenen J.; Bonafos C.; Lhostis S. (2011) Effect of ion implantation energy for the synthesis of Ge nanocrystals in SiN films with HfO2/SiO2 stack tunnel dielectrics for memory application. Nanoscale Res. Lett. 6, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolichnov I.; Riester S. W.; Mikheev E.; Setter N.; Rushforth A. W.; Edmonds K. W.; Campion R. P.; Foxon C. T.; Gallagher B. L.; Jungwirth T.; Trodahl H. J. (2011) Ferroelectric polymer gates for non-volatile field effect control of ferromagnetism in (Ga, Mn)As layers. Nanotechnology 22, 254004. [DOI] [PubMed] [Google Scholar]
- Mocatta D.; Cohen G.; Schattner J.; Millo O.; Rabani E.; Banin U. (2011) Heavily doped semiconductor nanocrystal quantum dots. Science 332, 77–81. [DOI] [PubMed] [Google Scholar]
- Fernandes M.; Nobre S. S.; Rodrigues L. C.; Gonçalves A. Z. (2011) Li+- and Eu3+-doped poly(ε-caprolactone)/siloxane biohybrid electrolytes for electrochromic devices. ACS Appl. Mater. Interfaces 3, 2953–2965. [DOI] [PubMed] [Google Scholar]
- Waser R.; Aono M. (2007) Nano-ionics-based resistive switching memories. Nat. Mater. 6, 833–840. [DOI] [PubMed] [Google Scholar]
- Weiner N. (1948) Cybernetics: Or Control and Communication in the Animal and Machine, Librairie Hermann & Cie.; MIT Press, Cambridge, MA. [Google Scholar]
- Shannon C. E. (1948) A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423; 623–656. [Google Scholar]
- Turing A. (1950) Computing machinery and intelligence. Mind 59, 433–460. [Google Scholar]
- Turing A. (1960) Computational theory of mind, Putnam & various publishers, New York, NY. [Google Scholar]
- Taub H. A., Ed. (1963) Design of Computers and Theory of Automata and Numerical Analysis, John von Neumann Collected Works, Vol V, Pergamon Press, London. [Google Scholar]
- Waldrop M. M. (1992) Complexity: The Emerging Science at the Edge of Order and Chaos, Viking- Penguin Press, New York. [Google Scholar]
- Wolfram S. (2002) A New Kind of Science, Wolfram Media, Champaign, IL. [Google Scholar]
- Piccinini G. (2004) The first computational theory of mind and brain: A close look at McCulloch and Pitts’s “logical calculus of ideas immanent in nervous activity”. Synthese 141, 175–215. [Google Scholar]
- Benenson Y. (2009) Biocomputers: From test tubes to live cells. Mol. BioSyst. 5, 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spormns O. (2011) Networks of the Brain, MIT Press, Cambridge, MA. [Google Scholar]
- Aho A. V. (2011) What is computation? Computation and computational thinking. Ubiquity Symposium, Association for Computing Machinery, New York, NY. [Google Scholar]
- Landon D N.; Langley O. K. (1971) The local chemical environment of nodes of Ranvier: A study of cation binding. J Anat. 108(Pt 3), 419–432. [PMC free article] [PubMed] [Google Scholar]
- Mozhaeva G. N.; Naumov A. P. (1973) Potassium stationary-state conductance of Ranvier’s node membrane in the presence of La3+, Zn2+ and Cu2+ in the external medium. Tsitologiia 15, 1431–1435. [PubMed] [Google Scholar]
- Fox J. M.; Rojas E.; Stämpfli R. (1974) Blocking of sodium and potassium conductance by internal application of Zn2+ in the node of Ranvier. Pflugers Arch. 351, 271–274. [DOI] [PubMed] [Google Scholar]
- Bogdanov K. Y. (1974) [Mathematical assessment of ephaptic interaction and the recording of transmembrane potential shifts.] [Russian]. Biull. Eksp. Biol. Med. 78(10), 48–51. [PubMed] [Google Scholar]
- Schmitt R. O.; Dev P.; Smith B. H. (1976) Electronic processing of information by brain cells. Science 193, 114–120. [DOI] [PubMed] [Google Scholar]
- Fritz L. C.; Brockes J. P. (1981) Clustering of ion channels at the node of Ranvier. Nature 291, 190–191. [DOI] [PubMed] [Google Scholar]
- Kandel E. R.; Schwartz J. H. (1982) Molecular biology of memory: Modulation of transmitter release. Science 218, 433–442. [DOI] [PubMed] [Google Scholar]
- Pasztor V. M.; Bush B. M. H. (1982) Impulse coded and analog signaling in single mechanoreceptor neurons. Science 215, 1635–1637. [DOI] [PubMed] [Google Scholar]
- Turner R. W.; Richardson T. L.; Miller J. J. (1984) Ephaptic interactions contribute to paired pulse and frequency potentiation of hippocampal field potentials. Exp. Brain Res. 54(3), 567–70. [DOI] [PubMed] [Google Scholar]
- Lynch G.; Baudry M. (1984) The biochemistry of memory: A new and specific hypothesis. Science 224, 1057–1063. [DOI] [PubMed] [Google Scholar]
- Kmjevik K. (1986) Ephaptic interactions: A significant mode of communications in the brain. NIPS 1, 28–29. [Google Scholar]
- Khulusi S. S.; Brown M. W.; Wright D. M. (1986) Zinc and paired-pulse potentiation in the hippocampus. Brain Res. 363, 152–155. [DOI] [PubMed] [Google Scholar]
- Wilson C. J.; Mastronarde D. N.; McEwen B.; Frank J. (1992) Measurement of neuronal surface area using high-voltage electron microscope tomography. NeuroImage 1, 11–22. [DOI] [PubMed] [Google Scholar]
- Vyvyan H. C.; Glen J.; Duncan S.; Alan; Wallén P.; Browne M. (1993) Measurement of total neuronal volume, surface area, and dendritic length following intracellular physiological recording. NeuroProtocols 2, 113–120. [Google Scholar]
- Jefferys J. G. R. (1995) Nonsynaptic modulation of neuronal activity in the brain: Electric currents and extracellular ions. Physiol. Rev. 75, 689–723. [DOI] [PubMed] [Google Scholar]
- Bokil H.; Laaris N.; Blinder K.; Ennis M.; Keller A. (2001) Ephaptic interactions in the mammalian olfactory system. J. Neurosci. 21, RC173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D.; Boudkkazi S.; Campanac E.; Cudmore R. H.; Giraud P.; Fronzaroli-Molinieres L.; Carlier E.; Caillard O. (2008) Paired-recordings from synaptically coupled cortical and hippocampal neurons in acute and cultured brain slices. Nat. Protoc. 3, 1559–1568. [DOI] [PubMed] [Google Scholar]
- Roth W. T., Ford J. M., Pfefferbaum A., and Elbert T. R. (2000) Methodological issues in event-related brain potential and magnetic field studies. In Psychopharmacology- 4th Generation (Bloom L. E., and Kupfer D. J., Eds.), Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- Rasband M. N.; Trimmer J. S. (2001) Developmental clustering of ion channels at and near the node of Ranvier. Dev. Biol. 236(1), 5–16. [DOI] [PubMed] [Google Scholar]
- Bear M. F., Connors B. W., and Pradiso M. A. (2001) Neuroscience: Exploring the Brain, 2nd ed., p 97, Lippincott Williams and Wilkins, Baltimore. [Google Scholar]
- Lindner B.; Chacron M. J.; Longtin A. (2005) Integrate-and-fire neurons with threshold noise: a tractable model of how interspike interval correlations affect neuronal signal transmission. Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys. 72(2 Pt 1), 021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T.; Schoffelen J. M.; Oostenveld R.; Singer W.; Desimone R.; Engel A. K.; Fries P. (2007) Modulation of neuronal interactions through neuronal synchronization. Science 316, 1609–1612. [DOI] [PubMed] [Google Scholar]
- Kampa B. M.; Letzkus J. J.; Stuart G. J. (2007) Dendritic mechanisms controlling spike-timing-dependent synaptic plasticity. Trends Neurosci. 30(9), 456–463. [DOI] [PubMed] [Google Scholar]
- Tzvetanov T.; Womelsdorf T. (2008) Predicting human perceptual decisions by decoding neuronal information profiles. Biol. Cybern. 98, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenz A.; Silver R. A.; Schaefer A. T.; Margrie T. W. (2008) The contribution of single synapses to sensory representation in vivo. Science 321, 977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. D. (2008) The neuron level phenomena underlying cognition and consciousness. Neuroscience 153, 556–570. [DOI] [PubMed] [Google Scholar]
- Perea G.; Navarrete M.; Araque A. (2009) Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 32, 421–31. [DOI] [PubMed] [Google Scholar]
- Kishida K. T., and Klann E. (2009) Reactive oxygen species, synaptic plasticity and memory. In Oxidative Neural Injury (Veasey S.C., Ed.), Chapter 1, pp 1–28, Humana Press: New York. [Google Scholar]
- Fries P. (2009) Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu. Rev. Neurosci. 32, 209–224. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W.; Rose C. R. (2010) Ion changes and signaling in perisynaptic glia. Brain Res. Rev. 63, 113–129. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. (2010) From the connectotome to the synaptosome: An epic love story. Science 330, 1198–1201. [DOI] [PubMed] [Google Scholar]
- Shelton J. T.; Elliott E. M.; Matthews R. A.; Hill B. D.; Gouvier W. D. (2010) The relationships of working memory, secondary memory, and general fluid intelligence: Working memory is special. J. Exp. Psychol. 36(3), 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesburguères E.; Gobbo O. L.; Alaux-Cantin S.; Hambucken A.; Trifilieff P.; Bontempi B. (2011) Early tagging of cortical networks is required for the formation of enduring associative memory. Science 331, 924–928. [DOI] [PubMed] [Google Scholar]
- Anastassiou C. A.; Montgomery S. M.; Barahona M.; Buzsáki G.; Koch C. (2010) The effect of spatially inhomogeneous extracellular electric fields on neurons. J. Neurosci. 30, 1925–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels T. P.; Sprekeler H.; Zenke F; Clopath C; Gerstner W. (2011) Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science 334, 1569–1573. [DOI] [PubMed] [Google Scholar]
- Levin E.; Arieff A.; Kleeman C. R. (1971) Evidence of different compartments in the brain for extracellular markers. Am. J. Physiol. 221, 1319–1325. [DOI] [PubMed] [Google Scholar]
- Nicholson C.; Phillips J. M. (1981) Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J. Physiol. (Oxford, U.K.) 321, 225–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S.; Edelman G. M. (1987) A proteoglycan with HNK-1 antigenic determinants is a neuron-associated. ligand for cytotactin. Proc. Natl. Acad. Sci. U.S.A. 84, 2523–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M.; Carlson S. S. (1993) A large chondroitin sulfate proteoglycan has the characteristics of a general extracellular matrix component of adult brain. J. Neurosci. 13, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M.; Wight T. N.; Carlsons S. S. (1993) A brain extracellular matrix proteoglycan forms aggregates with hyaluronan. J. Biol. Chem. 268, 15061–15069. [PubMed] [Google Scholar]
- Castillo G. M.; Ngo C.; Cummings J.; Wight T. N.; Snow A. D. (1997) Perlecan binds to the β-amyloid proteins (Aβ) of Alzheimer’ s disease, accelerates Aβ fibril formation, and maintains Aβ fibril stability. J. Neurochem. 69, 2452–2465. [DOI] [PubMed] [Google Scholar]
- Lander C; Zhang H; Hockfield S. (1998) Neurons produce a neuronal cell surface-associated chondroitin sulfate. J. Neurosci. 18, 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain C. J.; Traynelis S. F.; Dingledine R. (1990) Regional variation of extracellular space in the hippocampus. Science 249, 674–677. [DOI] [PubMed] [Google Scholar]
- Nicholson C; Syková E. (1998) Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21, 207–215. [DOI] [PubMed] [Google Scholar]
- Bukalo O; Schachner M; Dityatev A. (2001) Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 104(2), 359–369. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y. (2000) Lecticans: Organizers of the brain extracellular matrix. Cell. Mol. Life Sci. 57, 276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A.; Schachner M. (2003) Extracellular matrix molecules and synaptic plasticity. Nat. Rev. 4, 456–468. [DOI] [PubMed] [Google Scholar]
- Kleene R.; Schachner M. (2004) Glycans and neural cell interactions. Nat. Rev. Neurosci. 5, 195–208. [DOI] [PubMed] [Google Scholar]
- Syková E. (2003) Diffusion parameters of the extracellular space. Isr. J. Chem. 43, 55–69. [Google Scholar]
- Piet R.; Vargova L.; Sykova E.; Poulain D. A.; Oliet S. H. R. (2004) Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc. Natl. Acad. Sci. U.S.A. 101, 2151–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A.; Schachner M. (2006) The extracellular matrix and synapses. Cell Tissue Res. 326, 647–654. [DOI] [PubMed] [Google Scholar]
- Balter M. (2007) News Focus: Brain evolution studies. Science 315, 1208–1211. [DOI] [PubMed] [Google Scholar]
- Bukalo O. (2008) Introduction: Cell adhesion and extracellular matrix molecules in synaptic plasticity. Neuron Glia Biol. 4, 165–167. [DOI] [PubMed] [Google Scholar]
- Fischknecht R.; Seidenbecher C. I. (2008) The crosstalk of hyaluronan-based extracellular matrix and synapses. Neuron Glia Biol. 4, 249–257. [DOI] [PubMed] [Google Scholar]
- Vigetti D.; Andrini O.; Clerici M.; Negrini D.; Passi A.; Moriondo A. (2008) Chondroitin sulfates act as extracellular gating modifiers on voltage-dependent ion channels. Cell Physiol. Biochem. 22, 137–146. [DOI] [PubMed] [Google Scholar]
- Syková E; Vargová L. (2008) Extrasynaptic transmission and the diffusion parameters of the extracellular space. Neurochem. Int. 52(1–2), 5–13. [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D.; Wiley C. A. (2009) Brain extracellular matrix in neurodegeneration. Brain Pathol. 19, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrab̌etov S.; Masri D.; Tao L.; Xiao F.; Nicholson C. J. (2009) Calcium diffusion enhanced after cleavage of negatively charged components of brain extracellular matrix by chondroitinase ABC. Physiology 587, 4029–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger S. T.; Voytik-Harbin S. L. (2009) Hyaluronan concentration within a 3D collagen matrix modulates matrix viscoelasticity, but not fibroblast response. Matrix Biol. 28, 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N.; Caroni P.; Lüthi A.; Herry C. (2009) Perineuronal nets protect fear memories from erasure. Science 325, 1258–1261. [DOI] [PubMed] [Google Scholar]
- Dityatev A.; Schachner M.; Sonderegger P. (2010) The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat. Rev. Neurosci. 11, 735–746. [DOI] [PubMed] [Google Scholar]
- Dityatev A.; Seidenbecher C.; Schachner M. (2010) Compartmentalization from the outside: The extracellular matrix and functional microdomains in the brain. Trends .Neurosci. 33, 503–512. [DOI] [PubMed] [Google Scholar]
- Kochlamazashvili G.; Henneberger C.; Bukalo O.; Dvoretskova E.; Senkov O.; Lievens M. J.; Westenbroek R; Engel A. K.; Catterall W. A.; Rusakov D. A.; Schachner M.; Dityatev A. (2010) The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic l-type Ca2+ channels. Neuron 67, 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb J. A.; Mayford M. R.; Tsien J. Z.; Albertini C. M. (2010) Cognition enhancement strategies. J. Neurosci. 30, 14987–14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nörenberg U.; Hubert M.; Rathjen F. G. (1996) Structural and functional characterization of tenascin-R (restrictin), an extracellular matrix glycoprotein of glial cells and neurons. Int. J. Dev. Neurosci. 14, 217–231. [DOI] [PubMed] [Google Scholar]
- Milev P.; Fischer D.; Harig M.; Schulthess T.; Margolis R. K; Chicket Ehrismann R.; Margolis R. U. (1997) The fibrinogen like globe of tenascin-C mediates its interactions with neurocan and phophacan/protein-tyrosine phosphatase-β. J. Biol. Chem. 272, 15501–15509. [DOI] [PubMed] [Google Scholar]
- Weber P.; Rasband M. N.; Czaniera R.; Lang Y.; Bluethmann H.; Margolis R. U.; Levinson S. R.; Shrager P.; Montag D.; Schachner M. (1999) Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J. Neurosci. 19, 4245–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel G.; Schmidt R.; Schachner M. (2000) Involvement of L1.1 in memory consolidation after active avoidance conditioning in zebrafish. J. Neurobiol. 43, 389–403. [PubMed] [Google Scholar]
- Probstmeier R.; Braunewell K.; Pesheva P. (2000) Involvement of chondroitin sulfates on brain-derived tenascin-R in carbohydrate-dependent interactions with fibronectin and tenascin-C. Brain Res. 863, 42–51. [DOI] [PubMed] [Google Scholar]
- Bukalo O; Schachner M; Dityatev A. (2001) Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 104(2), 359–369. [DOI] [PubMed] [Google Scholar]
- Kappler J.; Baader S. L.; Franken S.; Pesheva P.; Schilling K.; Rauch U.; Gieselmann V. (2002) Tenascins are associated with lipid rafts isolated from mouse brain. Biochem. Biophys. Res. Commun. 294, 742–747. [DOI] [PubMed] [Google Scholar]
- Schumacher S.; Stübe E. M. (2003) Regulated binding of the fibrinogen-like domains of tenascin-R and tenascin-C to the neural EGF family member CALEB. J. Neurochem. 87, 1213–1223. [DOI] [PubMed] [Google Scholar]
- Freitag S.; Schachner M.; Morellini F. (2003) Behavioral alterations in mice deficient for the extracellular matrix glycoprotein tenascin-R. Behav. Brain Res. 145, 189–207. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M.; Montag D. (2003) Severe cognitive and motor coordination deficits in tenascin-R-deficient mice. Genes Brain Behav. 2, 20–31. [DOI] [PubMed] [Google Scholar]
- Dityatev A; Schachner M. (2003) Extracellular matrix molecules and synaptic plasticity. Nat. Rev. Neurosci. 4(6), 456–468. [DOI] [PubMed] [Google Scholar]
- Kjær M. (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 84, 649–698. [DOI] [PubMed] [Google Scholar]
- Day J.; Olin A.; Murdoch A.; Canfield A.; Sasaki T.; Timpl R.; Hardingham T.; Aspberg A. (2004) Alternative splicing in the aggrecan G3 domain influences binding interactions with tenascin-C and other extracellular matrix proteins. J. Biol. Chem. 279, 12511–12518. [DOI] [PubMed] [Google Scholar]
- Lundell A.; et al. (2004) Structural basis for interactions between tenascins and lectican C-type lectin domains: Evidence for a crosslinking role for tenascins. Structure 12, 1495–1506. [DOI] [PubMed] [Google Scholar]
- Woodworth A.; Pesheva P.; Fiete D.; Baenziger J. U. (2004) Neuronal-specific synthesis and glycosylation of tenascin-R. J. Biol. Chem. 279, 10413–10421. [DOI] [PubMed] [Google Scholar]
- Syková E.; Vorísek I.; Mazel T.; Antonova T.; Schachner M. (2005) Reduced extracellular space in the brain of tenascin-R- and HNK-1-sulphotransferase deficient mice. Eur. J. Neurosci. 22, 1873–1880. [DOI] [PubMed] [Google Scholar]
- Vargová L; Syková E. (2008) Extracellular space diffusion and extrasynaptic transmission. Physiol Res. 57(Suppl 3), S89–99. [DOI] [PubMed] [Google Scholar]
- Zimmermann D. R.; Dours-Zimmermann M. T. (2008) Extracellular matrix of the central nervous system: From neglect to challenge. Histochem. Cell Biol. 130, 635–653. [DOI] [PubMed] [Google Scholar]
- Gurevicius K.; Kuang F.; Stoenica L.; Irintchev A.; Gureviciene I.; Dityatev A.; Schachner M.; Tanila H. (2009) Genetic ablation of tenascin-C expression leads to abnormal hippocampal CA1 structure and electrical activity in vivo. Hippocampus 19, 1232–1246. [DOI] [PubMed] [Google Scholar]
- Morellini F.; Sivukhina E.; Stoenica L.; Oulianova E.; Bukalo O.; Jakovcevski I.; Dityatev A.; Irintchev A.; Schachner M. (2010) Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cereb. Cortex 20, 2712–2727. [DOI] [PubMed] [Google Scholar]
- Dityatev A.; Schachner M.; Sonderegger P. (2010) The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 11(11), 735–746. [DOI] [PubMed] [Google Scholar]
- Kawakami K.; Matsumoto K. (2011) Behavioral alterations in mice lacking the gene for tenascin-X. Biol. Pharm. Bull. 34(4), 590. [DOI] [PubMed] [Google Scholar]
- Henke G.; Möllmann H.; Alfes H. (1971) Comparative studies on the concentration of various trace elements in specific areas of the human brain using neutron activation analysis]. Z. Neurol. 199, 283–294(German). [PubMed] [Google Scholar]
- Höck A.; Demmel U.; Schicha H.; Kasperek K.; Feinendegen L. E. (1975) Trace element concentration in human brain. Activation analysis of cobalt, iron, rubidium, selenium, zinc, chromium, silver, cesium, antimony and scandium. Brain 98, 49–64. [DOI] [PubMed] [Google Scholar]
- Markesbery W. R.; Ehmann W. D.; Hossain T. I.; Alauddin M.; Goodin D. T. (1981) Instrumental neutron activation analysis of brain aluminum in Alzheimer disease and aging. Ann. Neurol. 10, 511–516. [DOI] [PubMed] [Google Scholar]
- Tholey G.; Ledig M.; Kopp P.; Sargentini-Maier L.; Leroy M.; Grippo A. A.; Wedler F. C. (1988) Levels and sub-cellular distribution of physiologically important metal ions in neuronal cells cultured from chick embryo cerebral cortex. Neurochem. Res. 13, 1163–1167. [DOI] [PubMed] [Google Scholar]
- Yinsong W.; Guisun Z.; Mingguang T.; Min Z.; Yuandi C. (1991) Distribution of some elements in human hair and internal organs, determined by neutron activation analysis. J. Radioanal. Nucl. Chem. 151, 301–311. [Google Scholar]
- Guisun Z.; Yinson W.; Mingguang T.; Min Z.; Yongjie W.; Fulin Z. (1991) Preliminary study of trace elements in human brain tumor tissues by instrumental neutron activation analysis (NAA). J. Radioanal. Nucl. Chem. 151, 327–335. [Google Scholar]
- Destasio G.; Perfetti P.; Oddo N.; Galli P.; Mercanti D.; Ciotti M. T.; Koranda S.; Hardcastle S.; Tonner B. P.; Margaritondo G. (1992) Metal uptake in neuron cultures: A systematic study. NeuroReport 3, 965–968. [DOI] [PubMed] [Google Scholar]
- Yoshida D.; Ikeda Y.; Nakazawa S. (1993) Quantitative analysis of copper, zinc and copper/zinc ratio in selected human brain tumors. J. Neuro-Oncol. 16, 109–115. [DOI] [PubMed] [Google Scholar]
- González R.; Guimaraes A.; Sachs G.; Rosenbaum J.; Garwood M.; Renshaw P. (1993) Measurement of human brain lithium in vivo by MR spectroscopy. Am. J. Neuroradiol. 14, 1027–1037. [PMC free article] [PubMed] [Google Scholar]
- Plenge P.; Stensgaar A.; Jensen H.; Thomsen C.; Mellerup E.; Henriksen O. (1994) 24-h lithium concentration in human brain studied by Li-7 magnetic resonance spectroscopy. Biol. Psychiatry 36, 511–516. [DOI] [PubMed] [Google Scholar]
- Sachs G.; Renshaw P.; Lafer B.; Stoll A.; Guimaraes A.; Rosenbaum J.; Gonzalez R. G. (1995) Variability of brain lithium levels during maintenance treatment: A magnetic resonance spectroscopy study. Biol. Psychiatry 38, 422–428. [DOI] [PubMed] [Google Scholar]
- Al-Saleh I.; Shinwari N. (2001) Levels of cadmium, lead, and mercury in human brain tumors. Biol. Trace Elem. Res. 79, 197–203. [DOI] [PubMed] [Google Scholar]
- Fitsanakis V. A.; Zhang N.; Anderson J.; Erikson K.; Avison M.; Gore J.; Aschner M. (2008) Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol. Sci. 103, 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J. S.; Zoriy M. V.; Pickhardt C.; Palomero-Gallagher N.; Zilles K. (2005) Imaging of copper, zinc, and other elements in thin section of human brain samples (hippocampus) by laser ablation inductively coupled plasma mass spectrometry. Anal. Chem. 77, 3208–3216. [DOI] [PubMed] [Google Scholar]
- Popescu B. F. G.; Robinson C. A.; Rajput A.; Rajput A. H.; Harder S. L.; Nichol H. (2009) Iron, Copper, and Zinc distribution of the cerebellum. Cerebellum 8, 74–79. [DOI] [PubMed] [Google Scholar]
- Becker J. S. (2010) Bioimaging of metals in brain tissue from micrometre to nanometre scale by laser ablation inductively coupled plasma mass spectrometry: State of the art and perspectives. Int. J. Mass Spectrom. 289, 65–75. [Google Scholar]
- Popescu B. F. G.; Nichol H. (2011) Mapping brain metals to evaluate therapies for neurodegenerative disease. CNS Neurosci. Ther. 17, 256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z.; Caruso J. A.; Lai B.; Matusche A.; Becker J. S. (2011) Trace metal imaging with high spatial resolution: Applications in biomedicine. Metallomics 3, 28–37. [DOI] [PubMed] [Google Scholar]
- Dwyer F. P., and Mellor D. P. (1964) Chelating Agents and Metal Chelates, Academic Press, New York. [Google Scholar]
- Balt S.; de Bolster M. W.; Booij M.; van Herk A. M.; Visser-Luirink G. (1983) Binding of metal ions to polysaccharides. V. Potentiometric, spectroscopic, and viscosimetric studies of the binding of cations to chondroitin sulfate and chondroitin in neutral and acidic aqueous media. J. Inorg. Biochem. 19(3), 213–26. [DOI] [PubMed] [Google Scholar]
- Clark R. W.; Bonicamp J. M. (1998) The Ksp–Solubility Conundrum. J. Chem. Educ. 75, 1182–1188. [Google Scholar]
- Nagy L.; Yamashita S.; Yamaguchi T.; Sipos P.; Wakita H.; Nomura M. (1998) The local structures of Cu(II) and Zn(II) complexes of hyaluronate. J. Inorg. Biochem. 72, 49–55. [Google Scholar]
- Barbucci R.; Magnani A.; Lamponi S.; Mitola S.; Ziche M.; Morbidelli L.; Bussolino F. (2000) Cu(II) and Zn(II) complexes with hyaluronic acid and its sulphated derivative. Effect on the motility of vascular endothelial cells. J. Inorg. Biochem. 81, 229–37. [DOI] [PubMed] [Google Scholar]
- Barbucci R.; Lamponi S.; Magnani A.; Piras F. M.; Rossi A; Weber E. (2005) Role of the Hyal-Cu (II) complex on bovine aortic and lymphatic endothelial cells behavior on microstructured surfaces. Biomacromolecules 6, 212–219. [DOI] [PubMed] [Google Scholar]
- Burger K.; Illés J.; Gyurcsik B.; Gazdag M.; Forrai E.; Dékány I.; Mihályfi K. (2001) Metal ion coordination of macromolecular bioligands: Formation of zinc(II) complex of hyaluronic acid. Carbohydr. Res. 332, 197–207. [DOI] [PubMed] [Google Scholar]
- Donati A.; Magnani A.; Bonechi C.; Barbucci R.; Rossi C. (2001) Solution structure of hyaluronic acid oligomers by experimental and theoretical NMR, and molecular dynamics simulation. Biopolymers 59, 434–445. [DOI] [PubMed] [Google Scholar]
- D’Auria G.; Flores G.; Falcigno L.; Oliva R.; Vacatello M.; Corsaro M. M.; Parrilli M.; Paolillo L. (2003) Hyaluronate tetrasaccharide- Cu(II) interaction: A NMR study. Biopolymers 70, 260–269. [DOI] [PubMed] [Google Scholar]
- Barbucci R.; Lamponi S.; Magnani A.; Piras F. M.; Rossi A.; Weber E. (2005) Role of the Hyal-Cu (II) complex on bovine aortic and lymphatic endothelial cells behavior on microstructured surfaces. Biomacromolecules 6, 212–219. [DOI] [PubMed] [Google Scholar]
- Tielrooij K. J.; Garcia-Araez N.; Bonn M.; Bakker H. J. (2010) Cooperativity in ion hydration. Science 328, 1006–1009. [DOI] [PubMed] [Google Scholar]
- González E.; Arbiol J.; Puntes V. F. (2011) Carving at the nanoscale: sequential galvanic exchange and Kirkendall growth at room temperature. Science 334, 1377–1380. [DOI] [PubMed] [Google Scholar]
- Schmitt F. O.; Dev P.; Smith B. H. (1997) Electronic processing of information by brain cells. Science 193, 114–120. [DOI] [PubMed] [Google Scholar]
- Gevins A. S.; Schaffer R. E.; Doyle J. C.; Cutillo B. A.; Tannehill R. S.; Bressler S. L. (1983) Shadows of thought: shifting lateralization of human brain electrical patterns during brief visuo-motor task. Science 220, 97–99. [DOI] [PubMed] [Google Scholar]
- Gevins A. S.; Doyle J. C.; Cutillo B. A.; Schaffer R. E.; Tannehill R. S.; Bressler S. L. (1985) Neurocognitive pattern analysis of a visuospatial task: Rapidly-shifting foci of evoked correlations between electrodes. Psychophysiology 22, 32–43. [DOI] [PubMed] [Google Scholar]
- Gevins A. S.; Morgan N. H.; Bressler S. L.; Cutillo B. A.; White R. M.; Illes J.; Greer D. S.; Doyle J. C.; Zeitlin G. M. (1987) Human neuroelectric patterns predict performance accuracy. Science 235, 580–585. [DOI] [PubMed] [Google Scholar]
- Su M. H.; Srinivasan V.; Ghanem A. H.; Higuchi W. I. (1994) Quantitative in vivo iontophoretic studies. J. Pharm. Sci. 83, 12–17. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G. R. (1995) Nonsynaptic modulation of neuronal activity in the brain: Electric currents and extracellular ions. Physiol. Rev. 75, 689–723. [DOI] [PubMed] [Google Scholar]
- Binczaka S.; Eilbeck J. C.; Scott A. C. (2001) Epiphaptic coupling of myelinated nerve fibers. Physica D 148(1–2), 159–174. [Google Scholar]
- Elinder F.; Arhem P. (2003) Metal ion effects on ion channel gating. Q. Rev. Biophys. 36, 373–427. [DOI] [PubMed] [Google Scholar]
- Kim S. J.; Kim H.; Park S. J.; Kim I. Y.; Lee S. H.; Lee T. S.; Kim S. I. (2005) Behavior in electric fields of smart hydrogels with potential application as bio-inspired actuators. Smart Mater. Struct. 14, 511. [Google Scholar]
- Pietruchaa K.; Marzecb E. (2005) Dielectric properties of the collagen–glycosaminoglycans scaffolds in the temperature range of thermal decomposition. Biophys. Chem. 118, 51–56. [DOI] [PubMed] [Google Scholar]
- Paradee N.; Sirivat A.; Niamlang S.; Prissanaroon-Ouajai W. (2012) Effects of crosslinking ratio, model drugs, and electric field strength on electrically controlled release for alginate-based hydrogel.. J. Mater. Sci. Mater. Med. 23, 999–1010. [DOI] [PubMed] [Google Scholar]
- Gelbard-Sagiv H.; Mukamel R.; Harel M.; Malach R.; Fried I. (2008) Internally generated reactivation of single neurons in human hippocampus during recall. Science 322, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlushkou D.; Dhopeshwarkar R.; Crooks R. M.; Tallarek U. (2008) The influence of membrane ion-permselectivity on electrokinetic concentration enrichment in membrane-based preconcentration units. Lab Chip 8, 1153–1162. [DOI] [PubMed] [Google Scholar]
- Kasha P. C.; Banga A. K. (2008) A review of patent literature for iontophoretic delivery and devices. Recent Pat. Drug Delivery Formulation 2, 41–50. [DOI] [PubMed] [Google Scholar]
- Grimnes S., and Martinsen O. M. (2008) Bioimpedance and Bioelectricity Basics, Academic Press, New York. [Google Scholar]
- Guhr G.; Schmidt H.; Weihnacht M. (2009) A new tool to assess mechanical and dielectric properties of tissues. Conf. Proc. IEEE Eng. Med. Biol. Soc. 729–732. [DOI] [PubMed] [Google Scholar]
- Lieleg O.; Baumgärtel R. M.; Bausch A. R. (2009) Selective filtering of particles by the extracellular matrix: An electrostatic bandpass. Biophys. J. 97(6), 1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righetti P. G. (2009) Happy bicentennial, electrophoresis!. J. Proteomics 73, 181–187. [DOI] [PubMed] [Google Scholar]
- Miller F. J.; Weaver K. E.; Ojemann J. G. (2009) Direct electrophysiological measurements of hyman default network areas. Proc. Natl. Acad. Sci. U.S.A. 106, 12174–12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R. R. (1988) The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 242, 1654–1663. [DOI] [PubMed] [Google Scholar]
- Lieleg O.; Baumgärtel R. M.; Bausch A. R. (2009) Selective filtering of particles by the extracellular matrix: an electrostatic bandpass. Biophys. J. 97(6), 1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiseppi-Eliea A. (2010) Electroconductive hydrogels: Synthesis, characterization and biomedical applications. Biomaterials 31, 2701–2716. [DOI] [PubMed] [Google Scholar]
- Paz R.; Gelbard-Sagiv H.; Mukamel R.; Harel M.; Fried I. (2010) A neural substrate in the human hippocampuss for linking successive events. Proc. Natl. Acad. Sci. U.S.A. 107, 6046–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. A., Faber D. S. (2010). Field effects in the CNS play functional roles. Front. Neural Circuits 18, DOI: 10.3389/fncir.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoo N. A.; Rahman Z.; Repka M. A.; Murthy S. N. (2010) Electroporation: An avenue for transdermal drug delivery. Curr. Drug Delivery 7, 125–36. [DOI] [PubMed] [Google Scholar]
- Gratieri T.; Kalaria D.; Kalia Y. N. (2011) Non-invasive iontophoretic delivery of peptides and proteins across the skin. Expert Opin. Drug Delivery 8, 645–663. [DOI] [PubMed] [Google Scholar]
- Tesselaar E.; Sjöberg F. (2011) Transdermal iontophoresis as an in-vivo technique for studying microvascular physiology. Microvasc. Res. 81, 88–96. [DOI] [PubMed] [Google Scholar]
- Smirnov A. Y.; Mourokh L. G.; Nori F. (2011) Electrostatic models of electron-driven proton transfer across a lipid membrane. J. Phys.: Condens. Matter 23, 234101. [DOI] [PubMed] [Google Scholar]
- Anastassiou C. A.; Perin R.; Markram H.; Koch C. (2011) Ephaptic coupling of cortical neurons. Nat. Neurosci. XX, 1–8. [DOI] [PubMed] [Google Scholar]
- Alexe-Ionescu A. L.; Barbero G.; Meyer C. (2012) Influence of the rheological properties on the electrical impedance of hydrogels. J. Appl. Phys. 111, 014905. [Google Scholar]
- Santiago R. C. (1906) (in German) Studien über die Hirnrinde des Menschen v.5, Johann Ambrosius Barth. [Google Scholar]
- Mazzarello P. (2010) Golgi: A Biography of the Founder o,f Modern Neuroscience (translated by Badiani A., and Buchtel H. A.), Oxford University Press, New York, ISBN 970195337846. [Google Scholar]
- Fairhall L. T. (1963) Industrial Toxicology, Hafner Publishing Co., London. [Google Scholar]
- Fink E. B. (1976). In Trace Elements in Human Health and Disease (Prasad A. S., and Oberlis D., Eds.), Vol II, pp 1–16, Academic Press, New York. [Google Scholar]
- Weiss B. (1978) The behavioral toxicology of metals. Fed. Proc. 37, 22–27. [PubMed] [Google Scholar]
- Johnson F. N. (1981) The influence of alkali metal chlorides on environmentally-linked behavioral stereotypes in the rat. Int. J. Neurosci. 13, 199–204. [DOI] [PubMed] [Google Scholar]
- Casdorph R. H., and Walker M. (1994) Toxic Metal Syndrome: How Metal Poisonings Can Affect Your Brain. Dr. Morton Walker Health Book, Penguin Putnam, New York. [Google Scholar]
- Chang L. W., Ed. (1996) Toxicology of Metals. CRC: Boca Raton, FL. [Google Scholar]
- Friberg L., Nordberg G. F., and Vouk V. B., Eds. (2007) Handbook on the Toxicology of Metals, Elsevier Publishing, New York. [Google Scholar]
- Kern J. K.; Grannemann B. D.; Trivedi M. H.; Adams J. B. (2007) Sulfhydryl-reactive metals in autism. J. Toxicol. Environ. Health, Part A 70, 715–721. [DOI] [PubMed] [Google Scholar]
- Popke E. J. (2008) Behavioral Toxicology. In AccessScience, McGraw-Hill Companies, http://www.accessscience.com. [Google Scholar]
- Priya L., and Geetha A. (2011) Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol. Trace Elem. Res. 142, 148−158. [DOI] [PubMed] [Google Scholar]
- Curtis J. T.; Hood A. N.; Chen Y.; Cobb G. P.; Wallace D. R. (2010) Chronic metals ingestion by prairie voles produces sex-specific deficits in social behavior: An animal model of autism. Behav. Brain Res. 213, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L., Daly F., Little M., and Cadgon M. (2011) Toxicology Handbook, 2nd ed., Elsevier, Amsterdam. [Google Scholar]
- Evans G. W.; Dubois R. S.; Hambidge K. M. (1973) Wilson’s Disease: Identification of an abnormal copper-binding protein. Science 181, 1175–1176. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. (1986) Metallothionein. Annu. Rev. Biochem. 55, 913–51. [DOI] [PubMed] [Google Scholar]
- Kägi J. H. R.; Schäffer A. (1988) Biochemistry of metallothionein. Biochemistry 27, 8509–8515. [DOI] [PubMed] [Google Scholar]
- Nishimura N.; Nishimura H.; Ghaffar L.; Tohyama O. (1992) Localization of Metallothionein in the Brain of Rat and Mouse. J. Histochem .Cytochem. 40, 309–311. [DOI] [PubMed] [Google Scholar]
- Aschner M. (1996) The functional significance of brain metallothioneins. FASEB J. 10, 1129–1139. [DOI] [PubMed] [Google Scholar]
- Erickson J. C.; Hollopeter G.; Thomas S. A.; Froelick G. J.; Palmiter R. D. (1997) Disruption of the metallothionein-III gene in mice: Analysis of brain zinc, behavior, and neuron vulnerability to metals, aging, and seizures. J. Neurosci. 17, 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E. H.; Davie E. W. (1998) Recent excitement regarding metallothionein Commentary. Proc. Natl. Acad. Sci. U.S.A. 95, 3333–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. (1998) The elusive function of metallothioneins. Proc. Natl. Acad. Sci. U.S.A. 95, 8428–8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V. K.; Hanson J. (2006) Assessment of metallothionein and antibodies to metallothionein in normal and autistic children having exposure to vaccine-derived thimerosal (Hg). Pediatr. Allergy Immunol. 17, 291–296. [DOI] [PubMed] [Google Scholar]
- Russo A. J. (2008) Anti-Metallothionein IgG and levels of metallothionein in autistic families. Swiss Med. Wkly. 138, 70–77. [DOI] [PubMed] [Google Scholar]
- Chung R. S.; Hidalgo J.; West K. A. (2008) New insight into the molecular pathways of metallothionein-mediated neuroprotection and regeneration. J. Neurochem. 104, 14–20. [DOI] [PubMed] [Google Scholar]
- Russo A. J. (2008) Anti-Metallothionein IgG and levels of metallothionein in autistic families. Swiss Med. Wkly. 138, 70–77. [DOI] [PubMed] [Google Scholar]
- McAuliffe J. J.; Joseph B.; Hughes E.; Miles L.; Vorhees C. V. (2008) Metallothionein I,II deficient mice do not exhibit significantly worse long-term behavioral outcomes following neonatal hypoxia-ischemia: MT-I,II deficient mice have inherent behavioral impairments. Brain Res. 1190, 175–185. [DOI] [PubMed] [Google Scholar]
- Koumura A.; Kakefuda K.; Honda A; Ito Y; Tsuruma K.; Shimazawa M.; Uchida Y.; Hozumi I.; Satoh M.; Inuzuka T.; Hara H. (2009) Metallothionein-3 deficient mice exhibit abnormalities of psychological behaviors. Neurosci. Lett. 467, 11–14. [DOI] [PubMed] [Google Scholar]
- Levin E. D.; Perrauta C.; Pollarda N.; Freedmanb J. H. (2006) Metallothionein expression and neurocognitive function in mice. Physiol. Behav. 87, 513–518. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D.; Cole T. B.; Quaife C. J.; Findley S. D. (1996) ZnT- 3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. U.S.A. 93, 14934–14939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T. B.; Wenzel H. J.; Kafer K. E.; Schwartzkroin P.; Palmiter R. D. (1999) Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad .Sci. U.S.A. 96, 1716–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T. B.; Martyanova A.; Palmiter R. D. (2001) Removing zinc from synaptic vesicles does not impair spatial learning, memory, or sensorimotor functions in the mouse. Brain Res. 891, 253–265. [DOI] [PubMed] [Google Scholar]
- Martel G.; Hevi C.; Friebely O.; Baybutt T; Shumyatsky G. P. (2010) Zinc transporter 3 is involved in learned fear and extinction, but not in innate fear. Learn. Mem. 17(11), 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindreu C.; Palmiter R. D.; Storm D. R. (2011) Zinc transporter ZnT-3 regulates presynaptic Erk1/2 signaling and hippocampus-dependent memory. Proc. Natl .Acad. Sci. U.S.A. 108, 3366–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A. (2001) Significance of transferrin in iron delivery to the brain. J. Health Sci. 47, 520–524. [Google Scholar]
- Tinoco A. D.; Incarvito C. D.; Valentine A. M. (2007) Calorimetric, spectroscopic, and model studies provide insight into the transport of Ti(IV) by human serum transferrin. J. Am. Chem. Soc. 129, 3444–3454. [DOI] [PubMed] [Google Scholar]
- Nörenberg U.; Hubert M.; Rathjen F. G. (1996) Structural and functional characterization of tenascin-R (restrictin), an extracellular matrix glycoprotein of glial cells and neurons. Int. J. Dev. Neurosci. 14, 217–231. [DOI] [PubMed] [Google Scholar]
- Srinivasan J.; Schachner M.; Catterall W. A. (1998) Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc. Natl. Acad. Sci. U. S. A. 95, 15753–15757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z. C.; Ragsdale D. S.; Malhotra J. D.; Mattei L. N.; Braun P. E.; Schachner M.; Isom L. L. (1999) Tenascin-R is a functional modulator of sodium channel beta subunits. J. Biol. Chem. 274, 26511–26517. [DOI] [PubMed] [Google Scholar]
- Weber P.; Bartsch U.; Rasband M. N.; Czaniera R.; Lang Y.; Bluethmann H.; Margolis R. U.; Levinson S. R.; Shrager P.; Montag D.; Schachner M. (1999) Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J. Neurosci. 19, 4245–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel G.; Schmidt R.; Schachner M. (2000) Involvement of L1.1 in memory consolidation after active avoidance conditioning in zebrafish. J. Neurobiol. 43, 389–403. [PubMed] [Google Scholar]
- Probstmeier R.; Braunewell K.; Pesheva P. (2000) Involvement of chondroitin sulfates on brain-derived tenascin-R in carbohydrate-dependent interactions with fibronectin and tenascin-C. Brain Res. 863, 42–51. [DOI] [PubMed] [Google Scholar]
- Bukalo O; Schachner M; Dityatev A. (2001) Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 104(2), 359–369. [DOI] [PubMed] [Google Scholar]
- Freitag S.; Schachner M.; Morellini F. (2003) Behavioral alterations in mice deficient for the extracellular matrix glycoprotein tenascin-R. Behav. Brain Res. 145, 189–207. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M.; Montag D. (2003) Severe cognitive and motor coordination deficits in tenascin-R-deficient mice. Genes Brain Behav. 2, 20–31. [DOI] [PubMed] [Google Scholar]
- Vargová L; Syková E. (2008) Extracellular space diffusion and extrasynaptic transmission. Physiol Res. 57(Suppl 3), S89–99. [DOI] [PubMed] [Google Scholar]
- Zimmermann D. R.; Dours-Zimmermann M. T. (2008) Extracellular matrix of the central nervous system: From neglect to challenge. Histochem. Cell Biol. 130, 635–653. [DOI] [PubMed] [Google Scholar]


