Abstract
J Clin Hypertens (Greenwich). 2012; 14:675–679. ©2012 Wiley Periodicals, Inc.
The authors quantified the impact of hypertension on gout incidence in middle‐aged white and African American men and women. The Atherosclerosis Risk in Communities Study (ARIC) was a prospective population‐based cohort that recruited patients between 1987 and 1989 from 4 US communities. Using a time‐dependent Cox proportional hazards model, the authors estimated the adjusted hazard ratio (HR) of incident gout by time‐varying hypertension and tested for mediation by serum urate level. There were 10,872 participants among whom 45% had hypertension during follow‐up; 43% were men and 21% were African American. Over 9 years, 274 (2.5%) participants developed gout (1.8% of women and 3.5% of men). The unadjusted HR of incident gout was approximately 3 times (HR, 2.87; 95% confidence interval [CI], 2.24–3.78) greater for those with hypertension. Adjusting for confounders resulted in an attenuated but still significant association between hypertension and gout (HR, 2.00; 95% CI, 1.54–2.61). Adjustment for serum urate level further attenuated but did not abrogate the association (HR, 1.36, 95% CI, 1.04–1.79). There was no evidence of effect modification by sex (P=.35), race (P=.99), or obesity at baseline (P=.82). Hypertension was independently associated with increased gout risk in middle‐aged African American and white adults. Serum urate level may be a partial intermediate on the pathway between hypertension and gout.
There is a high prevalence of hypertension in the United States 1 and this chronic condition is associated with morbidity and mortality. 2 One condition associated with hypertension is gout, a metabolic disorder characterized by an inflammatory arthritis. Previous studies found that hypertension is associated with a 2‐ to 3‐fold increase in the risk of gout. 3 , 4 , 5 , 6 , 7 However, these previous studies have been limited in their ability to draw inference to broader populations. They were unable to generalize the results to the US population because these studies involved only a small number of patients with gout, 7 included only men, 4 , 5 , 6 , 7 or included only white participants. 8 Many of these studies have focused on the association of a baseline history of hypertension, 4 , 5 , 6 , 7 rather than time‐varying measures of both treated and untreated hypertension. The prevalence of hypertension increases with age and thus studying a time‐fixed measure of hypertension in a longitudinal cohort will misclassify participants as being normotensive. Previous studies have not adjusted for factors such as kidney function, 4 , 6 , 7 , 8 which may explain part of the association of hypertension and gout. Additionally, other studies have adjusted for diuretic use when assessing the impact of hypertension. 6 , 8 However, diuretics are first‐line treatment for hypertension and thus are on the causal pathway between hypertension and gout. Adjusting for diuretic use measures the direct effect of hypertension on the incidence of gout but does not confound this association.
In this study, we addressed the previous gaps in the literature surrounding the association of hypertension and gout. We quantified the hazard of incident gout by hypertension and hypertension risk factors during 9 years of follow‐up in a longitudinal population‐based cohort of middle‐aged adults. Additionally, we tested whether serum urate levels mediated the association of hypertension and gout.
Materials and Methods
Setting and Participants
The Atherosclerosis Risk in Communities Study (ARIC) was a prospective population‐based cohort study of 15,792 individuals recruited from 4 US communities (Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and the suburbs of Minneapolis, Minnesota). The institutional review board of the participating institutions approved the ARIC study protocol and study participants provided written informed consent. Participants aged 45 to 64 years were recruited to the cohort in 1987 to 1989. This cohort was established to study the natural history of atherosclerosis, and consisted of 1 baseline visit (visit 1) between 1987 and 1989 and 3 follow‐up visits (visits 2, 3, and 4) administered 3 years apart.
This analysis was limited to participants who were Caucasian or African American; few participants reported other races (n=48). Gout status was ascertained at visit 4. We excluded participants who did not report their gout status at visit 4 (n=4269) and those with prevalent gout at cohort entry, defined as the self‐report of gout onset prior to the baseline visit (n=419). Additionally, we limited the population to those with no missing data on baseline hypertension or hypertension risk factors (n=184). Participants who were older, had diabetes, lower education, and higher serum urate were less likely to attend the fourth visit. However, baseline hypertension, higher body mass index (BMI), lower estimated glomerular filtration rate (eGFR), use of an antihypertensive medication (including diuretic use), or sex were associated with not attending visit 4 and responding to the gout query.
Exposure: Hypertension
At each visit, technicians used a random‐zero sphygmo‐manometer to take 3 blood pressure (BP) measures. An average of the second and third measurements was recorded for the first 3 visits and the first and second measurement for the fourth visit. Hypertension was defined as the self‐report of medication to treat hypertension or a measured systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg. Participants with diabetes (fasting glucose >126 mg/dL) or chronic kidney disease (<60 mL/min/1.73m2) were considered to have diabetes if their measured systolic BP was ≥130 mm Hg or diastolic BP ≥80 mm Hg or the self‐report of medication to treat hypertension. If hypertension status was missing at visit 2 or 3, hypertension status was carried forward from the previous visit. Hypertension prior to gout onset, ascertained at visits 1, 2, and 3, was considered the exposure of interest.
Outcome: Incident Gout
At visit 4, participants were asked, “Has a doctor ever told you that you had gout?” Participants who answered, “Yes,” to the gout query then reported the age of gout diagnosis. The outcome of interest was incident gout based on self‐report. Incident gout was considered to be onset of gout after baseline. Our previous research suggests that self‐report of a physician diagnosis of gout is a reliable (3‐year reliability κ=0.73) and sensitive (sensitivity=84%) measure of gout. 9
Other Measures
Other covariates of interest that were assessed at baseline included age (in years), sex (male or female), race (white or African American), alcohol intake (g/wk), and BMI (kg/m2). eGFR was estimated by using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐Epi) equation 10 and categorized as ≥90, 60 to 90, or <60 mL/min/1.73m2. These variables were considered to be risk factors for both hypertension and gout and, thus, potential confounders. The categories were chosen to reduce residual confounding.
Serum urate concentrations were measured with the uricase method at visits 1 and 2 in mg/dL. The reliability coefficient of serum urate was 0.91, and the coefficient of variation was 7.2% in a sample of 40 individuals with repeated measures taken at least 1 week apart. 11 In the complete ARIC cohort, the mean serum urate levels were 0.36 mg/dL higher at visit 2 due to laboratory drift compared with visit 1 after adjustment for age at the visit. Therefore, we subtracted 0.36 mg/dL from the visit 2 serum urate levels to make them comparable to visit 1 values to correct for laboratory drift.
Statistical Methods
First, the mean and standard deviation (SD) as well as the prevalence of the covariates were calculated and compared by baseline hypertension. The mean of continuous variables in patients with hypertension was compared with the mean of those who did not have hypertension using a t test; the prevalence of categorical factors was compared using chi‐square tests.
Using a time‐dependent Cox proportional hazards model, the hazard rate ratio (HR) and 95% confidence intervals (CIs) of incident gout by time‐varying hypertension status (at visit 1, 2, and 3) was estimated. Age was used as the time scale and we allowed for late entries by age at cohort enrollment. All confounding variables were considered time‐fixed at baseline. Models were adjusted for confounders of the association of hypertension and incident gout including sex, race, BMI, alcohol intake, and categorical eGFR. The unadjusted cumulative incidence functions were plotted using a Kaplan‐Meier approach. Proportional hazards assumptions were confirmed by visual inspection of the complimentary log‐log plots and Schoenfeld residuals.
We tested whether there was evidence that serum urate levels mediated the association of hypertension and incident gout through a qualitative assessment. We added visit 2 serum urate level to the adjusted Cox proportional hazards model to test for mediation. If the effect estimate of hypertension was closer to the null after including serum urate level in the model, then serum urate was considered to be a partial mediator. Furthermore, if the effect estimate of hypertension was null (HR=1), then this was considered to be full mediation.
We used sensitivity analyses to further investigate the association of hypertension and incident gout among subgroups. We limited the study population to those: (1) without missing hypertension status at visit 2 or 3, (2) without diuretic use, (3) adjusting for diuretic use, and (4) without baseline hyperuricemia (>7.0 mg/dL).
All analyses were performed in SAS, version 9.3 (SAS Institute, Cary, NC).
Results
Study Population Characteristics
A total of 10,872 ARIC participants met the study criteria. The study population was 43% men and 21% African American. The mean age at cohort entry was 54 years (SD=5.7) and the mean BMI was 27 kg/m2 (SD=5.1). During 9 years of follow‐up, 274 (2.5%) participants developed gout; 1.8% of women and 3.5% of men.
Table I lists the study population characteristics of participants by baseline hypertension status. Participants with hypertension were older (55.2 vs 53.2 years, P<.001) and more likely to be male (44% vs 42%, P=.04) and African American (35% vs 15%, P<.001) than those without hypertension. Additionally, participants with hypertension had a higher BMI (29.4 vs 26.5 kg/m2, P<.001) and were more likely to have glomerular filtration <60 mL/min (5% vs 1%, P<.001). The mean BP values were in the normal range for those with hypertension since some hypertensive participants received treatment baseline.
Table I.
Baseline Gout Risk Factors and Number of Gout Cases by Baseline Hypertension in the Atherosclerosis Risk in Communities Study (N=10,872)
| Baseline Risk Factors | No Hypertension (n=6003) | Hypertension (n=4869) |
|---|---|---|
| Male sex, No. (%) | 3260 (44) | 1449 (42)a |
| Mean age (SD), y | 53.2 (5.6) | 55.2 (5.6)b |
| African American race, No. (%) | 1109 (15) | 1225 (35)b |
| Mean blood pressure, mm Hg (SD) | ||
| Systolic | 113 (12.1) | 133 (18.5)b |
| Diastolic | 70 (8.5) | 80 (11.2)b |
| Mean body mass index, kg/m2 (SD) | 26.5 (4.5) | 29.4 (5.7)b |
| eGFR, mL/min, No. (%) | ||
| <60 | 59 (1) | 158 (5)b |
| 60–90 | 2975 (40) | 1527 (44) |
| >90 | 4381 (59) | 1772 (51) |
| Mean alcohol intake, g/wk, (SD) | 40.4 (83) | 39.8 (95) |
| Mean serum urate, mg/dL, (SD) | 5.7 (1.4) | 6.4 (1.5)b |
| Incident gout, No. (%) | 114 (1.5) | 160 (4.6)b |
Abbreviations: eGFR, estimated glomerular filtration rate; SD, standard deviation. a P value <.05 comparing hypertension with no hypertension. b P value <.001 comparing hypertension with no hypertension.
There were 4869 (45%) participants who had hypertension during follow‐up. The prevalence of hypertension increased at each visit (Table II).
Table II.
Prevalence of Hypertension Status at Each Visit in the Atherosclerosis Risk in Communities Study (N=10,872)
| Visit 1 | Visit 2 | Visit 3 | |
|---|---|---|---|
| Overall, % | 32 | 34 | 40 |
| Gout‐free participants, % | 31 | 33 | 39 |
| Participants with gout, % | 59 | 64 | 70 |
Association of Hypertension and Gout
The data in Table I suggest that incident gout occurred more frequently in participants with hypertension during 9 years of follow‐up compared with those without hypertension (4.6% vs 1.5%, P<.001). The cumulative incidence of gout was higher for participants with hypertension (P<.001) (Figure). There was an increase in the prevalence of hypertension for both participants who developed gout and those who were free from gout. Furthermore, there was a higher prevalence of hypertension for participants who developed gout at each visit (Table II).
Figure FIGURE.
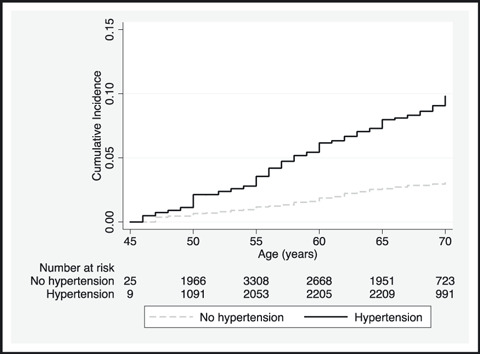
Cumulative incidence of gout by baseline hypertension. P value for log‐rank test <.001.
Table III lists the unadjusted and adjusted HR of incident gout by time‐varying hypertension. The unadjusted HR of incident gout was 3 times (HR, 2.87; 95% CI, 2.24–3.78) greater for those with hypertension compared with those without hypertension. After adjusting for sex and race, hypertension was associated with an increased risk of developing gout (HR, 2.49; 95% CI, 1.93–3.21). Additionally, adjusting for confounders resulted in an attenuated but still significant association between hypertension and the development of gout (HR, 2.00; 95% CI, 1.54–2.61). After adjusting for serum urate level, hypertension was marginally associated with incident gout (HR, 1.36, 95% CI, 1.04–1.79), suggesting that serum urate may be a partial mediator on the pathway between hypertension and incident gout.
Table III.
Hazard Rate Ratio of Incident Gout by Time‐Varying Hypertension Status and Hypertension Risk Factors in the Atherosclerosis Risk in Communities Study (N=10,872)
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Hypertension, time‐varying | 2.87 (2.24–3.78) | 2.49 (1.93–3.21) | 2.00 (1.54–2.61) | 1.36 (1.04–1.79) |
| Male sex | 2.12 (1.66–2.71) | 2.07 (1.60–2.68) | 1.09 (0.83–1.42) | |
| African American race | 2.33 (1.81–3.00) | 2.31 (1.78–3.00) | 1.78 (1.37–2.32) | |
| Body mass index, 5 kg/m2 | 1.39 (1.25–1.54) | 1.22 (1.09–1.36) | ||
| High alcohol intake | 2.50 (1.77–3.51) | 2.00 (1.42–2.82) | ||
| Estimated GFR, mL/min | ||||
| <60 | 2.43 (1.50–3.94) | 0.85 (0.51–1.43) | ||
| 60–90 | 1.15 (0.89–1.48) | 0.80 (0.62–1.04) | ||
| >90 | Reference | Reference | ||
| Serum urate, mg/dL | 1.93 (1.80–2.06) | |||
Abbreviation: eGFR, estimated glomerular filtration rate.
The hypertension risk factors were significantly associated with gout in the adjusted model. The strongest hypertension risk factor that was also associated with gout was eGFR <60 mL/min. However, after adjustment for serum urate level, the association of these risk factors and gout were all attenuated, suggesting that serum urate mediates the association between hypertension risk factors and gout.
There was no evidence of effect modification of the association of hypertension and gout by sex (P=.35), race (P=.99), or obesity at baseline (P=.82).
Sensitivity Analysis
Results did not change when we adjusted the final model for time‐varying BP and time‐varying BMI as confounders. Results were similar, although slightly stronger, when the analysis was restricted to participants without missing hypertension at visit 2 or 3 (confounder‐adjusted HR, 2.10; 95% CI, 1.60–2.74; confounder‐ and serum urate–adjusted HR, 1.41; 95% CI, 1.07–1.85). Additionally, restricting the analysis to participants without diuretic use hypertension was associated with incident gout (confounder adjusted HR, 1.61; 95% CI, 1.14–2.29). Hypertension was also associated with incident gout after adjusting for diuretic use (confounder‐adjusted HR, 1.50; 95% CI, 1.12–2.02). Finally, among participants without baseline hyperuricemia, hypertension was associated with incident gout (confounder‐adjusted HR, 1.61; 95% CI, 1.02–2.55).
Discussion
In a large prospective population‐based study of middle‐aged African American and white adults, patients with hypertension were at a 2‐fold higher risk of developing gout. The association of hypertension and gout was independent of gout risk factors including kidney function. Additionally, our results suggest that serum urate level may be a partial mediator of hypertension and gout. Finally, hypertension increased the risk of gout regardless of sex, race, and obesity status.
We were able to extend the previous findings that hypertension was associated with gout from cohorts that were limited to men or participants of white race to a population‐based cohort. The magnitude of the association, approximately a 2‐fold increase in risk, was similar to previous studies. 6 , 8 Campion and colleagues 5 reported that hypertension was not associated with gout after adjusting for serum urate in men. Similarly, our results suggest that serum urate is a mediator of the association of hypertension and gout. However, we did not observe that the presence of hypertension nullified the association of African American race and gout, as was observed in a previous study. 4 These differences in results may be due to the difference in the age of the cohorts, with ARIC being a study of adults at middle‐age rather than at early adult life.
The biological mechanism linking hypertension and gout is thought to be through kidney function. The excretion of uric acid occurs primarily via the kidney. 12 Hypertension is thought to be the cause of glomerular arteriolar damage and glomerulosclerosis. This will then lead to renal insufficiency and hyperuricemia. However, the directionality of hypertension and hyperuricemia is currently debated and it is possible that the relationship is bi‐directional. 13 Additionally, hypertension is a component of the metabolic syndrome, which has been associated with the development of gout. 14
ARIC was a well‐characterized cohort with very high response rates. This is one of the largest biracial studies of gout, which included both male and female participants with gout. Additionally, we used a broad definition of hypertension that included both measured BP and treatment. We were able to control for potential confounders of the association of hypertension and gout such as obesity, alcohol intake, and kidney function. Additionally, our results were robust to sensitivity analyses.
Study Limitations
The main limitation of our study was that gout was self‐reported by participants at visit 4. However, our previous work has suggested that self‐reported gout and age of onset is both sensitive and reliable. 9 Participants had to survive until visit 4 and be healthy enough to attend the clinic visit to be included in this study. However, participants who did not attend visit 4 and respond to the gout query were more likely to be older and have more comorbidities and thus would be more likely to develop gout than their healthy counterparts who attended the visit. Therefore, we would expect that our observed association between hypertension and gout be attenuated due to loss to follow‐up. We could not rule out the possibility that serum urate is a confounder and not a mediator of this association. Although serum urate has been found to predict the onset of hypertension, 15 a randomized controlled trial would be necessary to determine the directionality of the uric acid and hypertension association in middle‐aged adults. Our analysis was not designed to test whether the urate level was a consequence of changing hypertension.
Conclusions
The results from this population‐based, longitudinal study support the hypothesis that hypertension increases the risk of gout in African American and white men and women. As the prevalence of hypertension continues to rise in both developed and developing countries, 16 it is important for public health professionals to consider the debilitating nature of gout as a consequence of hypertension. The impact of reducing the prevalence of hypertension and hypertension risk factors on the incidence of gout warrants further investigation.
Acknowledgments and disclosure: The authors thank the staff and participants of the Atherosclerosis Risk in Communities Study (ARIC) study for their important contributions. ARIC is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Mara McAdams DeMarco was supported by a T32 training grant from the National Heart, Lung, and Blood Institute grant (5T32HL007024). The authors report no conflict of interest.
References
- 1. Cutler JA, Sorlie PD, Wolz M, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. [DOI] [PubMed] [Google Scholar]
- 2. Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153:598–615. [DOI] [PubMed] [Google Scholar]
- 3. Bhole V, de Vera M, Rahman MM, et al. Epidemiology of female gout: 52‐year follow‐up of a prospective cohort. Arthritis Rheum 2010;62(4):1069–1076. [DOI] [PubMed] [Google Scholar]
- 4. Hochberg MC, Thomas J, Thomas DJ, et al. Racial differences in the incidence of gout. The role of hypertension. Arthritis Rheum. 1995;38:628–632. [DOI] [PubMed] [Google Scholar]
- 5. Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82:421–426. [DOI] [PubMed] [Google Scholar]
- 6. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow‐up study. Arch Intern Med. 2005;165:742–748. [DOI] [PubMed] [Google Scholar]
- 7. Roubenoff R, Klag MJ, Mead LA, et al. Incidence and risk factors for gout in white men. JAMA. 1991;266:3004–3007. [PubMed] [Google Scholar]
- 8. Bhole V, de Vera M, Rahman MM, et al. Epidemiology of gout in women: fifty‐two‐year followup of a prospective cohort. Arthritis Rheum. 2010;62:1069–1076. [DOI] [PubMed] [Google Scholar]
- 9. McAdams MA, Maynard JW, Baer AN, et al. Reliability and sensitivity of the self‐report of physician‐diagnosed gout in the campaign against cancer and heart disease and the atherosclerosis risk in the community cohorts. J Rheumatol. 2011;38:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eckfeldt JH, Chambless LE, Shen YL. Short‐term, within‐person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118:496–500. [PubMed] [Google Scholar]
- 12. Gutman AB, Yu TF. Renal function in gout; with a commentary on the renal regulation of urate excretion, and the role of the kidney in the pathogenesis of gout. Am J Med. 1957;23:600–622. [DOI] [PubMed] [Google Scholar]
- 13. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen SY, Chen CL, Shen ML. Manifestations of metabolic syndrome associated with male gout in different age strata. Clin Rheumatol. 2007;26:1453–1457. [DOI] [PubMed] [Google Scholar]
- 15. Johnson RJ, Kang DH, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. [DOI] [PubMed] [Google Scholar]
- 16. Ezzati M, Lopez AD, Rodgers A, et al. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. [DOI] [PubMed] [Google Scholar]


