Abstract
Purpose
Toward the goal of designing a clinical trial using imaging parameters to treat stroke patients with unknown onset time, we investigated the timing of changes on MRI in patients with well-defined stroke onset.
Methods
Hypothesis generating (n=85) and confirmatory (n=111) samples were scored by blinded readers for FLAIR hyperintensity in diffusion-positive regions. Reader measured signal intensity ratio (SIR) of the lesion to contralateral tissue was compared with SIR measured by co-registration.
Results
Lesion conspicuity increased with time on FLAIR (p=0.006). Qualitative assessment of FLAIR-negative vs FLAIR hyperintensity (k= 0.7091, 95% CI 0.61–0.81) showed good interrater agreement. Subtle hyperintensity was less reliably categorized (k=0.59, 95% CI 0.47–0.71). Reader measured SIR <1.15 can identify patients within the treatable time window of 4.5 hours (positive predictive value= 0.90). The SIR was greater for right hemisphere lesions (p=0.04) for a given reported time from stroke symptom onset.
Conclusion
The SIR on FLAIR provides a quantitative tool to identify early ischemic strokes. In developing SIR thresholds, right hemisphere lesions may confound the accurate estimate of stroke onset time. Image co-registration for thrombolytic trial enrollment is not necessary. A SIR < 1.15 on FLAIR yields a practical estimate of stroke onset within 4.5 hours.
Keywords: DWI= Diffusion Weighted Imaging, FLAIR= Fluid Attenuation Inversion Recovery, Thrombolysis, Stroke
INTRODUCTION
As a matter of convention, time of stroke onset for thrombolytic eligibility is estimated as the time that a patient was last known to be normal. Although this conservative estimate assures that treatment is started within the established time window, it excludes potentially treatable stroke if the time of onset is uncertain, such as patients with symptoms first noted upon awakening or those with unwitnessed onset who are unable to give an accurate history. This factor likely contributes to low treatment percentage rates of IV tPA of 3 to 8.5% in a recent study of medical centers in the United States 1. The primary reason for low utilization rates of thrombolytics are that most patients (~77%) did not meet the 3-hour window of treatment or because their symptom onset time was unknown2.
Toward the goal of designing a clinical trial using imaging parameters to safely treat ischemic stroke patients with unknown onset time, we are interested in the timing of imaging changes on acute MRI in patients with well-defined stroke onset time. A recent study comparing CT changes in wake-up strokes to those in strokes of known onset time concluded that the time of true onset in wake-up strokes was shortly before awakening3, suggesting the potential use of thrombolytic treatment to this patient population . Another center’s experience with off-label, compassionate use of thrombolysis in wake-up stroke patients, given pre-treatment CT scans reports higher rates of good clinical outcomes when compared with non-treated wake-up strokes4. However, the results of ABESTT-II, a randomized, double-blind, placebo-controlled trial of abciximab using CT screening, gives reason for caution when treating wakeup strokes, since this cohort had higher mortality and symptomatic intracranial hemorrhage rates5,6 than the non-wake-up group.
Because fluid-attenuated inversion recovery (FLAIR) signal hyperintensity of an acute ischemic lesion is normal in the first hours after stroke, the DWI-positive, FLAIR-negative MRI pattern has been proposed as a marker for time when the precise time of symptom onset is unknown. Using diffusion MRI in conjunction with FLAIR has been reported to identify hyperacute ischemic strokes within the thrombolytic time window7–10. However lesion conspicuity on FLAIR is subjective with low interrater agreement7, especially when subtle changes are present. Lesion to normal signal intensity ratios on FLAIR co-registered to DWI images has been proposed as a means to quantify changes that occur with time 8, but such measurements may be impractical within the time constraints of thrombolytic treatment.
We consider whether early ischemic changes on FLAIR MRI in stroke of known onset permit identification of early strokes in patients with unknown onset time. Specifically, we investigate whether qualitative or quantitative assessments of FLAIR hyperintensity is better at making this determination, and whether off-line co-registration of diffusion-weighted (DWI) and FLAIR images is necessary for quantitative estimate of hyperintensity.
METHODS
Imaging
MRI Analysis
For the first MRI hypothesis generating sample, cases from April 2005 to December 2008 were selected from the Lesion Evolution in Stroke and Ischemia On Neuroimaging (LESION) Project registry, a prospective series of stroke patients screened with MRI within 24 hours from witnessed stroke onset, DWI-confirmed acute ischemic stroke, and admission NIH Stroke Scale (NIHSS) >3 or received an acute intervention. Inclusion was limited to patients with left anterior circulation stroke with witnessed stroke onset. Right hemisphere strokes were initially excluded because the risk of anosognosia may lead to incorrect tissue onset times11. Lacunar strokes and lesions with hemorrhagic transformation on acute imaging were also excluded.
For the confirmatory evaluation, a separate, second sample of consecutively screened patients from January 2009 through December 2010 were analyzed similarly to the first sample with the exception that right hemisphere strokes were included and the last seen normal and time first seen with symptoms were extended up to 30 minutes.
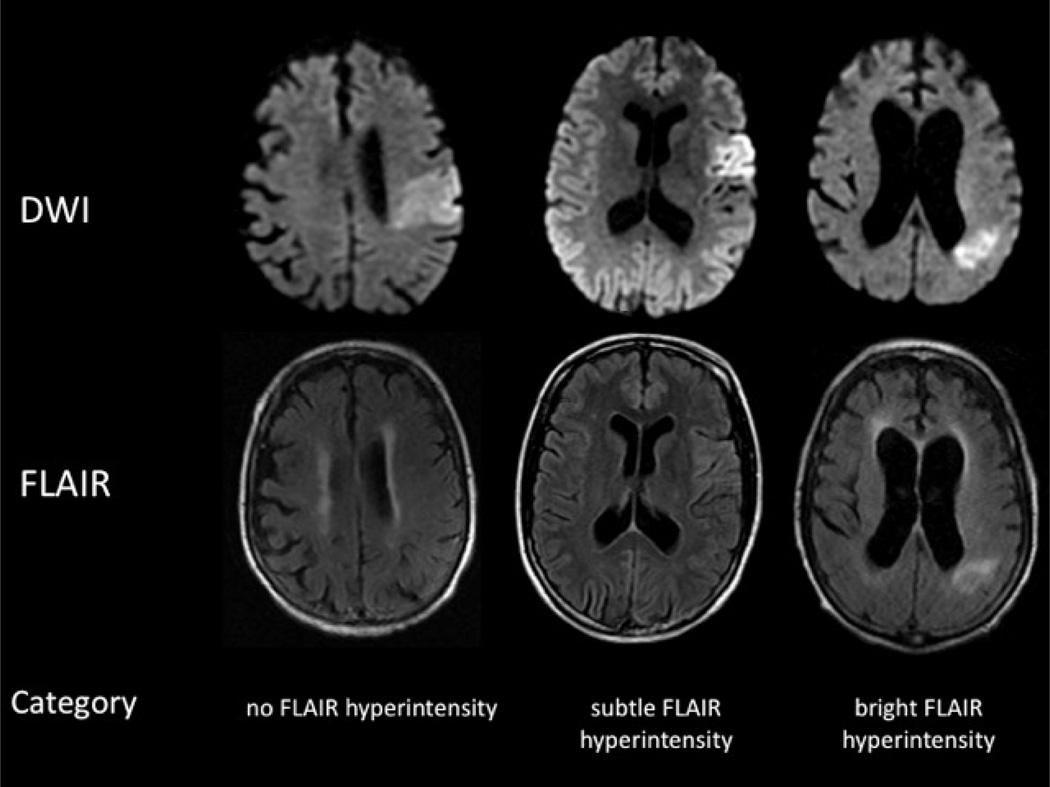
Blinded to stroke onset time, two readers (CHR and SSS) separately rated the region on FLAIR images corresponding to the acute DWI-bright lesion was rated as none (0), subtle (1), or bright (2) hyperintensity (Figure 1). A third reader (SW) was invoked as the tie-breaker when there was a discrepancy between the FLAIR rating and signal intensity ratio between the two readers. If the FLAIR signal was heterogeneous, the rating was based on the area of brightest signal intensity underlying the lesion on DWI. FLAIR scans were considered “negative” if the hyperintensity was scored as none (0). After qualitative scoring had been completed, a region of interest (ROI) was drawn corresponding to the area of brightest FLAIR signal intensity. A second ROI was drawn over homologous area of parenchyma in the contralateral hemisphere, with care to avoid areas of chronic infarction, leukoariosis, and cerebral spinal fluid (CSF). The ratio of mean signal intensity of the lesion to the signal intensity of the contralateral hemisphere was calculated and reported as the signal intensity ratio (SIR). The optimum SIR threshold for identifying stroke onset within 4.5 hours was chosen from the first sample using the approximate point where sensitivity and specificity were equivalent.
Figure 1.
Example images from three different patients representative of each of the three qualitative ratings of none, subtle, and bright FLAIR hyperintensity.
Co-registration
To verify the SIR method was indeed as accurate as a quantitative process a selection of patients from both samples was analyzed using MIPAV (Medical image Processing, Analysis and Visualization, version 4.4, National Institutes of Health, Bethesda, MD). Forty-nine patients were selected for the quantitative analysis based on 1) none to mild leukoariosis, 2) stroke lesion diameter >1 cm, and 3) none to minimal motion artifact. The intensity on FLAIR in a volume of interest (VOI) corresponding to the entire DWI region was identified. Next, the DWI and FLAIR images were acquired co-localized in order to identify a homologous region of healthy tissue on the contralateral hemisphere. The FLAIR was mid-sagitally aligned and the DWI was co-registered to the FLAIR. The parameters used were 9-specific rescale degrees of freedom, tri-linear interpolation and normalized mutual information cost function. Following co-registration, an independent reader blinded to the FLAIR, segmented the ischemic lesion on DWI. The resulting VOI were mirrored across midline to perform measurements in the contralateral hemisphere. Manual editing to remove regions overlapping the ventricles, sulci, or cerebrospinal fluid (CSF) was used in the contralateral hemisphere (<3%). The VOIs from both the ipsi- and contralateral hemispheres were copied to the FLAIR image. The SIR was calculated as the ratio of the average ipsilateral voxel intensity to that of the contralateral hemisphere. The volumes of the VOIs were recorded.
MRI Protocol
Imaging was performed using a 1.5T (Twinspeed, General Electric) or 3T (Achieva, Philips) clinical MRI scanner. Parameters for DWI spin-echo echo-planar series included either 40-3.5mm or 20-7mm thick contiguous axial oblique slices with b=0 and b=1000 s/mm2, trace or isotropically weighted, repetition time (TR)/echo time (TE)=6000-7000/72-90 ms, acquisition matrix of 96x96 or 128x128, and 22cm field of view (FOV). FLAIR images were acquired with commercially available 2D sequences, balanced across field strength for detecting chronic ischemic parenchyma with the following parameters at 1.5T: repetition time (TR)/echo time (TE) = 9000/145 msec, inversion time (TI) = 2200 msec, 20 contiguous slices with 0.9 × 0.9 × 7 mm resolution (zero filled) for a total acquisition of 2 minutes 25 seconds; at 3T: TR/TE = 9000/120 msec, TI= 2600 msec, 20 contiguous slices with 1 × 1 × 4 mm resolution (SENSE R=1.75), for a total acquisition of 2 minutes 15 seconds.
Statistical Analysis
Two sample t-test was used to compare the means of continuous variables. The Mann-Whitney U test was used when the data showed strong departure from normality. The means (standard deviation) are reported when data do not show departure from normality, as assessed by the Kolmogorov-Smirnov test statistic. Paired samples t-test were used to compare SIR means between left and right hemispheres. When the data was skewed, the Wilcoxon Signed Rank Test was used instead. SPSS (IBM, version 17.0) was used for testing. For evaluating interrater agreement for qualitative FLAIR rating, kappa values were used12 with 0.5 representing moderate to good agreement and 0.7 or higher representing excellent agreement between readers13. Both asymptotic and bootstrap 95% confidence intervals for kappa were used. These were calculated using the SAS macro of Vierkant14.
RESULTS
MRI Analysis
In the first sample, acute MRI scans were obtained within 24 hours for 466 patients with witnessed stroke onset. After excluding right hemisphere strokes, pure brainstem strokes, lacunar strokes, and hemorrhagic strokes, 85 patients who met the inclusion criteria were selected for analysis. In the second sample, 111 patients had both witnessed stroke onset time within 30 minutes and an acute MRI scan within 24 hours. Left hemisphere strokes comprised 41% (45) of the confirmatory sample and the remaining 51% (57) and 8% (9) were right hemisphere or bilateral hemispheric strokes, respectively.
Qualitative assessment of FLAIR using all three categories from no FLAIR hyperintensity (0), subtle (1), and bright hyperintensity (2) showed kappa using asymptotic estimate = 0.6876 (95% CI 0.60–0.77) and bootstrap estimate = 0.69 (95% CI 0.60–0.77). There was no difference between asymptotic and bootstrap confidence intervals for all kappa estimates. When dichotomized between no FLAIR hyperintensity and subtle and bright hyperintensity grouped together as positive, the kappa agreement, using asymptotic estimate = 0.7091 (95% CI 0.61, 0.81). Based on qualitative FLAIR rating, 89 patients (45%) had a scan interpreted as negative or no FLAIR hyperintensity. Of the 89 negative scans, 62 were within 3 hours, 73 were within 4.5 hours, and 78 were within 6 hours of stroke onset. In Table 1 are the descriptive statistics dichotomized based on qualitative rating of FLAIR hyperintensity. There was no significant difference for age, gender, and stroke severity between FLAIR-negative and FLAIR-positive patients (p=0.17). FLAIR hyperintensity was seen as early as 0.7 hours.
Table 1.
Demographics and comparisons data.
| Characteristics | FLAIR- Negativea (n= 89) |
FLAIR-Positivea (n= 107) |
Group Comparison pb |
|---|---|---|---|
| Median Age (IQR), yr | 72(61–81) | 73(61–82) | 0.952 |
| Female sex, n (%) | 49(55.1) | 63(58.9) | 0.664 |
| Median NIHSS (IQR) | 10(5.0–20.0) | 8(5.0–17.5) | 0.346 |
| Median time to LSN (IQR) | 2.1(1.4–3.7) | 3.5(1.8–10.5) | <0.001 |
| Left side of Infarction, n (%) | 71(79.8) | 68(63.6) | 0.017 |
Fluid Attenuation Inversion Recovery (FLAIR)
Group comparison was performed using either the Two sample t-test or Mann-Whitney U test for continuous variables. Fisher’s exact test was used for categorical variables.
IQR = InterQuartile Range; NIHSS = National Institutes of Health Stroke Scale; LSN= Last Seen Normal
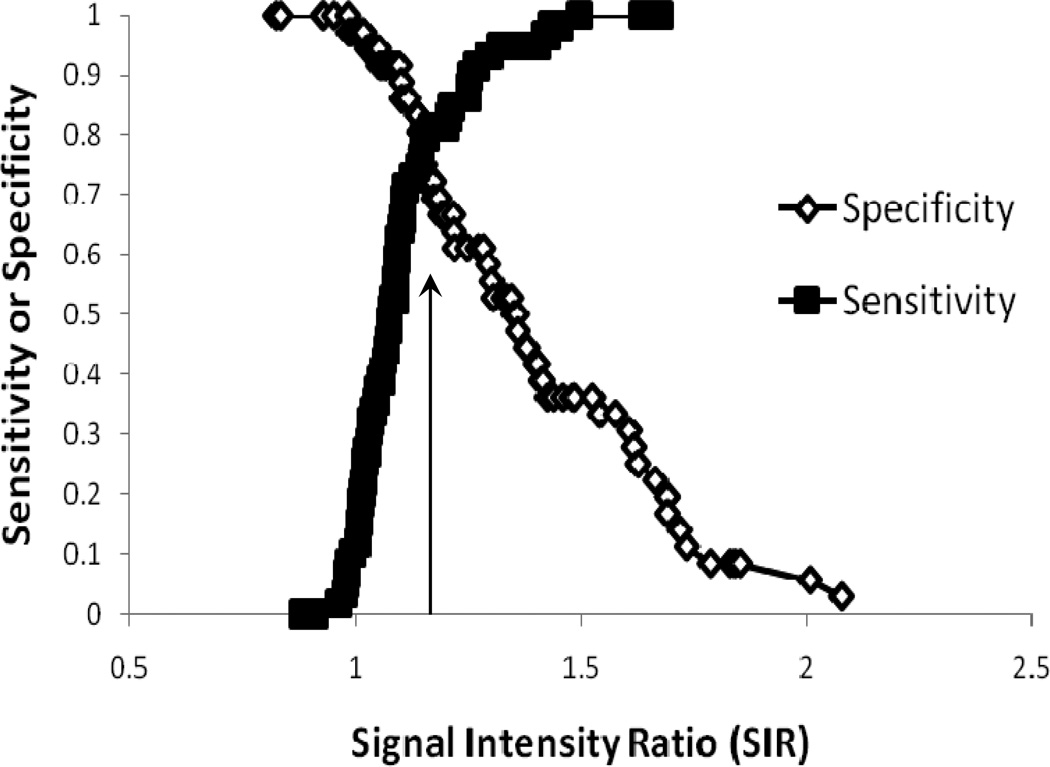
The optimum SIR threshold of 1.15 (Figure 2) determined from the first sample of left hemispheric lesions only for onset less than 4.5 hours had a sensitivity of 0.80, specificity of 0.82, accuracy of 0.73, and positive predictive value of 0.82. When comparing the confirmatory sample and then separating left and right hemispheric lesions, we found that there was a significant difference in optimum SIR thresholds for identifying ischemic strokes less than 4.5 hours (SIR left only= 1.14 versus SIR right only= 1.29, p=0.000). With an adjustment for time, there still remained a significant difference between left and right hemisphere mean-SIRs (p=0.000). The SIR from lesion relative to contralateral regions were correlated between readers (r=0.69, p=0.000) with no significant difference between mean SIRs.
Figure 2.
The sensitivity and specificity for binary classification based on signal intensity ratio (SIR) as a function of threshold for the time-window of 0–4.5 hours. The arrow indicates the optimum SIR threshold where sensitivity and specificity are approximately equal.
The mean SIR for FLAIR images rated as negative based on qualitative assessment was 1.05(0.09) in comparison to 1.30(0.21) for images considered to either have subtle or bright FLAIR hyperintense lesions. The mean SIR for subtle FLAIR hyperintense was 1.15(0.06). By combining qualitative FLAIR reading with the SIR threshold of <1.15, 85 of 196 patients were classified as DWI-positive and FLAIR-negative. Eighty-two percent of FLAIR-negative patients were within 4.5 hours from witnessed stroke onset. The prevalence of lesion conspicuity increases with time on FLAIR (p=0.006). The average time for FLAIR MRI to remain negative was 3.2h (SD=3.7h, range 0.75–22.22; 95%CI 2.0–4.3).
Using co-registered DWI-FLAIR measurements, the subtle FLAIR hyperintense lesions had a mean SIR of 1.08(0.07) which was significantly lower than the reader measured SIR of 1.15(0.06), p=0.008. This was expected since the co-registered DWI-FLAIR, SIR measures were taking into account the entire FLAIR region outlined by the DWI lesion whereas the reader selected the brightest areas of FLAIR hyperintensity for SIR measurement. There was no significant difference between mean values of the co-registered DWI-FLAIR measures and reader selected SIRs for FLAIR- negative scans (p=0.82). The co-registered DWI-FLAIR measure did not substantially increase sensitivity or specificity over reader measured SIRs. The accuracy of identifying stroke onset within 4.5 hours using reader measured SIR threshold of 1.15 was comparable to that of co-registered DWI-FLAIR-SIR at the same threshold (sensitivity=0.78, specificity=0.78 versus sensitivity=0.85, specificity=0.67, respectively).
DISCUSSION
Our findings derived from patients with well-defined stroke onset times show FLAIR MRI can accurately identifying patients with early stroke onset because the prevalence of lesion visibility increases with time on FLAIR. We found that on average, lesions determined to be qualitatively FLAIR-positive (subtle and bright hyperintense lesions) were 15–20% brighter than FLAIR- negative MRI. Subtle FLAIR hyperintensity was more difficult to interpret and this is shown by the lower interrater agreement. To circumvent the subjectivity of FLAIR hyperintensity by visual inspection only, SIR quantitatively measures what is visually identified. Using both qualitative FLAIR assessment and measuring SIR reduces the possibility of incorrectly predicting a patient to be within 4.5 hours of stroke onset.
In our study, with co-registration, subtle FLAIR hyperintensity was 8% brighter than normal tissue, which is similar to findings in a recent study that found patients imaged later than 3 hours from stroke onset had a FLAIR ratio of 7% or higher8. However, at this time, co-registration requires time-consuming post processing which is not feasible to do in an acute setting. We show that reader measured SIRs for the detection of FLAIR-negative MRI scans is comparable to co-registration of DWI-FLAIR. The co-registration technique does not substantially increase sensitivity and specificity for identifying stroke onset within 4.5 hours. Reader measured SIRs can be performed within a couple minutes on the MRI console. A recent, multicenter study showed interboserver agreement for FLAIR lesion visibility was moderate with positive predictive value of 83%, which is similar to our findings10. However, a higher sensitivity of 78% was seen using the SIR method. Instead of relying on qualitative consensus evaluations in which interrater reliability ranges between 65–66% 7, or may not be reliable when lesions are small7,15, we propose using a novel, quantitative signal intensity comparison that more objectively interprets FLAIR hyperintensity in specific ranges using lesion-to-normal ratios. The combination of using SIR along with qualitative evaluation for FLAIR- negative helps make the selection of patients for treatment trials more objective than relying solely on the interpretation of each reader. The proposed two-step FLAIR evaluation and SIR measurement is a conservative approach that is not unlike other imaging-based diagnosis where in the first step is a qualitative assessment for pathology and the second is a quantitative measurement to confirm what is visually seen. It has the advantage of interpreting what is subjectively seen to a quantified number that has good correlation among readers and is simple and practical to implement in the acute care setting.
The second novel approach in our study was the separation of left and right hemisphere strokes. There was a significant difference in the lesion hyperintensity between hemispheres which supports the argument that anosognosia in right hemisphere strokes11, 16, 17 may lead to incorrect stroke onset times. Inclusion of right hemisphere lesions may confound the accurate estimate of stroke onset time in developing imaging based thresholds. Another potential cause for SIR differences between hemispheres may be the size of lesions, which was not measured in our study. Larger DWI lesions on either hemisphere may be more conspicuous to readers on FLAIR then smaller ones1010.
A challenging aspect to the imaging evaluation is presented by the presence of severe background disease or leukoariosis, where “normal” tissue for comparison cannot be easily selected. In such cases where acute, subacute, and chronic diseases are in question, the experience of the MRI reader is important as there are other MRI sequences such as the apparent diffusion coefficient (ADC) that can help distinguish hyperacute lesions. A limitation with qualitative FLAIR interpretation and reader measured SIR, is that this model is not highly sensitive or specific in identifying patients in the 0–4.5 hour time window. The findings in our study are consistent with recent published reports which show FLAIR- negative MRI is indicative of hyperacute ischemic stroke but limitations exist with dilemmas such as low interrater agreement7–9. Lesion volumes were quite small in the second study (median DWI volume = 1.5 ml) which can make FLAIR reads for hyperintensity difficult to interpret. After exclusion of very small lesions (<0.5ml), the sensitivity and specificity of FLAIR-negative scans improved9.
In 2009, the NIH Stroke Team saw 86 patients who were within 24 hours of last known well time and within 4.5 hours from stroke symptom onset. Of these, 53 patients were within the age range of 18 to 80 years specified by prior thrombolysis study inclusion criteria. Thus, approximately one patient per week met the time and age eligibility criteria for MR WITNESS, a multi-center clinical trial (MR Witness- http://clinicaltrials.gov/ct2/show/NCT01282242). In 2008 at Massachusetts General Hospital, 488 patients were screened for stroke in the emergency department. Of these patients, 22 reached the emergency department within 3 hours from when they were discovered with symptoms but beyond the current thrombolytic time window. Therefore, approximately 75 patients would have been potentially eligible for MR WITNESS between the two enrollment sites.
In conclusion, the FLAIR-SIR evaluation can help identify patients who are within 4.5 hours of symptom onset. Using SIR in conjunction with qualitative FLAIR reads can improve the precision of identifying stroke patients within 4.5 hours of onset. Further prospective studies to determine safety in treating patients using the MRI-based tissue clock is underway in a multi-center clinical trial (MR Witness- http://clinicaltrials.gov/ct2/show/NCT01282242). The MR WITNESS study will enroll subjects whose last seen normal time is within 24 hours and who present within 4.5 hours from time of symptom awareness for stroke evaluation and treatment.
Acknowledgments
Source of Funding: This research was supported by the Intramural Research Program of the NIH, NINDS
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Zivin JA. Acute stroke therapy with tissue plasminogen activator (tPA) since it was approved by the U.S. Food and Drug administration (FDA) Ann Neurol. 2009;66:6–10. doi: 10.1002/ana.21750. [DOI] [PubMed] [Google Scholar]
- 2.Allen NB, Myers D, Watanabe E, Dostal J, Sama D, Goldstein LB, et al. Utilization of intravenous tissue plasminogen activator for ischemic stroke: Are there sex differences? Cerebrovasc Dis. 2009;27:254–258. doi: 10.1159/000196824. [DOI] [PubMed] [Google Scholar]
- 3.Todo K, Moriwaki H, Saito K, Tanaka M, Oe H, Naritomi H. Early CT findings in unknown-onset and wake-up strokes. Cerebrovasc Dis. 2006;21:367–371. doi: 10.1159/000091545. [DOI] [PubMed] [Google Scholar]
- 4.Barreto AD, Martin-Schild S, Hallevi H, Morales MM, Abraham AT, Gonzales NR, et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke. 2009;40:827–832. doi: 10.1161/STROKEAHA.108.528034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams HP, Jr, Effron MB, Torner J, Davalos A, Frayne J, Teal P, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: Results of an international phase III trial: Abciximab in emergency treatment of stroke trial (AbESTT-II) Stroke. 2008;39:87–99. doi: 10.1161/STROKEAHA.106.476648. [DOI] [PubMed] [Google Scholar]
- 6.Adams HP, Jr, Leira EC, Torner JC, Barnathan E, Padgett L, Effron MB, et al. Treating patients with 'wake-up' stroke: The experience of the AbESTT-II trial. Stroke. 2008;39:3277–3282. doi: 10.1161/STROKEAHA.107.508853. [DOI] [PubMed] [Google Scholar]
- 7.Thomalla G, Rossbach P, Rosenkranz M, Siemonsen S, Krutzelmann A, Fiehler J, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65:724–732. doi: 10.1002/ana.21651. [DOI] [PubMed] [Google Scholar]
- 8.Petkova M, Rodrigo S, Lamy C, Oppenheim G, Touze E, Mas JL, et al. Mr imaging helps predict time from symptom onset in patients with acute stroke: Implications for patients with unknown onset time. Radiology. 2010;257:782–792. doi: 10.1148/radiol.10100461. [DOI] [PubMed] [Google Scholar]
- 9.Ebinger M, Fiebach JB. Fluid-attenuated inversion recovery evolution within 12 hours from stroke onset: A reliable tissue clock? Stroke. 2010;41:250–255. doi: 10.1161/STROKEAHA.109.568410. [DOI] [PubMed] [Google Scholar]
- 10.Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, Wu O, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (pre-FLAIR): A multicentre observational study. Lancet neurology. 2011;10:978–986. doi: 10.1016/S1474-4422(11)70192-2. [DOI] [PubMed] [Google Scholar]
- 11.Sterzi R, Bottini G, Celani MG, Righetti E, Lamassa M, Ricci S, et al. Hemianopia, hemianaesthesia, and hemiplegia after right and left hemisphere damage. A hemispheric difference. J Neurol Neurosurg Psychiatry. 1993;56:308–310. doi: 10.1136/jnnp.56.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3rd Ed. Hoboken, NJ: Wiley; 2003. [Google Scholar]
- 13.Fiebach J, Jansen O, Schellinger P, Knauth M, Hartmann M, Heiland S, et al. Comparison of CT with diffusion-weighted MRI in patients with hyperacute stroke. Neuroradiology. 2001;43:628–632. doi: 10.1007/s002340100542. [DOI] [PubMed] [Google Scholar]
- 14.Vierkant R. A SAS macro for calculating bootstrapped confidence intervals about a kappa coefficient. SAS users group international online proceedings. 2000 [Google Scholar]
- 15.Luby M, Bykowski JL, Schellinger PD, Merino JG, Warach S. Intra- and interrater reliability of ischemic lesion volume measurements on diffusion-weighted, mean transit time and fluid-attenuated inversion recovery MRI. Stroke. 2006;37:2951–2956. doi: 10.1161/01.STR.0000249416.77132.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallar G, Bottini G, Sterzi R, Passerini D, Rusconi ML. Hemianesthesia, sensory neglect, and defective access to conscious experience. Neurology. 1991;41:650–652. doi: 10.1212/wnl.41.5.650. [DOI] [PubMed] [Google Scholar]
- 17.Vallar G, Sandroni P, Rusconi ML, Barbieri S. Hemianopia, hemianesthesia, and spatial neglect: A study with evoked potentials. Neurology. 1991;41:1918–1922. doi: 10.1212/wnl.41.12.1918. [DOI] [PubMed] [Google Scholar]