Abstract
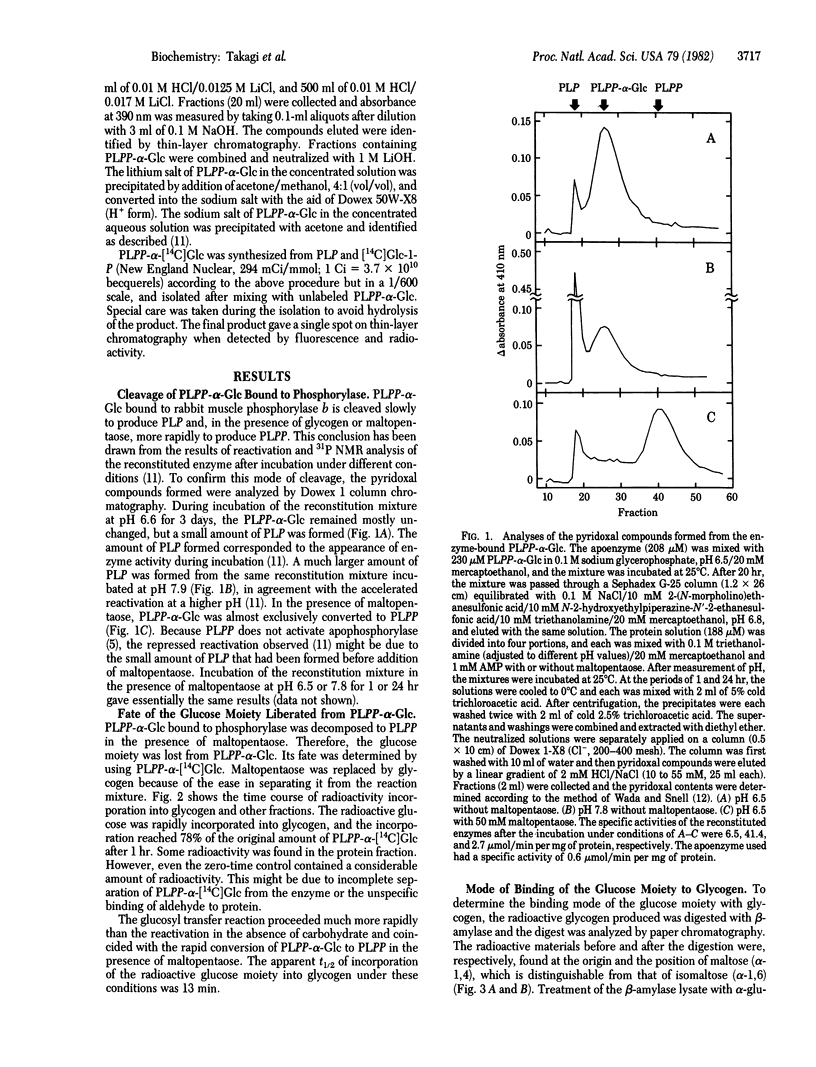
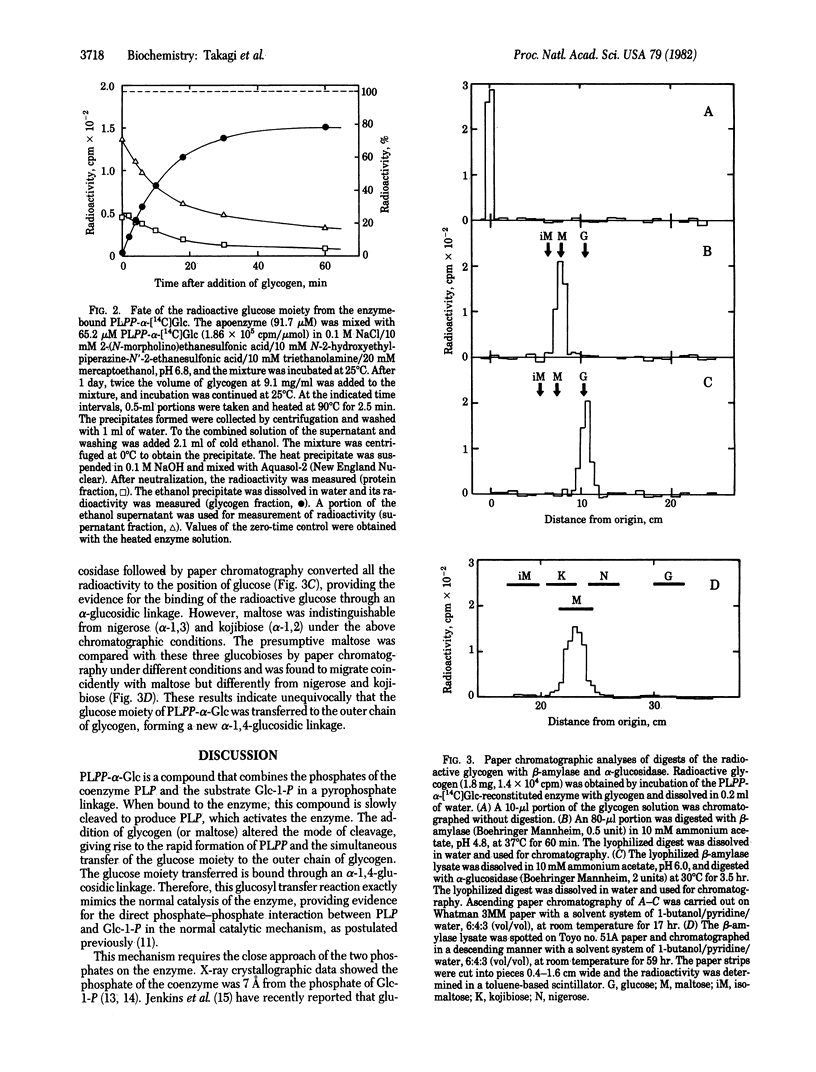
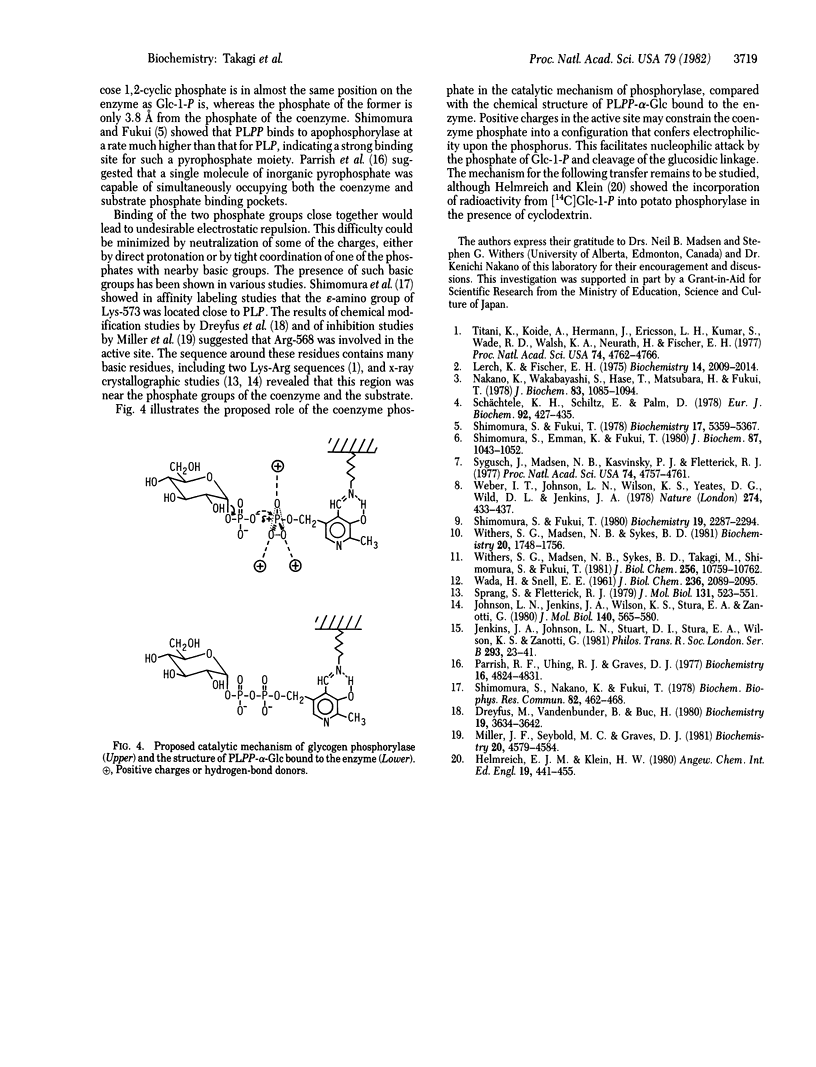
Pyridoxal(5')diphospho(1)-alpha-D-glucose was used to reconstitute glycogen phosphorylase beta (1,4-alpha-D-glucan:orthophosphate alpha-D-glucosyltransferase, EC 2.4.1.1) from rabbit muscle, replacing the natural pyridoxal 5'-phosphate coenzyme. Incubation of the reconstituted enzyme alone resulted in the gradual cleavage of the synthetic cofactor to pyridoxal 5'-phosphate, which caused slow reactivation of the enzyme. The addition of maltopentaose or glycogen altered the mode of cleavage; the cofactor was rapidly decomposed to pyridoxal 5'-diphosphate. The radioactive glucose moiety released from pyridoxal(5')diphospho(1)-alpha-D-[14C]glucose was incorporated into the outer chain of glycogen, forming an alpha-1,4-glucosidic linkage. These results show that the glucosyl transfer reaction discovered mimics the normal catalysis of this enzyme, and they strongly support the catalytic mechanism in which the coenzyme phosphate acts as a catalyst by direct interaction with the phosphate of the substrate, forming the pyrophosphate-like transition intermediate.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dreyfus M., Vandenbunder B., Buc H. Mechanism of allosteric activation of glycogen phosphorylase probed by the reactivity of essential arginyl residues. Physicochemical and kinetic studies. Biochemistry. 1980 Jul 22;19(15):3634–3642. doi: 10.1021/bi00556a033. [DOI] [PubMed] [Google Scholar]
- Helmreich E. J., Klein H. W. The role of pyridoxal phosphate in the catalysis of glycogen phosphorylases. Angew Chem Int Ed Engl. 1980;19(6):441–445. doi: 10.1002/anie.198004411. [DOI] [PubMed] [Google Scholar]
- Jenkins J. A., Johnson L. N., Stuart D. I., Stura E. A., Wilson K. S., Zanotti G. Phosphorylase: control and activity. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):23–41. doi: 10.1098/rstb.1981.0057. [DOI] [PubMed] [Google Scholar]
- Johnson L. N., Jenkins J. A., Wilson K. S., Stura E. A., Zanotti G. Proposals for the catalytic mechanism of glycogen phosphorylase beta prompted by crystallographic studies on glucose 1-phosphate binding. J Mol Biol. 1980 Jul 15;140(4):565–580. doi: 10.1016/0022-2836(80)90271-5. [DOI] [PubMed] [Google Scholar]
- Lerch K., Fischer E. H. Amino acid sequence of two functional sites in yeast glycogen phosphorylase. Biochemistry. 1975 May 6;14(9):2009–2014. doi: 10.1021/bi00680a031. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Seybold M. C., Graves D. J. Use of arginine compounds to examine the role of an essential arginine in the mechanism of glycogen phosphorylase. Biochemistry. 1981 Aug 4;20(16):4579–4584. doi: 10.1021/bi00519a010. [DOI] [PubMed] [Google Scholar]
- Nakano K., Wakabayashi S., Hase T., Matsubara H., Fukui T. Amino acid sequence around the pyridoxal 5'-phosphate binding site in potato phosphorylase. J Biochem. 1978 Apr;83(4):1085–1094. doi: 10.1093/oxfordjournals.jbchem.a131997. [DOI] [PubMed] [Google Scholar]
- Parrish R. F., Uhing R. J., Graves D. J. Effect of phosphate analogues on the activity of pyridoxal reconstituted glycogen phosphorylase. Biochemistry. 1977 Nov 1;16(22):4824–4831. doi: 10.1021/bi00641a011. [DOI] [PubMed] [Google Scholar]
- Schächtele K. H., Schiltz E., Palm D. Amino-acid sequence of the pyridoxal-phosphate-binding site in Escherichia coli maltodextrin phosphorylase. Eur J Biochem. 1978 Dec;92(2):427–435. doi: 10.1111/j.1432-1033.1978.tb12763.x. [DOI] [PubMed] [Google Scholar]
- Shimomura S., Emman K., Fukui T. The role of pyridoxal 5'-phosphate in plant phosphorylase. J Biochem. 1980 Apr;87(4):1043–1052. [PubMed] [Google Scholar]
- Shimomura S., Fukui T. A comparative study on alpha-glucan phosphorylases from plant and animal: interrelationship between the polysaccharide and pyridoxal phosphate binding sites by affinity electrophoresis. Biochemistry. 1980 May 27;19(11):2287–2294. doi: 10.1021/bi00552a001. [DOI] [PubMed] [Google Scholar]
- Shimomura S., Fukui T. Characterization of the pyridoxal phosphate site in glycogen phosphorylase b from rabbit muscle. Biochemistry. 1978 Dec 12;17(25):5359–5367. doi: 10.1021/bi00618a006. [DOI] [PubMed] [Google Scholar]
- Shimomura S., Nakano K., Fukui T. Affinity labeling of the cofactor site in glycogen phosphorylase b with a pyridoxal 5'-phosphate analog. Biochem Biophys Res Commun. 1978 May 30;82(2):462–468. doi: 10.1016/0006-291x(78)90897-5. [DOI] [PubMed] [Google Scholar]
- Sprang S., Fletterick R. J. The structure of glycogen phosphorylase alpha at 2.5 A resolution. J Mol Biol. 1979 Jul 5;131(3):523–551. doi: 10.1016/0022-2836(79)90006-8. [DOI] [PubMed] [Google Scholar]
- Sygusch J., Madsen N. B., Kasvinsky P. J., Fletterick R. J. Location of pyridoxal phosphate in glycogen phosphorylase a. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4757–4761. doi: 10.1073/pnas.74.11.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Koide A., Hermann J., Ericsson L. H., Kumar S., Wade R. D., Walsh K. A., Neurath H., Fischer E. H. Complete amino acid sequence of rabbit muscle glycogen phosphorylase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4762–4766. doi: 10.1073/pnas.74.11.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADA H., SNELL E. E. The enzymatic oxidation of pyridoxine and pyridoxamine phosphates. J Biol Chem. 1961 Jul;236:2089–2095. [PubMed] [Google Scholar]
- Weber I. T., Johnson L. N., Wilson K. S., Yeates D. G., Wild D. L., Jenkins J. A. Crystallographic studies on the activity of glycogen phosphorylase b. Nature. 1978 Aug 3;274(5670):433–437. doi: 10.1038/274433a0. [DOI] [PubMed] [Google Scholar]
- Withers S. G., Madsen N. B., Sykes B. D. Active form of pyridoxal phosphate in glycogen phosphorylase. Phosphorus-31 nuclear magentic resonance investigation. Biochemistry. 1981 Mar 31;20(7):1748–1756. doi: 10.1021/bi00510a007. [DOI] [PubMed] [Google Scholar]
- Withers S. G., Madsen N. B., Sykes B. D., Takagi M., Shimomura S., Fukui T. Evidence for direct phosphate-phosphate interaction between pyridoxal phosphate and substrate in the glycogen phosphorylase catalytic mechanism. J Biol Chem. 1981 Nov 10;256(21):10759–10762. [PubMed] [Google Scholar]



