Abstract
Recent advances in developmental genetics and human disease gene cloning have highlighted the essential roles played by cilia in developmental cell fate decisions, left–right asymmetry, and the pathology of human congenital disorders. Hedgehog signaling in sensory cilia illustrates the importance of trafficking receptors to the cilia membrane (Patched and Smoothened) and the concept of cilia ‘gatekeepers’ that restrict entry and egress of cilia proteins (Suppressor of fused: Gli complexes). Cilia-driven fluid flow in the embryonic node highlights the role of motile cilia in both generation and detection of mechanical signals in development. In this brief review I select examples of recent studies that have clarified and consolidated our understanding of the role of cilia in development.
Introduction
Apical cilia, once ignored as vestigial, are now recognized as essential cellular organelles in the regulation of development [1]. Cilia are microtubule based structures projecting from the apical surface of nearly all cell types [2]. Motile cilia are familiar as the driving force for bronchial mucus clearance and sperm motility while primary cilia are sensory and immotile. Both motile and sensory cilia are assembled and maintained by intra-flagellar transport (IFT), a microtubule motor based delivery of cilia component proteins to the growing tip (anterograde IFT) and retrieval of proteins to the cell body (retrograde IFT) [3]. Embryonic cells employ cilia to anchor membrane receptors and process incoming signals from morphogens (non-motile or sensory primary cilia) and to generate and respond to mechanical signals in the form of fluid flow and shear force in confined spaces (mechanosensory cilia, motile cilia) [1]. Even this simple distinction in cilia subtypes is breaking down since it is now appreciated that motile cilia also have sensory functions [4]. The wide spectrum of human pathologies, collectively termed ciliopathies, associated with mutations in genes required for cilia function includes Meckel-Gruber syndrome (MKS), nephronophthisis (NPHP), Bardet-Biedl syndrome (BBS), Joubert syndrome (JS), Senior-Løken syndrome, Leber congenital amaurosis (LCA), polycystic kidney disease (PKD), and oral–facial–digital syndrome (OFD) [5]. Most recently, Sensenbrenner Syndrome has been added to this growing list [6–8]. The overlap in clinical features of these syndromes, including cystic kidney, laterality defects, nervous system development defects, and retinal degeneration, along with the ever expanding number of gene mutations associated with ciliopathies highlights the complexity of cilia structure and the ubiquity of cilia function [5]. I concentrate here on advances made in the past two years that help clarify how cilia serve as embryonic signaling centers and new insights into the role of cilia generated fluid flow in morphogenesis.
Cilia as developmental signaling centers: recent advances in hedgehog signaling
By anchoring G-protein-coupled receptors in their membranes, primary cilia serve as a cellular ‘antenna’ for extracellular signaling molecules [9,10]. The best studied developmental signal linked to primary cilia is the Hedgehog (Hh) signaling system [11]. Genetic screens in mice lead to the unexpected finding that mutations in proteins required for cilia assembly, IFT proteins, disrupted neural tube patterning mediated by ventral sonic hedgehog signaling [12]. Subsequent studies implicated cilia IFT in Hedgehog signaling in endochondral bone formation [13]. Hedgehog signaling is surprisingly complex [11] and impossible to summarize in a brief review. Instead, I focus here on recent advances in our understanding of components of Hh signaling that highlight the unique functions of cilia as a ‘gated’ signaling center.
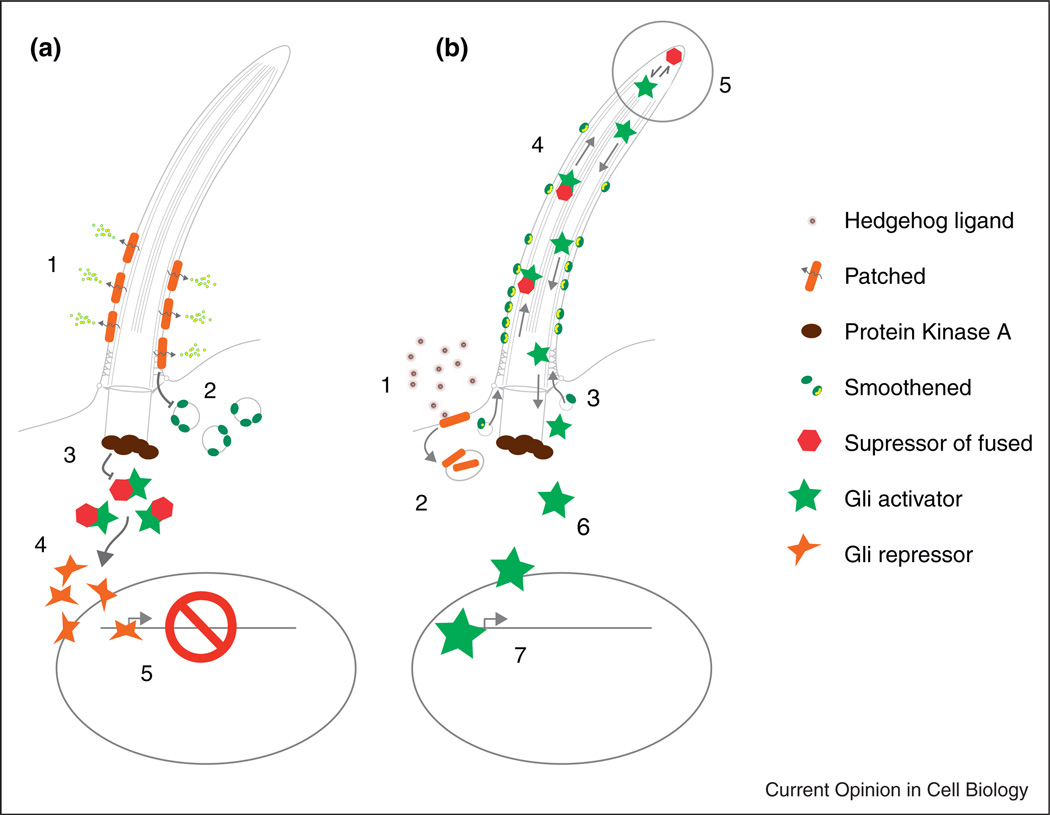
In brief, resting cells express the hedgehog receptor and sterol transporter Patched on membrane at the cilia base and axoneme (Figure 1) [14]. Once bound to Hedgehog ligand, Patched is removed from the cilia membrane and inhibition of the seven-transmembrane protein Smoothened (Smo) is relieved, allowing Smo to accumulate in the cilia membrane. Cilia-localized Smo promotes accumulation of Gli transcription factor effectors of hedgehog signaling (Gli2, Gli3) and their binding factor Suppressor of fused (Sufu), specifically at cilia tips [15]. Gli proteins exist in full-length activator forms (GliA) and proteolytically processed repressor forms (GliR) [16]. The output of hedgehog signaling is to shift the balance of Gli repressor to Gli activators and promote transcription of Hedgehog target genes. Cilia tip accumulation of Gli factors correlates with inhibition of Gli proteolytic processing and stabilization of activating forms of Gli proteins [15]. Thus an essential role of cilia is to regulate the ratio of GliA to GliR proteins to control transcription of hedgehog target genes.
Figure 1.
Recent advances in cilia-mediated Hedgehog signaling. For a complete overview of Hh signaling see Ref. [11]. (a) Resting cell state with Hh signaling inhibited. The Hedgehog receptor Patched acts as a sterol efflux transporter on the cilia membrane, restricting the amount of Smoothened seven transmembrane protein present on the cilium (2). Protein kinase A activity prevents access of Sufu:Gli complexes (3) to the cilium. Cytoplasmic processing of Gli to the Gli repressor form (4) and transit to the nucleus maintains Hh target genes in a repressed state (5). (b) Activation of Hh signaling in cilia by Hh ligand binding. (1) Hedgehog ligand binds Patched (1), promoting removal from the membrane and blocking its sterol efflux function (2). Sterol bound Smoothened accumulates in the cilia membrane (3), abrogates PKA inhibition of Sufu:Gli complex entry into the cilium, allowing transport of Sufu:Gli complexes to the cilia tip (4). Cilia tip accumulation of Sufu:Gli promotes dissociation of full-length activator Gli proteins by undefined mechanisms (5), allowing Gli activator egress from the cilia (6), transit to the nucleus (7) and activation of Hh target genes.
Clues as to how cilia regulate Gli protein activity have been provided by recent studies of the Gli interacting protein Suppressor of fused. Suppressor of fused (Sufu) is a Gli binding protein that inhibits Gli activity largely by sequestration in the cytoplasm [17–19]. Mutation in mammalian Sufu leads to ligand-independent activation of the Hh pathway, supporting the idea that Sufu acts as a repressor of Hh signaling [20]. The demonstration that deficiency of Sufu can lead to full activation of Hh signaling even in the absence of cilia (Ift88 mutant cells) indicated that a primary function of cilia is to remove Sufu inhibition of Gli activity [21••]. Recent biochemical studies support the idea that in resting cells, Sufu preferentially associates with full-length Gli3 and after proteolytic cleavage, GliR dissociates from the complex to represses Hh target genes [21••]. Activation of Hh signaling promotes dissociation of full-length Gli3 activator from Sufu, allowing subsequent Gli3 phosphorylation and activation of Hh target genes [21••]. Importantly, dissociation of Sufu:Gli3 complexes and Gli3A phosphorylation does not occur in cilia-deficient (Kif3a mutant) cells stimulated with SAG, a small molecule activator of smoothened [21••]. Thus a principal role of cilia in hedgehog signaling is to promote dissociation of inactive Sufu:Gli complexes, allowing the release of Gli activator proteins [23].
While many additional aspects of hedgehog signaling remain to be worked out, an intriguing question is how transit of Sufu:Gli complexes to cilia tips promotes Sufu:Gli dissociation. The cilia tip is the active zone of cilia growth; here, anterograde IFT raft cargo is released and re-assembled with other axonemal proteins to extend the cilium [24]; retrograde IFT then recycles transport proteins to the cilia base [25]. Cilia deficient in retrograde IFT accumulate proteins at the cilia tip resulting in bulbous tip membrane protrusions [26]. It will be interesting to see whether cilia tip associated mechanisms of Sufu:Gli dissociation have co-opted cellular machinery originally evolved for dissociating IFT raft cargo. It will also be important to resolve the somewhat puzzling role of retrograde IFT in Gli transport and processing since mutation in the retrograde IFTA motor Dync2h1 inhibits Hh signaling [27,28] while mutation in other retrograde IFTA proteins activates Hh signaling [26,29]. Clarification of these results is likely to come from modeling cilia association of Hh signaling proteins as the outcome of shifting the equilibrium toward cilia retention of proteins that are constantly shuttled in and out of the cilium [28], as opposed to a strictly gated process. For instance, the concept of cilia trafficking equilibria has recently been put forward to account for findings that hedgehog signaling deficits caused by the loss of the retrograde IFT A motor Dync2h1 can be compensated by reduction in the levels of anterograde IFT B proteins [30].
Gatekeepers of protein trafficking in the cilium
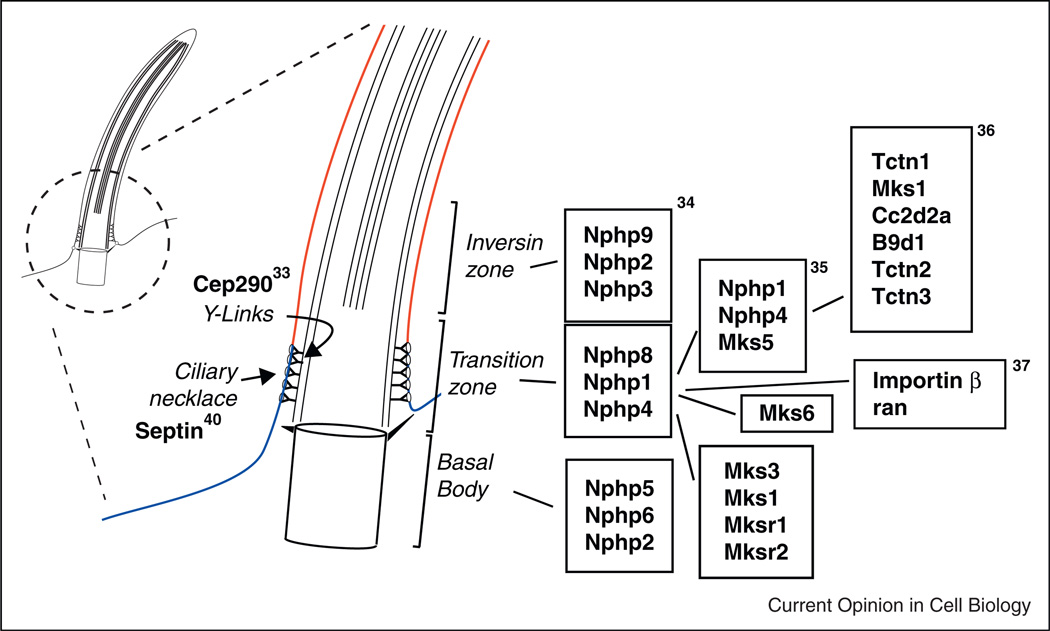
Control of both initiation of Hedgehog signaling and subsequent processing of Gli proteins is critically dependent on access of signaling receptors (Smo) and downstream effectors (Gli) to the ciliary compartment. For instance, Protein kinase A, a known inhibitor of hedgehog signaling, acts by preventing access of Sufu:Gli complexes to the cilia, thus preventing Sufu:Gli dissociation and Gli activation [23,31,32]. Access to the cilium is likely to be a general principle in cilia function affecting many cell-type-specific cilia signaling systems. The cilia transition zone, a short segment of the cilium just above the basal body, has recently been implicated as an essential ‘gatekeeper’ that limits or controls diffusion of membrane proteins in and out of the cilium (Figure 2) [33•]. This proposed function of the transition zone is correlated with the presence of ‘Y-links’, structures that link cilia microtubule doublets to the cilia membrane in position to restrict membrane protein diffusion or regulate IFT [33•]. The significance of the transition zone is highlighted by recent reports that a complex of proteins encoded by genes found to be mutated in syndromic ciliopathies (MKS/NPHP) function as a multiprotein complex in the cilia transition zone [34••] and act as a barrier to membrane protein diffusion [35••,36••]. Whether MKS/NPHP proteins are part of the Y-link structure or whether they perform a regulatory function in cilia trafficking remains to be determined.
Figure 2.
The ciliary gate. The ciliary necklace and transition zone lie just above the basal body and play important roles in limiting diffusion of cilia membrane proteins and regulating cilia protein traffic. Septin expression in the ciliary necklace demarcates the apical cell membrane (blue) from the cilia membrane (red). Other protein complexes contribute to Y-link structures (Cep290) and to regulated trafficking or retention of cilia proteins (Importin β/ran and complexes of NPHP/MKS proteins). Boxed proteins represent biochemically defined complexes identified in the noted references.
Another barrier or ‘gate’ controlling access to cilia is the so-called ‘ciliary necklace’, a ring of intramembrane proteins at the junction of the apical plasma membrane and the cilia base adjacent to the transition zone (Figure 2). Recent reports establish an unexpected function for components of the nuclear pore complex in transit of proteins past the ciliary necklace [37•,38]. The nuclear pore complex regulates traffic in and out of the nucleus [39]. Importin b and the small GTPase ran are key elements of this transport system that have now been shown to also function in regulating protein transit to the cilium via the ciliary necklace [37•,38]. Septin 2, a highly conserved protein responsible for maintaining distinct membrane domains in budding yeast, is also expressed in the ciliary necklace region and functions there as a barrier to membrane protein diffusion [40]. These observations indicate that multiple cilia membrane-associated barriers regulate trafficking of specific proteins to and from the cilium. In addition, specific apical membrane retention signals can restrict diffusion of proteins into the cilium [41]. Elucidation of the molecular nature of the transition zone and protein sorting signals should yield new insights into how cilia membrane composition is regulated and ultimately how cilia signaling is controlled.
Cilia membrane protein transport is mediated by cilia targeted coatamer-like proteins (the ‘BBSome’ [42]) and by the activity of multiple small GTPases including rab8, rab11, and rab23 [43,44]. The temporal sequence of rab8, rabin8, a rab8 guanine nucleotide exchange factor, and rab11 activities in ciliogenesis has recently been elegantly demonstrated by live cell imaging [45]. Further studies of other cilia-associated small GTPases including arf4, arl13b, arl2, arl3, arl6, rabl4/IFT27, and rabl5/IFT25 [46] are likely to identify molecular switches that regulate cilia gatekeeper functions, cilia membrane composition, and cilia structure [47,48].
Cilia, fluid flow, and development
Another major function of cilia in development is to generate fluid movement in confined embryonic spaces and to sense and interpret fluid shear force (mechanotransduction) as a developmental signal. The best studied example of this occurs in the embryonic node, or Kupffer’s vesicle (KV) in fish, where leftward fluid flow is harnessed as a developmental cue for breaking left–right symmetry. Seminal findings of left–right asymmetry disorders in human patients with immotile cilia syndromes [49] ultimately lead to the discovery of motile cilia and fluid flow in the mouse node [50], a transient embryonic midline structure. In the node, anterioRposterior polarity is transformed into left–right polarity by the action of planar polarity signaling on cilia basal body position and orientation (reviewed in [51]). Tipping the basal body to the posterior results in an asymmetric cilia beat stroke and a strong leftward flow and a weaker rightward return flow [52]. Asymmetric left-sided expression of Nodal is observed after the initiation of flow and artificial reversal of flow is sufficient to reverse situs [53]. So how might fluid flow be transduced into a morphogenetic signal? One view is the flow delivers lipid-encapsulated morphogens preferentially to the left side of the embryo [54]. However for this to work, released morphogens would have to be avidly removed from circulation on the left side since return rightward flow would randomize their distribution in the node. As an alternative, a compelling case for left-sided mechanotransduction by cilia can be made. This idea posits that both motile and sensory cilia exist in the node, with sensory cilia positioned at the edges of the node to transduce flow into an increase in intracellular calcium, ultimately signaling left-sided Nodal expression [55]. This model is supported by findings that calcium is elevated on the left side of the node [55] and knockdown or mutations in the cilia-associated calcium channel Polycystin2 (PKD2) result in randomized L/R asymmetry [56,57]. PKD2 is one of two genes mutated in Autosomal dominant polycystic kidney disease [58]. The other, PKD1, interacts with PKD2 to form active ion channels [59]. A somewhat puzzling finding was that PKD1 is not expressed in the node; so how do PKD2 proteins form an active channel? The answer recently reported by two groups is that a PKD1 ortholog, PKD1L1, is expressed in its place in the node and mutations in PKD1L1 lead to left–right asymmetry defects [60•,61•].
A surprising outcome of these studies is that PKD1L1 is expressed, at least in Medaka fish, on all KV cilia, including motile cilia [61•]. Can cilia be both motile and sensory? Precedent for this is seen in human airway cilia where bitter taste receptors are present on motile cilia and can signal changes in cilia beat rate [62]. Lung and oviduct motile cilia also respond to mechanical resistance and the viscosity of the medium by altering their beat rate, a homeostatic response that ensures continued medium propulsion (reviewed in [4]). In the node, motile cilia may encounter resistance to beating in areas of increased local flow and relay this mechanical signal by elevating cytoplasmic calcium. One further level of complexity in cilia mechanotransduction is that the calcium wave induced in ciliated epithelial cells by fluid flow is not directly propagated by cilium bending but rather by ATP secretion and stimulation of purinergic receptors on neighboring cells [63–65]. Since purinergic receptors are ubiquitous in embryos and signal via calcium release [66], it will be interesting to see whether they play a role in propagating node calcium signaling and establishment of L/R asymmetry.
In addition to signaling in the embryonic node, cilia-driven fluid flow impacts morphogenesis of the larval zebrafish kidney as well as development of the mammalian brain. In the zebrafish pronephros, motile cilia-driven fluid flow signals collective migration of epithelial tubule cells, resulting in convolution of the nephron and functional association with the vasculature [67]. In the brain, cerebrospinal fluid (CSF) is circulated by motile cilia and cilia defects cause fluid back pressure leading to hydrocephalus or swelling of the brain ventricles [68]. In addition, directional migration of neuroblasts from the subventricular zone to the olfactory bulb occurs in response to CSF flow and establishment of a concentration gradient of guidance molecules [69]. Also, in the developing central nervous system, spontaneous rhythmic firing of neurons is required to establish proper neuronal connectivity. A recent report presents provocative findings that rhythmic neuronal activity is dependent on fluid flow, in this case most likely providing a fluid shear mechanical signal that stimulates neural activity [70].
Conclusions
Cilia serve multiple roles in development and function in highly context dependent ways. Sensory cilia display a unique spectrum of receptors depending on the cell type and can impact development in diverse ways. Motile cilia exert long range, non-cell autonomous effects via generating fluid flow, and thereby guiding tissue morphogenesis under the control of mechanical signals. That cilia can be both motile and sensory offers new possibilities for interpreting cilia function. Now that proteomic analysis of cilia has identified over 1,000 cilia-associated proteins, we can expect many new insights on cilia gene function in the next few years. The field has moved beyond simple association of cilia gene mutations with pathology; our current and future goals should be to determine how different complexes of proteins function together to carry out specific cilia functions or build cilia substructures. To this end, biochemical protein interaction studies, analysis of compound genetic mutants, and high resolution electron microscopic structural analysis will all play essential roles in determining how cilia work. Finally, cilia are not static structures; they adapt to signals in their environment and alter their structure or activity in response [71•,72]. Future studies promise to integrate subcellular structure of the cilium with cell physiology, developmental patterning, and morphogenesis while revealing new aspects of the dynamic nature of these organelles.
Acknowledgements
The author gratefully acknowledges the contributions of Narendra Pathak and Danica Rili for editorial suggestions. This work was funded by the National Institututes of Health grant number DK053093 to IAD.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol. 2009;111:p39–p53. doi: 10.1159/000208212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisch C, Dupuis-Williams P. Ultrastructure of cilia and flagella — back to the future! Biol Cell. 2011;103:249–270. doi: 10.1042/BC20100139. [DOI] [PubMed] [Google Scholar]
- 3.Pazour GJ, Rosenbaum JL. Intraflagellar transport and ciliadependent diseases. Trends Cell Biol. 2002;12:551–555. doi: 10.1016/s0962-8924(02)02410-8. [DOI] [PubMed] [Google Scholar]
- 4.Bloodgood RA. Sensory reception is an attribute of both primary cilia and motile cilia. J Cell Sci. 2010;123:505–509. doi: 10.1242/jcs.066308. [DOI] [PubMed] [Google Scholar]
- 5.Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. 2011;26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arts HH, Bongers EM, Mans DA, van Beersum SE, Oud MM, Bolat E, Spruijt L, Cornelissen EA, Schuurs-Hoeijmakers JH, de Leeuw N, et al. C14ORF179 encoding IFT43 is mutated in Sensenbrenner syndrome. J Med Genet. 2011;48:390–395. doi: 10.1136/jmg.2011.088864. [DOI] [PubMed] [Google Scholar]
- 7.Walczak-Sztulpa J, Eggenschwiler J, Osborn D, Brown DA, Emma F, Klingenberg C, Hennekam RC, Torre G, Garshasbi M, Tzschach A, et al. Cranioectodermal Dysplasia. Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet. 2010;86:949–956. doi: 10.1016/j.ajhg.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bredrup C, Saunier S, Oud MM, Fiskerstrand T, Hoischen A, Brackman D, Leh SM, Midtbo M, Filhol E, Bole-Feysot C, et al. Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A Gene WDR19. Am J Hum Genet. 2011;89:634–643. doi: 10.1016/j.ajhg.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domire JS, Green JA, Lee KG, Johnson AD, Askwith CC, Mykytyn K. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell Mol Life Sci. 2011;68:2951–2960. doi: 10.1007/s00018-010-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet- Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 13.Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- 14.Bidet M, Joubert O, Lacombe B, Ciantar M, Nehme R, Mollat P, Bretillon L, Faure H, Bittman R, Ruat M, et al. The hedgehog receptor patched is involved in cholesterol transport. PLoS ONE. 2011;6:e23834. doi: 10.1371/journal.pone.0023834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 17.Dunaeva M, Michelson P, Kogerman P, Toftgard R. Characterization of the physical interaction of Gli proteins with SUFU proteins. J Biol Chem. 2003;278:5116–5122. doi: 10.1074/jbc.M209492200. [DOI] [PubMed] [Google Scholar]
- 18.Pearse RV, 2nd, Collier LS, Scott MP, Tabin CJ. Vertebrate homologs of Drosophila suppressor of fused interact with the gli family of transcriptional regulators. Dev Biol. 1999;212:323–336. doi: 10.1006/dbio.1999.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding Q, Fukami S, Meng X, Nishizaki Y, Zhang X, Sasaki H, Dlugosz A, Nakafuku M, Hui C. Mouse suppressor of fused is a negative regulator of sonic hedgehog signaling and alters the subcellular distribution of Gli1. Curr Biol. 1999;9:1119–1122. doi: 10.1016/s0960-9822(99)80482-5. [DOI] [PubMed] [Google Scholar]
- 20.Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, Ericson J, Toftgard R, Teglund S. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 21. Jia J, Kolterud A, Zeng H, Hoover A, Teglund S, Toftgard R, Liu A. Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia. Dev Biol. 2009;330:452–460. doi: 10.1016/j.ydbio.2009.04.009.. Using Ift88 mutant cells, the authors show that cilia signaling can occur in cells lacking cilia if Suppressor of fused is also mutated. This work points to a key step in cilia-mediated hedgehog signaling: removal of Sufu repression of Gli.
- 22. Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev. 2010;24:670–682. doi: 10.1101/gad.1902910.. An in depth dissection of the role of Suppressor of fused in Gli processing, stability, and phosphorylation.
- 23.Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu–Gli protein complexes. J Cell Biol. 2010;191:415–428. doi: 10.1083/jcb.201004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen LB, Miller MS, Geimer S, Leitch JM, Rosenbaum JL, Cole DG. Chlamydomonas IFT172 is encoded by FLA11, interacts with CrEB1, and regulates IFT at the flagellar tip. Curr Biol. 2005;15:262–266. doi: 10.1016/j.cub.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 25.Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehogdependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci U S A. 2009;106:21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin J, Lin Y, Norman RX, Ko HW, Eggenschwiler JT. Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components. Proc Natl Acad Sci U S A. 2011;108:1456–1461. doi: 10.1073/pnas.1011410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ocbina PJ, Eggenschwiler JT, Moskowitz I, Anderson KV. Complex interactions between genes controlling trafficking in primary cilia. Nat Genet. 2011;43:547–553. doi: 10.1038/ng.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuson M, He M, Anderson KV. Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube. Development. 2011;138:4921–4930. doi: 10.1242/dev.070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng H, Jia J, Liu A. Coordinated translocation of mammalian Gli proteins and suppressor of fused to the primary cilium. PLoS ONE. 2010;5:e15900. doi: 10.1371/journal.pone.0015900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105.. Using a Chlamydomonas mutant in the ciliopathy gene Cep290, the authors show that cilia membrane protein is randomized, pointing to a central role for NPHP/MKS/BBS proteins in regulating cilia membrane protein trafficking.
- 34. Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, et al. Mapping the NPHP–JBTS–MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019.. Using comprehensive biochemical and genetic approaches, this work demonstrated that NPHP–JBTS–MKS proteins can be be clustered into three biochemically and functionally distinct modules localized to cilia substructures.
- 35. Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192:1023–1041. doi: 10.1083/jcb.201012116.. An indepth study of C. elegans NPHP/MKS proteins revealed a consistent transition zone localization of these proteins, biochemical interactions between different complex members, and genetic interactions that defined their role in regulating cilia structure.
- 36. Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet. 2011;43:776–784. doi: 10.1038/ng.891.. This paper and [34••,35••] defined the function of syndromic ciliopathy proteins as regulators of cilia transition zone function.
- 37. Dishinger JF, Kee HL, Jenkins PM, Fan S, Hurd TW, Hammond JW, Truong YN, Margolis B, Martens JR, Verhey KJ. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat Cell Biol. 2010;12:703–710. doi: 10.1038/ncb2073.. An unexpected and novel relationship is defined between mechanisms of nuclear protein trafficking and cilia protein trafficking.
- 38.Hurd TW, Fan S, Margolis BL. Localization of retinitis pigmentosa 2 to cilia is regulated by Importin beta2. J Cell Sci. 2011;124:718–726. doi: 10.1242/jcs.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8:195–208. doi: 10.1038/nrm2114. [DOI] [PubMed] [Google Scholar]
- 40.Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francis SS, Sfakianos J, Lo B, Mellman I. A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J Cell Biol. 2011;193:219–233. doi: 10.1083/jcb.201009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 43.Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eggenschwiler JT, Bulgakov OV, Qin J, Li T, Anderson KV. Mouse Rab23 regulates hedgehog signaling from smoothened to Gli proteins. Dev Biol. 2006;290:1–12. doi: 10.1016/j.ydbio.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 45.Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. 2011;108:2759–2764. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Hu J. Small GTPases and cilia. Protein Cell. 2011;2:13–25. doi: 10.1007/s13238-011-1004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol. 2010;189:1039–1051. doi: 10.1083/jcb.200912001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Afzelius BA. Genetical and ultrastructural aspects of the immotile-cilia syndrome. Am J Hum Genet. 1981;33:852–864. [PMC free article] [PubMed] [Google Scholar]
- 50.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left–right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 51.Hashimoto M, Hamada H. Translation of anterior–posterior polarity into left–right polarity in the mouse embryo. Curr Opin Genet Dev. 2010;20:433–437. doi: 10.1016/j.gde.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. De novo formation of left–right asymmetry by posterior tilt of nodal cilia. PLoS Biol. 2005;3:e268. doi: 10.1371/journal.pbio.0030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left–right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418:96–99. doi: 10.1038/nature00849. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left–right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- 55.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left–right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 56.Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B. The ion channel polycystin-2 is required for left–right axis determination in mice. Curr Biol. 2002;12:938–943. doi: 10.1016/s0960-9822(02)00869-2. [DOI] [PubMed] [Google Scholar]
- 57.Obara T, Mangos S, Liu Y, Zhao J, Wiessner S, Kramer-Zucker AG, Olale F, Schier AF, Drummond IA. Polycystin-2 immunolocalization and function in zebrafish. J Am Soc Nephrol. 2006;17:2706–2718. doi: 10.1681/ASN.2006040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 59.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 60. Field S, Riley KL, Grimes DT, Hilton H, Simon M, Powles-Glover N, Siggers P, Bogani D, Greenfield A, Norris DP. Pkd1l1 establishes left–right asymmetry and physically interacts with Pkd2. Development. 2011;138:1131–1142. doi: 10.1242/dev.058149.. Using a genetic approach, this work resolves a mystery about the function of polycystin ion channel complexes in mechanotransduction of nodal fluid flow by identifying PKD1L1 as a key element in generating left–right asymmetry.
- 61. Kamura K, Kobayashi D, Uehara Y, Koshida S, Iijima N, Kudo A, Yokoyama T, Takeda H. Pkd1l1 complexes with Pkd2 on motile cilia and functions to establish the left–right axis. Development. 2011;138:1121–1129. doi: 10.1242/dev.058271.. Published along with Ref [60•], this work arrives at similar findings from a completely different angle: positional cloning a Medaka fish mutant. The authors also present the provocative finding that the proposed Pkd2:PKD1L1 sensory ion channel is present exclusively on motile cilia, challenging the idea that the only non-motile ciila can be sensory and offering chemosensory or mechanosensory roles for motile cilia.
- 62.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hovater MB, Olteanu D, Hanson EL, Cheng N-L, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, et al. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Puriner Signal. 2007;4:155–170. doi: 10.1007/s11302-007-9072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL. Attenuated, flow-induced ATP release contributes to absence of flowsensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol. 2009;296:F1464–F1476. doi: 10.1152/ajprenal.90542.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Praetorius HA, Leipziger J. Released nucleotides amplify the cilium-dependent, flow-induced [Ca2+]i response in MDCK cells. Acta Physiol. 2009;197:241–251. doi: 10.1111/j.1748-1716.2009.02002.x. [DOI] [PubMed] [Google Scholar]
- 66.Burnstock G, Ulrich H. Purinergic signaling in embryonic and stem cell development. Cell Mol Life Sci. 2011;68:1369–1394. doi: 10.1007/s00018-010-0614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vasilyev A, Liu Y, Mudumana S, Mangos S, Lam PY, Majumdar A, Zhao J, Poon KL, Kondrychyn I, Korzh V, et al. Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 2009;7:e9. doi: 10.1371/journal.pbio.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ibanez-Tallon I, Pagenstecher A, Fliegauf M, Olbrich H, Kispert A, Ketelsen UP, North A, Heintz N, Omran H. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum Mol Genet. 2004;13:2133–2141. doi: 10.1093/hmg/ddh219. [DOI] [PubMed] [Google Scholar]
- 69.Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 70.Yvert B, Mazzocco C, Joucla S, Langla A, Meyrand P. Artificial CSF motion ensures rhythmic activity in the developing CNS ex vivo: a mechanical source of rhythmogenesis? J Neurosci. 2011;31:8832–8840. doi: 10.1523/JNEUROSCI.1354-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hellman NE, Liu Y, Merkel E, Austin C, Le Corre S, Beier DR, Sun Z, Sharma N, Yoder BK, Drummond IA. The zebrafish foxj1a transcription factor regulates cilia function in response to injury and epithelial stretch. Proc Natl Acad Sci U S A. 2010;107:18499–18504. doi: 10.1073/pnas.1005998107.. This work demonstrates that signals in injured cells rapidly stimulate a transcriptional cascade initiated by foxj1 that controls motile ciliogenesis, highlighting a novel role for cilia dynamics in injury and regeneration.
- 72.Mukhopadhyay S, Lu Y, Shaham S, Sengupta P. Sensory signaling-dependent remodeling of olfactory cilia architecture in C. elegans. Dev Cell. 2008;14:762–774. doi: 10.1016/j.devcel.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]