Abstract
The evolutionarily conserved, non-coding ~800 base-pair zone of polarizing activity (ZPA) regulatory sequence (ZRS) controls Shh expression in the posterior limb. We report that the chicken mutant oligozeugodactly (ozd), which lacks limb Shh expression, has a large deletion within the ZRS. Furthermore, the preaxial polydactylous, Silkie Breed chicken, which develops ectopic anterior limb Shh expression, has a single base-pair change within the ZRS. Using an in vivo reporter assay to examine enhancer function in the chick limb, we demonstrate that the wild-type ZRS drives β-galactosidase reporter expression in the ZPA of both wild-type and ozd limbs. The Silkie ZRS drives β-galactosidase in both posterior and anterior Shh domains in wild-type limb buds. These results support the hypothesis that the ZRS integrates positive and negative prepatterned regulatory inputs in the chicken model system and demonstrate the utility of the chicken limb as an efficient genetic system for gene regulatory studies.
Keywords: limb development, limb bud prepattern, Sonic hedgehog (SHH), LMBR1, oligozeugodactyly (ozd), zone of polarizing activity (ZPA), cis-enhancer, reporter analysis, ZPA regulatory sequence (ZRS), mammals-fishes-conserved-sequence-1 (MFCS-1), Acheiropodia (ACHP), preaxial polydactyly (PPD), Dorking Breed chicken, Silkie Breed chicken
INTRODUCTION
Patterning along the anteroposterior (A/P) axis of the developing vertebrate limb is controlled by asymmetric expression of the Sonic hedgehog (Shh) gene (reviewed in Towers and Tickle, 2009; Zeller et al., 2009). Shh expression is restricted to a specific region of mesenchyme along the border of the posterior limb bud, corresponding to the zone of polarizing activity (ZPA). Misregulation of Shh expression in the limb bud occurs and results in preaxial polydactyly (PPD) (Chan et al., 1995; Masuya et al., 1995; Masuya et al., 1997; Sharpe et al., 1999), or other abnormalities (Krebs et al., 2003). The genetic characterization of animal models with limb defects has permitted an understanding of the underlying mechanisms surrounding some of these disorders, as well as insights into the molecular mechanisms of normal limb development.
A highly conserved long-range limb-specific Shh enhancer is present within intron 5 of the LMBR1 gene, located approximately 1 megabase upstream of the Shh gene (Lettice et al., 2002; Lettice et al., 2003; Sagai et al., 2004). This enhancer is called the ZPA regulatory sequence (ZRS) (Lettice et al., 2003) and is also known as mammals-fishes-conserved-sequence-1 (MFCS-1) (Sagai et al., 2005). The ZRS is syntenic with the Shh locus in all tetrapod species, teleost fish (Sagai et al., 2004), and cartilaginous fish (sharks, skates and rays) (Dahn et al., 2007) that have been analyzed, suggesting an evolutionarily conserved mechanism for limb and fin patterning along the A/P axis. Targeted removal of the ZRS sequence in mouse (Sagai et al., 2005) leads to limb-specific defects indistinguishable from those in the Shh−/− mouse (Chiang et al., 2001; Kraus et al., 2001) and demonstrates that the ZRS provides positive regulatory input for Shh expression in the limb.
There is an increasing number of reports of human families, mouse mutants and other mammals with preaxial polydactyly (PPD), or ectopic anterior digits, that have single nucleotide changes scattered throughout the conserved ZRS region (Lettice et al., 2002; Lettice et al., 2003; Sagai et al., 2004). In some PPD mouse models, Shh is expressed in an ectopic anterior domain, in addition to the posterior domain (Chan, et al., 1995; Masuya et al., 1995; Masuya et al., 1997; Sharpe et al., 1999). Experimentally induced anterior Shh expression results in the development of PPD in both chick and mouse (Riddle et al., 1993; Lopez-Martinez et al., 1995; Liu et al., 1998). It is proposed that the ZRS integrates regulatory inputs necessary for proper localization of Shh expression in the ZPA (Lettice et al., 2002; Lettice et al., 2003; Sagai et al., 2004; Maas and Fallon, 2005; Sagai et al., 2005). The ZRS is sufficient to drive reporter gene expression in the ZPA in the mouse limb (e.g., Lettice et al., 2003; Maas and Fallon, 2005). Extensive experiments using PPD-ZRS-LacZ mouse transgenic reporter assays (Lettice et al., 2008) show that the ZRS point mutations in human, mouse and cat drive anterior and posterior limb bud reporter expression in the mouse, similar to Shh expression in PPD mutant limb buds. This has been interpreted to mean the wide spread single base pair mutations in the ZRS produce a gain of function in the PPD-ZRS enhancer sequence (Lettice et al., 2008).
Although there are many reported cases of PPD in animal models and human families, examples of limb-specific Shh loss-of-function are rare. Acheiropodia (ACHP) is a recessive human disorder where affected individuals have distally truncated limbs but no other apparent defects in the body (Ianakiev et al., 2001). The mutation for ACHP has been mapped to a deletion of 4–6 kilobases in the LMBR1 gene, which removes exon 4 and several kilobases of flanking intronic sequence, distinct from the limb-specific Shh enhancer in intron 5 (Ianakiev et al., 2001). In the chicken mutant oligozeugodactyly (ozd), Shh is never expressed in the developing limbs, but is expressed normally elsewhere in the body, leading to limb-specific defects reminiscent of those found in ACHP (Ros et al., 2003). This phenotype is similar to limbs of the Shh−/− mouse (Chiang et al., 2001; Kraus et al., 2001). In ozd, these limb-specific defects include the absence of the ulna and all digits in the wing, and the absence of the fibula and all digits but digit 1 in the foot. However, unlike ACHP and the Shh−/− mouse, the bones that develop in the ozd limbs are morphologically normal. We previously showed that the ozd mutation is linked to the LMBR1-Shh genomic region, and that the open reading frame of LMBR1 and intronic regions corresponding to the ACHP deletion are normal in the ozd mutant (Maas and Fallon, 2004).
Here we report that the spontaneously occurring chicken ozd mutant contains a large deletion in LMBR1-intron 5, eliminating most of the limb-specific enhancer conserved sequence. In addition, we have developed and utilized a powerful reporter assay designed to analyze enhancer function in the vascularized mesenchyme of the developing limb bud. This assay allows localization of the β-galactosidase (β-gal) activity in the Shh domain of the chicken limb bud, representing the first reported enhancer analysis in a vascularized mesenchyme undergoing dynamic growth in the chick embryo. We further extended the potential of this reporter assay, using the ozd mutant to show that the early limb bud is prepatterned with Shh competence and that a distinct cohort of pre-ZPA cells exists in the early limb bud and this competence does not require Shh expression. Lastly, we report that the spontaneously occurring Silkie Breed and Polydactyly (Po) chicken PPD mutants have an identical single base pair change within the limb-specific Shh enhancer. Introduction of this point mutation into the reporter assay construct is sufficient to drive β-gal activity in both posterior and anterior domains in normal limb buds. This provides strong support for the hypothesis that the nucleotide change is the genetic mechanism for polydactyly in these mutants and also suggests negative regulatory functions for the ZRS.
RESULTS
Analysis of the conserved ZRS in the chicken ozd mutant
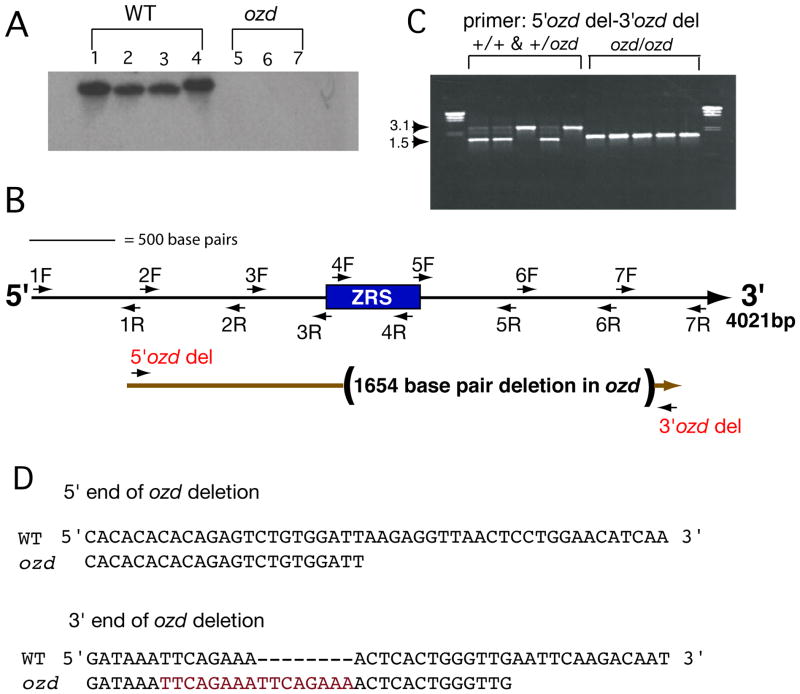
Initially we focused our attention on the conserved ZRS in the ozd chicken mutant because ozd mutants lack Shh expression specifically in the limb bud (Ros et al., 2003) and the LMBR1, intron 3 segregates with the ozd phenotype (Maas and Fallon, 2004). We attempted to amplify a small 477 base pair region from the ZRS from ozd mutants and phenotypically normal chickens from the ozd flock, corresponding to the region reported in Sagai et al., 2004. However, PCR-product was not obtained in any ozd sample, even using several different primer combinations, indicating that the ozd mutation likely had a deletion within the conserved region in LMBR1, intron 5. Southern hybridization was performed to determine whether the ZRS was present in ozd using the 477 base pair sequence as a probe. Although a strong band was detectable from all phenotypically normal samples, this band was not detectable in ozd mutant embryos, indicating that most, or all, of this region is deleted in ozd (Figure 1A).
Figure 1. The chicken oligozeugodactyly (ozd) mutant contains a deletion of the ZRS.
(A) Genomic DNA from phenotypically normal (normal, +/+, and carriers, +/ozd, lanes 1–4) or known ozd mutants (lanes 5–7) was digested with SalI and BamHI. The resulting blot was probed with a 477 base pair ZRS fragment. A distinct band is present in all of the phenotypically normal lanes, but not in any ozd lane. (B) A BAC clone containing the conserved 477 base pair intron 5 region (rectangle) was obtained and ~4 kilobases of intron 5 was sequenced. Approximate locations of primers are shown as arrows, where the direction of the arrowhead depicts whether the primer was used to sequence (forward primers- arrows facing away from conserved region) or to amplify a PCR product from ozd samples (reverse primers- arrows facing towards conserved region). Primer pairs are numbered. The mutation in ozd was determined to be a 1654 base pair deletion within LMBR1 intron 5, deleting all but the first 135 base pairs of the ZRS and several hundred base pairs of intronic sequence (shown in parentheses). (C) 5′ozd del and 3′ozd del primers were used to amplify fragments from normal, heterozygous, and ozd genomic DNA samples. The normal band is ~3.1 kilobases and the mutant band is ~1.5 kilobases, for further explanation, see Results section of text. (D) Shown here are the 5′ and 3′ breakpoints in ozd mutants. Note the duplicated octamer (shown in red) near the 3′ breakpoint of the ozd deletion.
To determine the exact sequence that was deleted in ozd mutants, we isolated a BAC clone containing the wild type LMBR1, intron 5-region by screening high-density replica filters from a chicken BAC library. Primers were designed, 1F to 7R (Figure 1B), within the conserved ZRS and used to sequence in both forward and reverse directions (e.g. 1F and 1R, 2F and 2R, and so on, Figure 1B). Sequence analysis of the PCR product obtained in ozd mutant samples using these primers showed that the ozd mutant contains a deletion of 1654 base pairs within LMBR1, intron 5 (Figure 1B) that eliminates all but the first 135 base pairs of the ~800 base pair conserved ZRS sequence reported in Lettice et al., 2003, as well as several hundred base pairs of nonconserved intronic sequence (Figure 1D). When we used 5′ozd del and 3′ozd del primers (Figure 1B), we observed ~3.1kb non-carrier band and ~1.5kb ozd carrier band (Figure 1C). The same PCR primers were used to generate both the large (wild type or +/+) and small (ozd or −/−) PCR fragment in ozd or phenotypically normal (+/+ or +/−) DNA samples. The reaction conditions were identical, but because the small fragment was more robustly amplified than the larger fragment, the small fragment appears brighter than the large fragment (Figure 1C), even though they are present in equimolar amounts; this is not a quantitative assay. Near the 3′ breakpoint (Figure 1D), the ozd sequence contains a duplicated octamer that is not present in normal White Leghorn or Brown Leghorn chickens (current carrier and original ozd strains, respectively). The sequence does not correspond to a known transcription factor binding-site; the significance of the duplication is unknown.
Development of an in vivo reporter assay to analyze ZRS function in the chicken limb
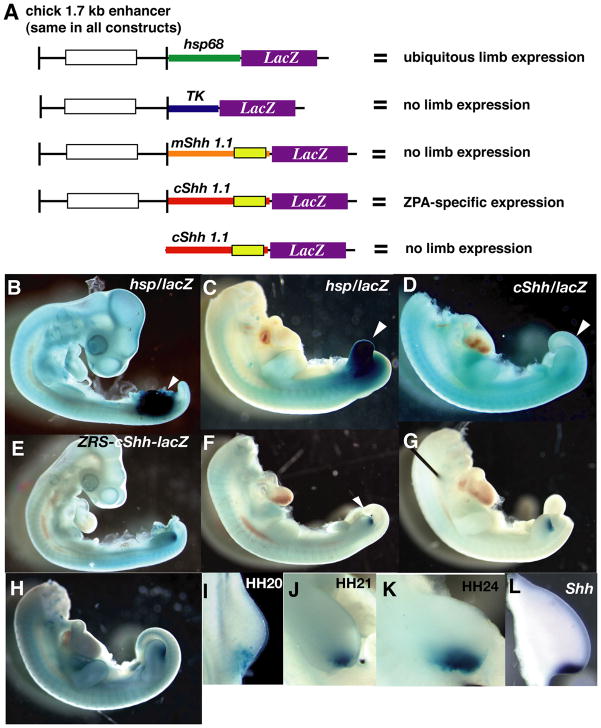
To rapidly and accurately study ZRS function, we developed an in vivo reporter assay using electroporation in the chicken limb field. Although enhancer analysis has been used successfully in the chicken avascular neural tube (Uchikawa et al., 2003), to our knowledge, the results described below represent the first reported enhancer analysis in a chicken vascularized mesenchyme. We cloned a 1.7 kilobase region of LMBR1, intron 5 containing the ZRS sequence, using genomic DNA from the progeny of Hyline White Leghorn × New Hampshire Red chicken cross (Figure 2A). Although only the core enhancer region is homologous between mouse and chicken, we chose to use the 1.7-kilobase fragment since it correlated with the fragment used successfully in previous mouse reporter analyses (Lettice et al., 2003; Maas and Fallon, 2005). The enhancer fragment was first tested in the HSP68-lacZ reporter construct used in mouse reporter gene experiments (Maas and Fallon, 2005). However, in chick, the HSP68 promoter in this construct was induced upon electroporation, causing widespread β-galactosidase (β-gal) activity at all stages analyzed (n=26/33; Figure 2B-St. 20; Figure 2C- St. 24). Therefore, this construct was used as a control to assess the extent of electroporated limb tissue expected to occur with other constructs in subsequent experiments.
Figure 2. An in vivo reporter assay analyzing the ZRS function in the chicken limb drives reporter gene expression in the ZPA.
(A) A summary of constructs tested in reporter assay. Reporter constructs containing a 1.7 kilobase fragment containing the conserved ZRS (conserved 800 base pair region represented by white rectangle) upstream of either the HSP68 (~800 base pairs), thymidine kinase (TK- ~500 base pairs), mouse minimal Shh (~1.1 kilobase), or chicken minimal Shh (~1.1 kilobase) promoters and lacZ, as well as a control construct containing the chicken minimal Shh promoter and lacZ but no enhancer sequence, were tested in an in vivo reporter assay in the chicken. The yellow rectangle in the mShh and cShh promoters represents a region of high homology in both promoters. Of these constructs, only the ZRS-cShh-lacZ construct drives reporter gene expression in the ZPA. (B, C) The HSP68-lacZ construct reproducibly drives lacZ expression throughout the entire limb bud at all stages analyzed (arrowheads point to experimental limb), including early (B, St. 20) and later (C, St. 24) stages. (D) No β-galactosidase was detectable in limbs electroporated with a control cShh-lacZ construct lacking the ZRS (n=9/9). (E-K) A high percentage of embryos electroporated with the ZRS-cShh-lacZ construct showed β-gal activity in the limb (n=43/59), and in all positively staining embryos, β-gal activity is restricted to the posterior limb in the endogenous Shh ZPA expression domain at all stages (St.) analyzed, including St. 20 (E, I), St. 21 (F, J), St. 22 (G), St. 26 (H, K). Also shown is Shh mRNA expression for comparison (L). In I-L, posterior is on the bottom.
Epstein et al. (1999) showed that a 1.1 kilobase fragment of the mouse Shh promoter drives reporter gene expression in regions of the central nervous system where Shh normally is expressed only when combined with neural specific enhancers. We inserted the 1.7 kilobase chicken sequence containing the ZRS into the mouse (m) SHH promoter/lacZ construct (ZRS-mShh-lacZ: Figure 2A). However, β-gal activity was not detected in the chicken limb upon electroporation of this construct.
We hypothesized that species-specific promoters may be necessary for regulatory control analysis in the chick limb bud, and replaced the 1.1 kilobase mouse Shh promoter fragment with a 1.1 kilobase region of the chicken (c) Shh promoter (ZRS-cShh-lacZ: Figure 2A). Embryos electroporated with the ZRS-cShh-lacZ construct showed β-gal staining in the ZPA region of the experimental limb through stage 24 (n=43/59; Figures 2E-K). The control cShh-lacZ construct, without the ZRS enhancer (Figure 2A) did not show β-gal activity (n=9/9, Figure 2D). Taken together these data raise the possibility that, contrary to results in the chick neural tube where the TK promoter was used successfully to drive Sox2 expression, (Uchikawa et al., 2003), species-specific promoters may be necessary in reporter assays in the chick limb bud vascularized mesenchyme. Our results demonstrate that enhancer analysis can be performed effectively in the vascularized mesenchyme of the chicken limb bud.
A cohort of ZPA-competent cells is present in the ozd mutant
Next, we extended our reporter analysis to examine whether β-gal activity could be detected in ozd mutant limbs buds, which lack most of the conserved ZRS sequence. It has been proposed that the early limb bud is prepatterned to establish a domain of Shh competence (Ros et al., 1996; Chiang et al., 2001;te Welscher et al., 2002; Zákány, 2004). The factors necessary for asymmetric Shh expression include both activators, such as HAND2, TBX3, and 5′ HOXD genes, and repressors, GLI3 and ALX4 (see for example: te Welscher, 2002; Zákány et al., 2004). The activator genes are initially expressed in ozd limb buds, but later are down regulated (Ros et al., 2003). Therefore, we reasoned that if these transcription factors, proposed to prepattern the limb bud, act through the ZRS to establish a domain of Shh expression then β-gal activity would be detectable in this cellular cohort in the ozd limb bud. Embryos from the ozd flock were electroporated with the ZRS-cShh-lacZ reporter construct. Experimental embryos were harvested from St. 19/20 to St. 26 and processed for β-gal staining.
We found that reporter activity is present in posterior ozd limb bud mesoderm at all stages analyzed, indicating that ZRS sequence is sufficient to initiate β-gal expression in the ozd mutant in cells where Shh would be expressed in wild type limb buds. At early stages of limb development, when the ozd limb is indistinguishable from normal limbs, reporter activity is detectable in the same region in all chick limbs from matings of heterozygote carrier parents, coinciding with the Shh expression domain (data not shown). Based on our examination of thousands of ozd heterozygous matings (Ros et al., 2003), 25% of these embryos would be homozygous for the ozd mutation, but indistinguishable from wild type in the reporter assay used.
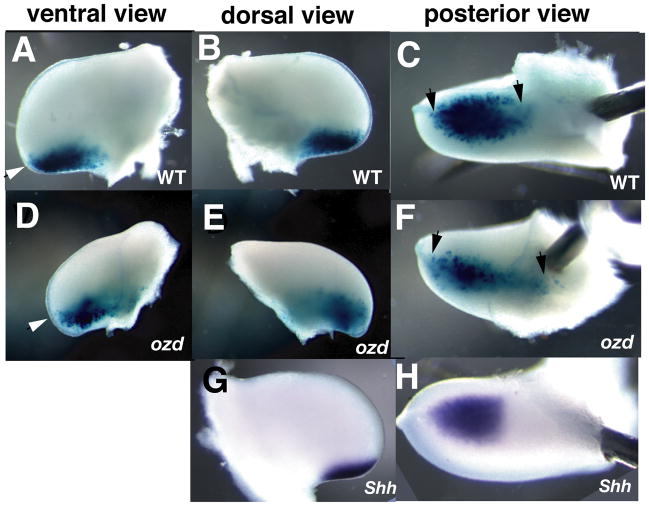
At later stages of development when the ozd limb begins to exhibit a characteristic ozd-shape (Ros et al., 2003), the domain of β-gal activity in ozd limbs is noticeably different from that in normal limbs (n=5). Specifically, when compared to normal limbs, the β-gal expression domain in the ozd limb bud occupies a greater portion of the developing limb bud along both the anterior-posterior (compare Figure 3A, B with 3D, E) and proximal-distal axes (compare Figures 3C with 3F), but is narrower along the dorsal-ventral axis (compare Figures 3C with 3F). It is possible that these observations relate to the change in size and shape of the ozd limb bud beginning at St. 23. The detection of β-gal expression after stage (St.) 23 when HAND2 mRNA is down-regulated in ozd limb buds is likely due to: 1) perdurance of the β-gal protein, prolonging enzymatic activity (Echelard et al.; 1994; 2) a potentially long half-life of HAND2 protein, which at this time is unknown; or 3) the auto-regulatory initiation of Shh, which has been reported in both the Shh null mouse and chick (Scherz et al., 2007, Sanz-Ezquerro and Tickle, 2000.
Figure 3. Expression of the reporter gene in ozd mutant limbs indicates the presence of a cohort of ZPA precursor cells in the early limb.
Limbs at stage 23–24 were harvested from equivalently staged normal (A, B, C) or ozd (D, E, F) embryos electroporated with the ZRS-cShh-lacZ construct. The same limb is shown from ventral (A, D), dorsal (B, E), or posterior (C, F) views to visualize the extent of β-gal activity. In posterior views, ventral is to the top and dorsal is to the bottom. Shh mRNA staining in normal limb buds is also shown (G, H) for reference. Note the expanded β-gal staining domain in the ozd limb buds (indicated by white and black arrowheads). Scale bars are included for comparison between samples. St refers to Hamburger and Hamilton 1951, stages of chick development.
The important conclusions from our observations is that detection of β-gal activity in ozd limbs demonstrates a cohort of pre-ZPA cells along the emerging limb bud border and that the ZRS is required for Shh expression in the chicken limb bud in this cellular cohort, establishing the positive regulatory input of the ZRS in the chicken model system.
Analysis the ZRS in PPD chicken mutants
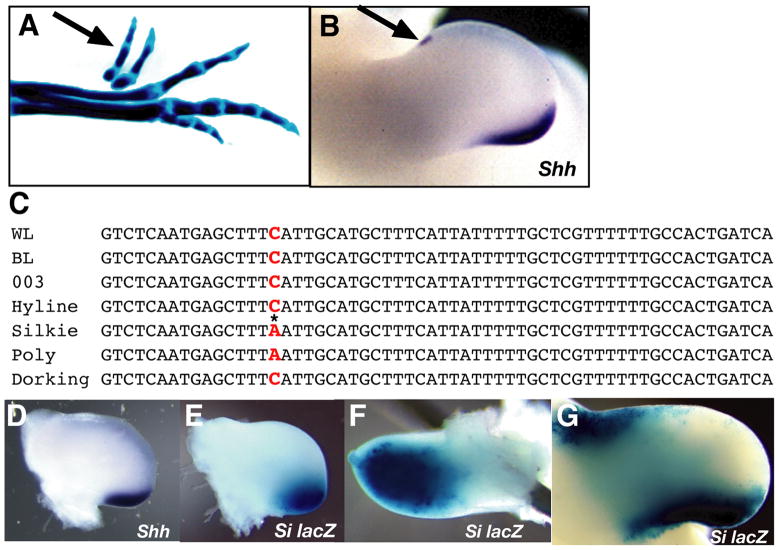
The Silkie Breed of chicken carries several characteristic, heritable traits that are not linked genetically, including semi-dominant PPD with variable penetrance. The normal (wild type) chicken foot has four toes on each foot, a total of 8-toes. As reported in the classical literature (see Sturkie, 1943; Warren, 1944, for quantitation and more references) Silkie Breed feet most commonly have 5 toes on each foot, (Figure 4A, arrow points to duplicated preaxial digit 1) for a total of 10-toes, but in rare cases Silkie may have between 8 toes total (normal phenotype) and very rarely, 12-toes total. The reason for this variability remains unknown.
Figure 4. Identification of a unique single base pair change in the ZRS of the Silkie and Polydactyly chicken PPD mutants.
(A) Day E10 cartilage stained Silkie foot with preaxial extra digit 1 (arrow-top). (B) St. 26 Silkie Breed limb demonstrating ectopic anterior Shh mRNA expression (arrow-top), in addition to broadened posterior Shh expression in the ZPA (bottom). (C) The normal conserved ZRS sequence in the nonpolydactylous chicken strains: White Leghorn (WL), Brown Leghorn (BL), strain 003 and Hyline (HL). An identical base pair change C to A within the ZRS region (red) denoted by an asterisk, was found in the PPD Silkie and Polydactyly (Po) chicken strains. The PPD Dorking strain was found to have a normal sequence throughout the ZRS. (D) Posterior Shh expression was expanded distally and anteriorly in Silkie limb buds at all stages analyzed (shown here, St. 23–24). (E-G)β-galactosidase staining in wild type limbs electroporated with a reporter construct containing the ZRS sequence from Silkie chickens [Si-ZRS-cShh-lacZ (Si lacZ)]. (E, F) St 23–24 showed no detectable anterior staining, but posterior β-gal activity was expanded similar to the expansion of Shh expression in the Silkie limb at St 23–24. (G) St. 25+ limb showed both ectopic anterior and expanded posterior β-gal activity.
Shh expression in Silkie limb buds extended farther distally and anteriorly than in normal limb buds along the posterior sub-apical ectodermal ridge boundary (Figure 4B) this pattern change has also been reported in the PPD mouse mutant Hemimelic-extra toes (Blanc et al., 2002). At later stages, posterior Shh expression was prolonged and remains more robust in Silkie Breed limbs compared with normal chick limb buds (not shown). Shh is also expressed ectopically at the anterior border of the developing limb bud and was first detectable at St. 25+/26 (Figure 4B). Another polydactylous chicken mutant that we studied, Polydactyly (Po), had identical PPD and a range of phenotypes similar to those in Silkie, but did not exhibit other inherited traits carried by the Silkie Breed.
First, we cloned the ZRS region from the Wisconsin White Leghorn, Brown Leghorn, HiLine, and 003 lines of non-polydactylous chickens to examine genetic variability unrelated to PPD in this genomic region. All lines exhibited occasional polymorphisms within the 1.7 kilobase region, but only the Silkie Breed and Po had a single base pair change within the conserved 800 base pair ZRS. This change was identical in Silkie and Po (Figure 4C *). We also analyzed genomic DNA samples for the presence of the base pair change from 8- (n=6), 9- (n=1), 10-(n=9), 11- (n=2), and 12- (n=1) toed Silkie chicks and found that all Silkie birds tested were homozygous for this mutation. However, all Po mutants that were analyzed (n=4) were heterozygous for the mutation. This reflects the breeding strategies for the two strains of chicken, since the Silkie Breed was always mated with other Silkie Breed birds, while Po carriers were outcrossed when the flock was reproduced. It is possible that Po originated from an outcross of the Silkie breed and that these identical mutations did not arise independently.
Bouldin and Harfe, 2009 reported that partially penetrant, preaxial polydactyly in the feet of Dorking Breed chickens was accompanied by ectopic anterior apical ectodermal ridge expression of FGF4, along with repression of normal cell death in the mesodermal anterior necrotic zone and variable anterior ectopic Shh expression; Shh was variably expressed. They proposed that PPD in the Dorking Breed was caused by an increase in the autopod cellular progenitor pool brought about by ectopic AER-FGF4 signaling preventing normal cell death. It was proposed further that in the absence of ectopic Shh an extra digit 1 would form, whereas in the presence of ectopic Shh a digit 2 would form (Bouldin and Harfe, 2009). To explore this further we sequenced (n=4) the Dorking Breed ZRS. Interestingly, and distinct from the Silkie Breed and Po, we found the conserved ZRS region was identical through out its length to the wild type sequence (Figure 4C). We conclude that Dorking Breed and Silkie Breed PPD represent fundamentally different developmental genetic mechanisms for PPD.
While our research was ongoing, (Dorshorst et al., 2010) published data demonstrating the same nucleotide change in the ZRS of Silkie and Po chickens and the lack of any change in the ZRS of the Dorking Breed, all of which we confirm and draw attention to here in the context of the Bouldin and Harfe, (2009) report.
To investigate further the function of the single nucleotide change in the Silkie Breed, we tested a reporter construct similar to that used in the previous ozd experiments above, replacing the 1.7 kilobase region from the non-polydactylous White Leghorn chicken with the same region from the Silkie breed (Si-ZRS-cShh-lacZ) and assayed at stages 23–24, several stages before anterior ectopic Shh is expressed in Silkie limbs. Interestingly, the posterior domain of Shh expression is wider in Silkie Breed than in normal limbs at all stages analyzed (compare Figure 3G with Figure 4D). In agreement with this observation, we consistently detected broader posterior and dorsal-ventral domains of β-gal activity in White Leghorn limbs electroporated with the Si-ZRS-cShh-lacZ construct. This is similar to the expanded posterior Shh expression domain in Silkie Breed compared with limbs electroporated with the wild type construct (compare Figures 3B, C with Figures 4E, F). No anterior β-gal activity was detected at these early stages, possibly indicating a lack necessary conditions for ectopic Shh expression. Importantly, at stages when anterior ectopic Shh would be expressed in Silkie limbs, normal embryos electroporated with the Si-ZRS-cShh-lacZ construct show β-gal expression in both posterior and anterior domains (n= 16/26; Figure 4G). Expression of the control HSP68-lacZ construct throughout the limb (Figure 2B and 2C) indicate that the Si-ZRS-cShh-lacZ construct is present throughout the entire limb, but necessary conditions in the limb bud only permit reporter gene expression in regions that correlate with Shh expression in Silkie limb buds.
DISCUSSION
We report that the chicken ozd mutation is caused by a spontaneous deletion of the majority of the highly conserved zone of polarizing activity (ZPA) regulatory sequence (ZRS), supporting the hypothesis that the ZRS is necessary to activate Shh expression in the posterior of the developing chick limb model system. This is the only documented report of a spontaneous loss of function mutation of the ZRS. We developed a novel reporter bioassay to test the function of the enhancer in the developing chicken limb. The results of these experiments establish the chicken limb as an efficient system in which to conduct gene regulatory studies. The in vivo reporter assay was extended to show that the ozd limb has a cohort of pre-ZPA cells that are prepatterned by trans-acting factors necessary for Shh expression in the limb. Lastly, we provide evidence supporting the hypothesis that negative regulatory elements within the enhancer are necessary for normal repression of Shh at the anterior of the limb bud.
Positive regulatory function of the long-range limb-specific Shh enhancer
Gene-targeted disruption of the ZRS enhancer in the mouse leads to a limb phenotype indistinguishable from that of the Shh−/− mouse (Sagai et al., 2005). Thus removal of the ZRS leads to a limb-specific loss of Shh expression in both mouse and chicken. It is interesting that the ozd phenotype is not the same as the mouse enhancer knockout phenotype, even though both deletions remove a functional enhancer. A main difference is in the stylopod-zeugopod interface where the mouse shows the initiation of the tibia and fibula, which are severely truncated, but their interaction with the femur shows a rudimentary knee joint. The ozd leg shows a recognizable tibia with normal indication of a knee joint, but the absence of the fibula. In the forelimb zeugopod, the mouse develops one long bone fused to the humerus, while the chick forms an independent long bone recognizable as the radius and a normal relationship with the proximal humerus. These phenotypic differences may represent a fundamental difference in the effect of the loss of Shh expression between mouse and chicken limb development; and/or may be a fundamental difference in morphogenesis, reflecting mammalian versus reptile-bird lineages, which were separated 300 million years ago. An unlikely alternative is that the sequence within the first 135 base pairs, which is present in ozd but not in the mouse knockout, is sufficient for controlling parallel, Shh independent morphogenetic functions during skeletal patterning.
The precise control of ZPA-restricted Shh expression may require regulatory inputs from regions outside of the 800 base pair limb-specific enhancer. Sagai et al., 2005 identified two additional blocks of homology within the LMBR1 locus that are highly conserved between teleost fish and mammals. Although these homologous sequences are located in regions other than intron 5, the ordering and spacing of the elements is conserved. As already noted, human patients affected with ACHP have a phenotype similar to ozd mutants, but show a unique, very large deletion in the region of intron 3 and associated coding region of the LMBR1 gene that is outside of the limb-specific Shh enhancer. Although the ACHP deletion removes part of the coding region of LMBR1, we previously showed that the LMBR1 protein is not required for normal chick limb development (Maas and Fallon, 2004). Sequence analysis has revealed no apparent regions of homology between other species within the ACHP deletion, and it has been proposed that the ACHP deletion eliminates the human-specific Shh limb enhancer (Sagai, 2005). However, to our knowledge, the ZRS within LMBR1, intron 5 has not been sequenced in ACHP and it is possible that additional regions of LMBR1 are mutated in ACHP. It is also possible that the ACHP deletion could affect Shh expression in the limb without removing the limb-specific Shh enhancer. Specifically, the very large ACHP deletion could alter spacing in the LMBR1 region of the chromosome, causing a steric limitation that interferes with ZRS physical contact with the Shh locus and or prevents the chromosomal looping associated with initiating Shh transcription (Amano et al., 2009). In this elegant study in the mouse, the authors demonstrate that chromosome contact in posterior limb bud border cells continues in the ΔZRS mouse. This indicates that the ZRS itself does not control this contact, which the authors propose must be controlled by other determinants (Amano et al., 2009); these determinants could be in LMBR1, intron 3 and associated coding region of the ACHP deletion. Either one of these factors could prevent normal ZRS function, or necessary interaction between the ZRS and the Shh promoter, precluding activation of limb specific Shh in the ACHP limb buds.
Does the absence of the limb-specific enhancer in ozd prevent prepatterning of the prospective ZPA cellular cohort?
Uncovering the genetic mechanism of Shh expression in the limb has allowed closer examination of multiple facets of gene regulation in limb development. The transcription factors HAND2 and the 5-prime HOXD genes are expressed along the emerging limb bud posterior border and both are required for Shh expression (Charite et al., 2000; Fernandez-Teran et al. 2000; Zákány et al., 2004). In parallel, loss of function studies indicate that HAND2 is required to exclude the expression of the repressors GLI3 and ALX4 from the posterior limb bud border, restricting them to the anterior limb mesenchyme (te Welscher et al., 2002). A model has been proposed (te Welscher et al., 2002) that the mutually exclusive expression of anterior GLI3 and posterior HAND2 at limb bud emergence represents a prepattern of gene expression that result in the posterior asymmetric expression of Shh. Follow-up studies have demonstrated strong support for this hypothesis, in that co-immuno-precipitation of HAND2 shows interaction with HOXD13. Also, HAND2 binding to the ZRS was demonstrated with chromatin immunoprecipitation, (Galli et al., 2010). Further support for a prepattern is that the Shh null mouse (Chiang et al., 2001; Kraus, 2001) and the chick ozd mutant (Ros et al., 2003) show initial posterior limb bud expression of HAND2 and HOXD13, which subsequently fails to reach the higher levels of expression and extensive distribution that develops with the presence of Shh. Importantly for our study and conclusions is the fact that all six of the HAND2 binding sites in the ZRS (Galli et al., 2010), are eliminated by the ozd ZRS mutation.
With the reporter assay described here, detection of β-gal activity in the ozd limbs that lack the enhancer and never express Shh, supports the hypothesis that the early limb bud contains a cohort cells expressing HAND2 that are normally destined to become the ZPA. Shh expression is not necessary for initiating HAND2 expression. Our data indicate that the ozd phenotype probably is caused solely by the loss of the enhancer region, which is necessary for limb Shh expression. Nevertheless, the emerging ozd early limb bud is prepatterned to establish a domain of Shh competence. This supports the hypothesis that the activator transcription factors necessary for Shh expression in the limb act through the ZRS conserved sequence and are stabilized by SHH. In addition, our reporter assay represents an important advance for the study of regulatory elements in the chicken, since transgenic strategies, a common mode of enhancer analysis in other systems, are not possible in the chicken. Additionally, our reporter assay could be readily adapted for the study of other regulatory elements necessary for limb development.
Are negative ZRS functions disrupted in PPD mutants?
In the early limb bud, posterior Shh expression is noticeably expanded in PPD limb buds compared to nonpolydactylous limb buds. It is only much later in limb development that ectopic Shh expression becomes detectable at the anterior of the limb bud of PPD mutants. At the same time posterior Shh expression persists for a longer time in PPD limb buds than in normal limb buds. These observations demonstrate that the point mutations, allowing Shh expression at the anterior border, also increases the extent of normal posterior Shh expression.
Amano et al., (2009) propose that there are three chromosome conformations associated with the ZRS in the limb bud mesoderm. The first is an active conformation, found in the ZPA region, where the ZRS is physically in contact with the Shh start site, followed by chromosome looping and initiation of Shh transcription. The second is a silent conformation, found in the middle of the limb bud where the ZRS is far from the Shh locus and Shh transcription has not been observed in this region. The third is a poised conformation, found in the limb bud anterior border mesoderm where physical interaction of the ZRS with the Shh locus is observed, but looping is not observed and transcription of Shh does not occur. Because the ZRS interacts with the Shh start site in anterior mesoderm, it is possible that the single base pair changes in the ZRS associated with PPD increase the affinity of the ZRS for the Shh start site and Shh transcription is initiated. Why this would occur only late in limb development and not parallel with the posterior ZPA Shh expression, needs to be evaluated.
Because the same genes are probably required for Shh activation in both posterior and anterior locations, one possibility for the delayed anterior expression is that it takes longer to establish a domain of Shh competence in the anterior limb in PPD mutants, since genes required for Shh expression are normally repressed in this location. In this context the possibility must be explored that the factors that repress Shh expression in limb cells other than the ZPA, also require the ZRS for function. Recent studies show that loss of ETV4 and ETV5 function and reduction of TWIST1 function result in anterior limb bud Shh expression and subsequent PPD (Firulli et al., 2005; Firulli et al., 2007; Zhang et al., 2009; Mao et al., 2009; Zhang et al., 2010; Krawchuk et al., 2010). These genes are required for posterior restriction of Shh. It is proposed that a balance at least of TWIST1 levels with HAND2 levels of expression from anterior to posterior, results in restriction of Shh to the posterior border and its repression anteriorly. While the interaction of the ZRS with HAND2 to initiate and maintain ZPA-Shh expression is established (Galli et al., 2010), it remains to be explored whether the ZRS plays a second role in the function of different factors such as TWIST1 or ETV4/5 that repress Shh expression in the rest of the limb bud mesoderm. The observation of the anterior poised ZRS-bearing chromosome configuration in anterior limb mesoderm (Amano et al., 2009) should be integral to approaches designed to understand the mechanisms that allow anterior Shh expression leading to PPD.
EXPERIMENTAL PROCEDURES
Chicken strains and embryos
White Leghorn, Brown Leghorn, Hyline, Silkie Breed, and oligozeugodactyly (ozd) embryos were obtained from mating flocks at the University of Wisconsin Poultry Research Lab (Madison, WI). Polydactyly (Po) and normal carrier strain 003 eggs were obtained from UC-Davis. Eggs were incubated, opened, and staged as described (Hamburger and Hamilton, 1951; Ros et al., 2000). Dorking chicken blood was obtained from the Pat Handrick Farm, Belleville WI.
Southern blot analysis
Genomic DNA was prepared from St. 26 normal or ozd mutant chicken embryos. 10 μg of each sample was digested overnight, electrophoresed, and transferred by capillary action onto uncharged nylon membranes. Membranes were hybridized with 1×106 cpm denatured 477 base pair ZRS probe and hybridization and washing was performed according to standard procedure.
Identification of a chicken BAC clone containing Lmbr1 intron 5
A High Density Replica Filter Library of chicken BAC clones (CHORI-Oakland, CA) was prehybridized for 1 hour at 65°C in Church’s buffer and then hybridized with the chicken ZRS sequence and a control probe from C. briggsae (to locate “anchor spots” on the developed autoradiograph) at 65°C overnight. Filters were washed with Church’s wash several times at 65°C and exposed to film. Positive BAC clones were grown in LB medium containing 20-μg/ml chloramphenicol.
BAC sequencing and identification of ozd mutation
BAC DNA was purified using the Qiagen Large Construct Purification Kit and sequenced with intron 5-1F (5′-CACAGAGTCTGTGGATTAAGAGG-3′) and intron 5-7R (5′-CACTCATTTCCAACAATTTATGG-3′) primers (1 μg DNA per reaction). Bands were amplified from both wild type and ozd genomic DNA samples (fragments of 3.1 and 1.5 kilobases, respectively) using 5′ ozd del (5′-CCACTGCTAACAGAGTACCTTGG-3′) and 3′ ozd del (5′-GATAGCACAGAGATTGGTATTCC-3′) primers.
Generation of chicken 1.7 kilobase fragment reporter constructs
The mouse 1.7 kb fragment containing the 800 base pair ZRS was aligned with the chicken genome. ~1000 base pairs in the center were homologous and ~300 base pairs on either side of the homologous region were included so chicken and mouse constructs were of similar size. 1.7 kilobase fragments were PCR amplified with primers containing NotI (5′) or BamHI (3′) restriction sites from both normal White Leghorn and Silkie breed samples (primers used: 5′ NotI primer- 5′-ATAAGAATGCGGCCGCGACACTGGAATAGCCTGAAG- 3′; 3′ BamHI primer- 5′-CGGGATCCCAGGGAATTTCAGTACTGG- 3′). The fragments were cloned into a pBR322 construct upstream of a 1.1 kilobase chicken Shh promoter fragment (primers- 5′ BamHI primer-5′-CGGGATCCGCCATTCACTTTGCCGGGAT- 3′; 3′ SpeI primer- 5′-GGACTAGTCCAATTACTTCACAGCTCTCTGTG- 3′).
Electroporation experiments for reporter gene analysis in chicken
DNA was purified using Qiafilter maxiprep kits (Qiagen) and resuspended at a concentration of ~4–5 μg/μl in TE, pH 8.0. We followed the protocol for limb-field electroporation described in Krull, 2004. Briefly, DNA solution was injected into the presumptive right hind limb region of stage (St.) 13–14 embryos using a glass pipette followed by several drops of chicken Ringer’s solution. 0.5 mm, electrodes consisted of a 0.5mm straight platinum wire cathode held over the top of the hind limb-forming region and a “hockey stick” anode (0.5mm platinum wire bent at a 90 degree angle) under the hind limb-forming region containing the injected DNA solution. Injected limbs were electroporated with 3 pulses of 13V, 50 ms pulse length and 100 ms interval length using a BTX, ECM 830 electroporator. Embryos were harvested 1–3 days after electroporation for visualization of reporter gene expression.
β-galactosidase staining of embryos
Embryos were harvested into ice cold phosphate buffered saline (PBS) and fixed in 2% formaldehyde, 0.2% glutaraldehyde, 0.2% NP-40 in PBS for 1–1.5 hours at 4°, washed 3 times in PBS, and incubated in 5mM K++FeCN, 5mM KFe+++CN, 0.2% NP-40, 2 mM MgCl2, 0.5 mg/ml XGAL in PBS at 37°C overnight. Embryos were washed in PBS and stored in PBS+ 10mM EDTA.
Acknowledgments
Grant Information: NICHD HD032551 to J.F.F.; JSPS Fellowship to T.S., PRESTO Grant-in-Aid to T.S.; KAKENHI 21770226 to T.S.
We thank Grace Boekhoff-Falk, Sean Carroll, Allen Laughon, Xin Sun, and members of the Fallon laboratory for helpful comments and discussions on this manuscript; Diana Myers for typing and formatting; Ralph DiLeone for the HSP/lacZ construct; Douglas Epstein for the mouse Shh/lacZ construct; Toshihiko Shiroishi for providing the chicken ZRS sequence prior to publication; Joseph Lancman for providing embryos stained for Shh mRNA expression for Figures 2 and 3; Ron Kean and Pat Handrick for Dorking Breed chicken blood and Joe Doolen for sequencing the Dorking Breed ZRS.
References
- Amano T, Sagai T, Tanabe H, Mizushina Y, Nakazawa H, Shiroishi T. Chromosomal dynamics at the Shh locus: limb bud-specific differential regulation of competence and active transcription. Dev Cell. 2009;16:47–57. doi: 10.1016/j.devcel.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Blanc I, Bach A, Robert B. Unusual pattern of Sonic hedgehog expression in the polydactylous mouse mutant Hemimelic extra-toes. Int J Dev Biol. 2002;46:969–974. [PubMed] [Google Scholar]
- Bouldin CM, Harfe BD. Aberrant FGF signaling, independent of ectopic hedgehog signaling, initiates preaxial polydactyly in Dorking chickens. Dev Biol. 2009;334:133–141. doi: 10.1016/j.ydbio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Chan DC, Laufer E, Tabin C, Leder P. Polydactylous limbs in Strong’s Luxoid mice result from ectopic polarizing activity. Development. 1995;121:1971–1978. doi: 10.1242/dev.121.7.1971. [DOI] [PubMed] [Google Scholar]
- Charite J, McFadden DG, Olson EN. The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development. 2000;127:2461–2470. doi: 10.1242/dev.127.11.2461. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, Fallon JF. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- Dahn RD, Davis MC, Pappano WN, Shubin NH. Sonic hedgehog function in chondrichthyan fins and the evolution of appendage patterning. Nature. 2007;445:311–314. doi: 10.1038/nature05436. [DOI] [PubMed] [Google Scholar]
- Dorshorst B, Okimoto R, Ashwell C. Genomic regions associated with dermal hyperpigmentation, polydactyly and other morphological traits in the Silkie chicken. J Heredity. 2010;101:339–350. doi: 10.1093/jhered/esp120. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, McMahon AP, Joyner AL. Regionalization of Sonic hedgehog transcription along the anteroposterior axis of the mouse central nervous system is regulated by Hnf3-dependent and -independent mechanisms. Development. 1999;126:281–292. doi: 10.1242/dev.126.2.281. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teran M, Piedra ME, Kathiriya IS, Srivastava D, Rodriguez-Rey JC, Ros MA. Role of dHAND in the anterior-posterior polarization of the limb bud: implications for the Sonic hedgehog pathway. Development. 2000;127:2133–2142. doi: 10.1242/dev.127.10.2133. [DOI] [PubMed] [Google Scholar]
- Firulli BA, Krawchuk D, Centonze VE, Vargesson N, Virshup DM, Conway SJ, Cserjesi P, Laufer E, Firulli AB. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nat Genet. 2005;37:373–381. doi: 10.1038/ng1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firulli BA, Redick BA, Conway SJ, Firulli AB. Mutations within helix I of Twist1 result in distinct limb defects and variation of DNA binding affinities. J Biol Chem. 2007;282:27536–27546. doi: 10.1074/jbc.M702613200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli A, Robay D, Osterwalder M, Bao X, Benazet JD, Tariq M, Paro R, Mackem S, Zeller R. Distinct roles of Hand2 in initiating polarity and posterior Shh expression during the onset of mouse limb bud development. PLoS Genet. 2010;6:e1000901. doi: 10.1371/journal.pgen.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin C. Evidence for an Expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Ianakiev P, van Baren MJ, Daly M, Toledo S, Cavalcanti MG, Neto JC, Silveria EL, Freire-Maia A, Heutink P, Kilpatrick MW, Tsipouras P. Acheiropodia is caused by a genomic deletion in C7orf2, the human orthologue of the Lmbr1 gene. Am J Hum Genet. 2001;68:38–45. doi: 10.1086/316955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus P, Fraidenraich D, Loomis CA. Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mech of Dev. 2001;100:45–58. doi: 10.1016/s0925-4773(00)00492-5. [DOI] [PubMed] [Google Scholar]
- Krawchuk D, Weiner SJ, Chen YT, Lu BC, Costantini F, Behringer RR, Laufer E. Twist1 activity thresholds define multiple functions in limb development. Dev Biol. 2010;347:133–146. doi: 10.1016/j.ydbio.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs O, Schreiner CM, Scott WJ, Bell SM, Robbins DJ, Goetz JA, Alt H, Hawes N, Wolf E, Favor J. Replicated anterior zeugopod (raz): a polydactylous mouse mutant with lowered Shh signaling in the limb bud. Development. 2003;10:6037–6047. doi: 10.1242/dev.00861. [DOI] [PubMed] [Google Scholar]
- Krull CE. A Primer on using In Ovo electroporation to analyze gene function. Dev Dyn. 2004;229:433–439. doi: 10.1002/dvdy.10473. [DOI] [PubMed] [Google Scholar]
- Lettice LA, Heaney SJ, Purdie LA, de Beer P, Oostra BA, Goode D, Elgar G, Hill RE, de Graaff E. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 2003;12:1725–1735. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- Lettice LA, Hill AE, Devenney PS, Hill RE. Point mutations in a distant sonic hedgehog cis-regulator generate a variable regulatory output responsible for preaxial polydactyly. Hum Mol Genet. 2008;17:978–985. doi: 10.1093/hmg/ddm370. [DOI] [PubMed] [Google Scholar]
- Lettice LA, Horikoshi T, Heaney SJH, van Baren MJ, van der Linde HC, Breedveld GJ, Joosse M, Akarsu N, Oostra BA, Endo N, Shibata M, Suzuki M, Takahashi E, Shinka T, Nakahori Y, Ayusawa D, Nakabayashi K, Scherer SW, Heutink P, Hill RE, Noji S. Disruption of a long-range cis-acting regulator for Shh causes preaxial polydactyly. Proc Natl Acad Sci USA. 2002;99:7548–7553. doi: 10.1073/pnas.112212199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Joyner AL, Turnbull DH. Alteration of limb and brain patterning in early mouse embryos by ultrasound-guided injection of Shh-expressing cells. Mech of Dev. 1998;75:107–115. doi: 10.1016/s0925-4773(98)00090-2. [DOI] [PubMed] [Google Scholar]
- Lopez-Martinez A, Chang DT, Chiang C, Porter JA, Ros MA, Simandl BK, Beachy PA, Fallon JF. Limb-patterning activity and restricted posterior localization of the amino-terminal product of Sonic hedgehog cleavage. Curr Biol. 1995;5:791–796. doi: 10.1016/s0960-9822(95)00156-4. [DOI] [PubMed] [Google Scholar]
- Maas SA, Fallon JF. Isolation of the chicken Lmbr1 coding sequence and characterization of its role during chick limb development. Dev Dyn. 2004;229:520–528. doi: 10.1002/dvdy.10502. [DOI] [PubMed] [Google Scholar]
- Maas SA, Fallon JF. Single base pair change in the long-range Sonic hedgehog limb-specific enhancer is a genetic basis for preaxial polydactyly. Dev Dyn. 2005;232:345–348. doi: 10.1002/dvdy.20254. [DOI] [PubMed] [Google Scholar]
- Mao J, McGlinn E, Huang P, Tabin CJ, McMahon AP. Fgf-dependent Etv4/5 activity is required for posterior restriction of Sonic Hedgehog and promoting outgrowth of the vertebrate limb. Dev Cell. 2009;16:600–606. doi: 10.1016/j.devcel.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuya H, Sagai T, Moriwaki K, Shiroishi T. Multigenic control of the localization of the zone of polarizing activity in limb morphogenesis in the mouse. Dev Biol. 1997;182:42–51. doi: 10.1006/dbio.1996.8457. [DOI] [PubMed] [Google Scholar]
- Masuya H, Sagai T, Wakana S, Moriwaki K, Shiroishi T. A duplicated zone of polarizing activity in polydactylous mouse mutants. Genes Dev. 1995;9:1645–1653. doi: 10.1101/gad.9.13.1645. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Ros MA, Dahn RD, Fernandez-Teran M, Rashka K, Caruccio NC, Hasso SM, Bitgood JJ, Lancman JJ, Fallon JF. The chick oligozeugodactyly (ozd) mutant lacks sonic hedgehog function in the limb. Development. 2003;130:527–537. doi: 10.1242/dev.00245. [DOI] [PubMed] [Google Scholar]
- Ros MA, Lopez-Martinez A, Simandl BK, Rodriguez C, Izpisua Belmonte JC, Dahn R, Fallon JF. The limb field mesoderm determines initial limb bud anteroposterior asymmetry and budding independent of sonic hedgehog or apical ectodermal gene expressions. Development. 1996;122:2319–2330. doi: 10.1242/dev.122.8.2319. [DOI] [PubMed] [Google Scholar]
- Ros MA, Simandl BK, Clark AW, Fallon JF. Methods for manipulating the chick limb bud to study gene expressions, tissue interactions and patterning. In: Lo CW, editor. Development Bioogy Protocols. Humana Press; 2000. pp. 245–266. [DOI] [PubMed] [Google Scholar]
- Sagai T, Hosoya M, Mizushina Y, Tamura M, Shiroishi T. Elimination of a long-range cis-regulatory module causes complete loss of limb-specific Shh expression and truncation of the mouse limb. Development. 2005;132:797–803. doi: 10.1242/dev.01613. [DOI] [PubMed] [Google Scholar]
- Sagai T, Masuya H, Tamura M, Shimizu K, Yada Y, Wakana S, Gondo Y, Noda T, Shiroishi T. Phylogenetic conservation of a limb-specific, cis-acting regulator of Sonic hedgehog (Shh) Mammalian Genome. 2004;15:23–34. doi: 10.1007/s00335-033-2317-5. [DOI] [PubMed] [Google Scholar]
- Sanz-Ezquerro JJ, Tickle C. Autoregulation of Shh expression and Shh induction of cell death suggest a mechanism for modulating polarising activity during chick limb development. Development. 2000;127:4811–4823. doi: 10.1242/dev.127.22.4811. [DOI] [PubMed] [Google Scholar]
- Sharpe J, Lettice L, Hecksher-Sørensen J, Fox M, Hill RE, Krumlauf R. Identification of Sonic hedgehog as a candidate gene responsible for the polydactylous mouse mutant Sasquatch. Curr Biol. 1999;9:97–100. doi: 10.1016/s0960-9822(99)80022-0. [DOI] [PubMed] [Google Scholar]
- Scherz PJ, McGlinn E, Nissim S, Tabin CJ. Extended exposure to sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev Biol. 2007;308:343–354. doi: 10.1016/j.ydbio.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturkie PD. Suppression of polydactyly in the domestic fowl by low temperature. J Exp Zool. 1943;93:325–346. [Google Scholar]
- te Welscher P, Fernandez-Teran M, Ros MA, Zeller R. Mutual genetic antagonism involving GLI3 and dHAND prepatterns the vertebrate limb bud mesenchyme prior to SHH signaling. Genes Dev. 2002;16:421–426. doi: 10.1101/gad.219202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers M, Tickle C. Growing models of vertebrate limb development. Development. 2009;136:179–190. doi: 10.1242/dev.024158. [DOI] [PubMed] [Google Scholar]
- Uchikawa M, Ishida Y, Takemoto T, Kamachi Y, Kondoh H. Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev Cell. 2003;4:509–519. doi: 10.1016/s1534-5807(03)00088-1. [DOI] [PubMed] [Google Scholar]
- Warren DC. Inheritance of polydactylism in the fowl. Genetics. 1944;29:217–231. doi: 10.1093/genetics/29.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zákány J, Kmita M, Duboule D. A dual role for Hox genes in limb anterior-posterior asymmetry. Science. 2004;304:1669–1672. doi: 10.1126/science.1096049. [DOI] [PubMed] [Google Scholar]
- Zeller R, Lopez-Rios J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet. 2009;10:845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sui P, Dong A, Hassell J, Cserjesi P, Chen Y-T, Behringer RR, Sun X. Preaxial polydactyly: interactions among ETV, TWIST1 and HAND2 control anterior- posterior patterning of the limb. Development. 2010;137:3417–3426. doi: 10.1242/dev.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Verheyden JM, Hassell JA, Sun X. FGF-regulated Etv genes are essential for repressing Shh expression in mouse limb buds. Dev Cell. 2009;16:607–613. doi: 10.1016/j.devcel.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]