Abstract
Background
Organic agriculture involve plants which are cultivated without using synthetic pesticides, herbicides or fertilizers and promotes biodiversity, biological cycles and improve the product quality. The carotenoids, total polyphenols and the antioxidant activity from skins of some wine and table grapes cultivated in organic and conventional agriculture were studied.
Results
The main carotenoids identified using high performance liquid chromatography were lutein and ß-carotene. Muscat Ottonel variety has the highest ß-carotene concentration 504.9 μg/kg for organic and 593.2 μg/kg for conventional grapes. For the organic farming, the total polyphenols content were in the range of 163.23 – 1341.37 mg GAE/kg fresh weight (FW) and 148.47 – 1231.38 mg GAE/kg FW for the conventional grapes. The highest ORAC values were obtained for blue-black variety Napoca in both farming system (43.5 ± 0.95 μmol TE/g organic; 40.4 ± 0.5 μmol TE/g conventional) and lowest for Aromat de Iaşi (16.8 ± 0.6 μmol TE/g organic; 14.7 ± 1.6 μmol TE/g conventional). Napoca variety showed also the highest antioxidant activity measured by DPPH method in both cultivated system.
Conclusion
Nine grape varieties cultivated in organic and conventional systems were compared regarding the carotenoids, total polyphenols and antioxidant activity. The white grape varieties have a higher carotenoids content compared with the blue-black cultivars while the blue-black varieties contain higher TPC and exhibit higher antioxidant activity (except for Muscat Hamburg-ORAC). Vitis vinifera grape skins originating from wine or table grape varieties can be used as a potential source of natural antioxidants.
Keywords: Grapes-Vitis vinifera, Carotenoids, HPLC, Total polyphenols, Antioxidant activity, Organic farming
Background
The grape berries are important since they are consumed as fruits, wine, juice or raisins and are largely cultivated for the wine industry. Grapes contain a wide range of chemical substances such as sugars, organic acids, mineral salts, vitamins, enzymes and also phytochemicals which are responsible for the sensory characteristics of wines [1] and for their health properties [2].
Antioxidant activities of grapes are due to the presence of antioxidant components such as flavonoids, phenolic acids, anthocyanins and carotenoids. Carotenoids play important roles in human nutrition through their provitamin A activity, but also by acting as antioxidants, for prevention of age-related macular degeneration or skin protection against UV radiation [3]. The antioxidant capacity of carotenoids was proved for pure compounds as well as for plant extracts and food [4,5]. Recent studies investigate the profile of carotenoids in some Italian grape varieties [6] and the effect of lightening and irrigation on carotenoids concentration [7,8].
Organic agriculture does not use synthetic pesticides and fertilizers [9] just ecological products during the cultivation. Higher concentration of bioactive compounds in plants grown in organic agriculture may be the results of the plant exposure to situation resulting from the absence of pesticides that leads to an increase of natural defense substances [10]. There are some studies concerning the influence of organic cultivation on the secondary metabolites in strawberries, yellow plumes and tomatoes [11,12]. It was shown that organic crop influenced the phenolic content and the antioxidant activity of white and purple grape juices [13]. Mulero et al. [14] studied the phenolic content and the antioxidant activity in Monastrell grapes obtained by organic and conventional agriculture. Vinković Vrček et al. [15] evaluate the antioxidant capacity, the polyphenols and metal content of conventionally and organically Croatian wines. The antioxidant activity of organically grown crops is reported to be higher [16] or there are no differences reported in the organic or conventional foods [17]. In eggplant cultivars grown with organic and conventional farming practices there were found no significant trend regarding the phenolic acids content [18]. The aim of this study was to compare the carotenoids, the total polyphenols content and the antioxidant activity from skins of some wine and table grapes cultivated in organic and conventional systems.
Results and discussions
Carotenoids
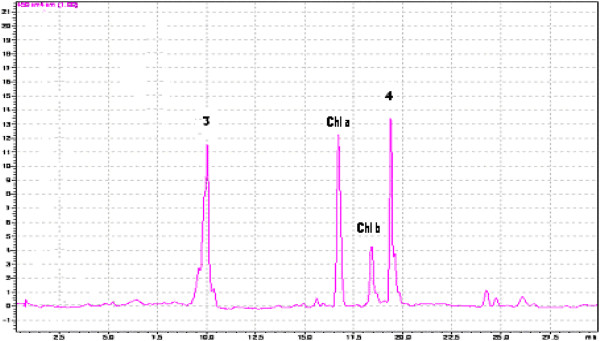
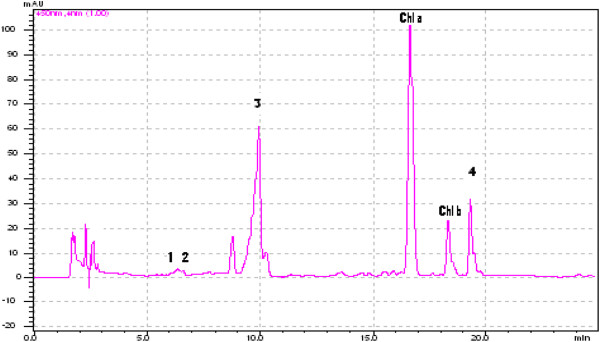
The HPLC chromatograms for the carotenoids separated from the skin of Muscat Ottonel and Muscat Hamburg grapes are presented in Figure 1 and Figure 2. The main carotenoids identified were lutein and β-carotene. The most important carotenoids (85% from all the carotenoids present) identified in grapes are ß-carotene and lutein, the rest of them being neochrome, neoxanthin, violaxanthin, luteoxanthin, flavoxanthin, zeaxanthin and cis-isomers of lutein and ß-carotene [19]. Carotenoids are directly involved in grapes aroma because they can suffer degradation reactions followed by apparition of norisoprenoid compounds [20].
Figure 1.
HPLC-PDA chromatogram of carotenoids separated from fresh skin grapes (Muscat Ottonel variety-conventional). The carotenoids identified are: (3) lutein and (4) b-carotene.
Figure 2.
HPLC-PDA chromatogram of carotenoids separated from fresh skin grapes (Muscat Hamburg variety-organic). Four carotenoids were identified: (1) neoxanthin, (2) violaxanthin, (3) lutein and (4) ß-carotene.
In Table 1 are presented the carotenoids concentration (ß-carotene and lutein) in grape skin extracts. When organic system was used it can be observed that the highest concentration of lutein was recorded in Fetească regală variety (855 μg/kg) followed at significant difference by Timpuriu de Cluj (799 μg/kg) and Muscat Ottonel (723 μg/kg) varieties, while the lowest concentration was obtained for Aromat de Iaşi (512 μg/kg). In conventional type of culture, Fetească regală (825 μg/kg) and Timpuriu de Cluj (803 μg/kg) has the highest lutein content. Regarding the ß-carotene content, Muscat Ottonel variety has the highest concentration (504.9 μg/kg-organic and 593.2 μg/kg-conventional, respectively) in both systems. The lowest ß-carotene content in organic system was found in Chasselas doré variety (273.5 μg/kg). In the conventional system, Chasselas doré and Riesling Italian varieties has statistically no differences between the values obtained for ß-carotene. The lowest ß-carotene values in conventional system were obtained for Napoca (229 μg/kg) and Aromat de Iaşi varieties (232 μg/kg).
Table 1.
The carotenoids concentration (μg/kg)in grape skin extract
| Variety | Color |
Organic |
Conventional |
||
|---|---|---|---|---|---|
|
ß-carotene |
Lutein |
ß-carotene |
Lutein |
||
| (μg/kg sample) | (μg/kg sample) | (μg/kg sample) | (μg/kg sample) | ||
| Aromat de Iaşi |
white |
304.0 ± 49de |
512.0 ± 69g |
232.0 ± 46e |
470.9 ± 46e |
| Traminer roz |
white |
331.9 ± 23c |
551.0 ± 35f |
356.8 ± 34bc |
598.0 ± 53d |
| Riesling italian |
white |
365.2 ± 34b |
632.0 ± 56e |
264.5 ± 47d |
709.0 ± 87b |
| Feteasca regală |
white |
328.3 ± 31cd |
855.0 ± 66a |
344.3 ± 32bc |
825.0 ± 39a |
| Muscat Ottonel |
white |
504.9 ± 43a |
723.0 ± 56c |
593.2 ± 35a |
602.0 ± 87cd |
| Timpuriu de Cluj |
white |
290.4 ± 14ef |
799.0 ± 23b |
334.1 ± 12c |
803.0 ± 52a |
| Napoca |
blue-black |
369.0 ± 26b |
701.0 ± 34cd |
229.6 ± 18e |
689.0 ± 19b |
| Chasselas doré |
white |
273.5 ± 23f |
552.0 ± 56f |
363.6 ± 45b |
589.0 ± 21d |
| Muscat Hamburg |
blue-black |
302.1 ± 56e |
685.0 ± 67d |
265.0 ±32d |
662.0 ± 12bc |
| LSD 0.05 | 25.77 | 31.27 | 22.86 | 51.57 | |
Values are mean ± SD, n = 3; in each column, mean values with different letters are significantly different at P < 0.05.
There are relatively few studies regarding grape carotenoids composition. The quantitative carotenoids data obtained are in agreement with the literature data. Crupi et al. [21] studied the carotenoids concentration in some wine grapes and obtained values for lutein concentration in the range 130–682 μg/kg and for ß-caroten between 590–1370 μg/kg depending on the grape variety studied. Another study reported a lutein concentration between 218–1044 μg/kg and ß-carotene concentration of 168–910 μg/kg for different varieties [22]. The lutein to ß-carotene ratio in grapes, are highly variable depending on the viticulture region and on the variety analyzed. In some varieties (Sauvignon, Pinot Noir and Merlot) some authors found that lutein level was almost double compared to ß-carotene while in other varieties the concentration of this two carotenoids are similar or even higher for ß-carotene [23].
The profile and the amount of grape carotenoids could be influenced by several factors, such as: plant variety, climate conditions, stage of maturation, soil features [24].There are studies showing that soil irrigation has less influence on carotenoids profile compared with the type of soil and its water holding capacity. Soil with a low water holding capacity can lead to an increase of carotenoids concentration [25].
Total polyphenols
In Table 2 is presented the total polyphenols content in grape skin extracts. The total polyphenols content (TPC) was determined using the Folin-Ciocâlteu method. Gallic acid was used as standard and the results (as gallic acid equivalents) were expressed as means ± standard deviation of triplicate analysis. When the organic system was applied the TPC values were in the range of 163.26 – 1341.37 mg GAE/kg fresh weight (FW) while for conventional cultivated grapes the TPC values were between 148.47 – 1231.38 mg GAE/kg FW. Among the grape variety cultivated in organic system, Napoca revealed the highest TPC as 1341.37 mg GAE/kg FW followed at significant difference by Muscat Hamburg (978.08 mg GAE/kg FW). The values obtained were higher compared with those obtain by Mulero et al. [14] for Monastrell variety (600 mg GAE/kg) and closer to the values obtained by Valls et al. [26]. Between grapes cultivated in conventional system, Napoca has the highest TPC (1231.38 mg GAE/kg) followed by Muscat Hamburg (953.04 mg GAE/kg) while the lowest value was found for Chasselas doré (148.47 mg GAE/kg). Regarding white conventional grape varieties, Fetească regală has the highest TPC content (575.71 mg GAE/kg FW) followed by Muscat Ottonel, Riesling italian, Traminer roz, Aromat de Iaşi and Timpuriu de Cluj. Significant differences were found in TPC for all the grape varieties analyzed (p < 0.05). Dani et al. [13] reported higher polyphenols content in red grape juices from Vitis labrusca compared with white ones. The range of total polyphenols in skin is lower than those reported by Lutz et al. [27] who analyzed four grape varieties and obtained values between 63.2-129 mg GAE/g. Junh et al. [28] and Katalinić et al. [29] also obtain higher values. The last one reported 875 mg GAE/kg (average) in seven white grape varieties grown in Dalmatia (Croatia). Previous studies reported lower total polyphenol values than those found in the present study [14,30]. This diversity of results can be due to the grape variety analyzed, growing conditions and/or the methods of extraction and analysis used [27].
Table 2.
Total polyphenol concentration in grape skin extracts
| Variety | Colour |
Organic |
Conventional |
|---|---|---|---|
|
Total polyphenols content |
Total polyphenols content |
||
| (mg GAE/kg sample) | (mg GAE/kg sample) | ||
| Aromat de Iaşi |
white |
220.38 ± 14.21g |
228.84 ± 6.23h |
| Traminer roz |
white |
219.33 ± 4.02g |
330.37 ± 2.36g |
| Riesling italian |
white |
423.43 ± 10.36e |
436.12 ± 11.02e |
| Feteasca regală |
white |
579.94 ± 11.12d |
575.71 ± 9.06c |
| Muscat Ottonel |
white |
631.38 ± 21.0c |
541.27 ± 32.3d |
| Timpuriu de Cluj |
white |
331.34 ± 3.52f |
380.62 ± 23.5f |
| Napoca |
blue-black |
1341.37 ± 21.1a |
1231.38 ± 21.0a |
| Chasselas doré |
white |
163.26 ± 3.66h |
148.47 ± 1.69i |
| Muscat Hamburg |
blue-black |
978.08 ± 12.8b |
953.04 ± 10.9b |
| LSD 0.05 | 12.93 | 19.43 |
Values are mean ± SD, n = 3; in each column, mean values with different letters are significantly different at P < 0.05.
Antioxidant activity
DPPH - scavenging activity assay
The DPPH· scavenging activity for organic and conventional grapes extracts is presented in Table 3. Significant differences between all the grape samples were obtained, the highest value being recorded for Napoca variety in both culture systems (32.12 ± 1.4 μg Trolox/g for organic and 25.07 ± 1.3 μg Trolox/g for conventional) followed at significant difference by Muscat Hamburg and Muscat Ottonel. The lowest value was obtained for Aromat de Iaşi variety (3.1 ± 0.2 μg Trolox/g organic, 2.1 ± 0.6 μg Trolox/g conventional). Anastasiadi et al. [31] reported DPPH values between 55.7-274.2 μg/g extract in grape skins on four varieties. An interesting study made on conventional and organic grapes in three stages of maturation showed that in stage one of ripening the antioxidant activity was higher for organic grapes (5.70 mM Trolox/g) compared with conventional grapes (4.40 mM Trolox/g [14].
Table 3.
Antioxidant activity for grape skin using DPPH method
| Variety | Colour |
Organic |
Conventional |
|---|---|---|---|
|
Antioxidant activity |
Antioxidant activity |
||
| (μg Trolox/g sample) | (μg Trolox/g sample) | ||
| Aromat de Iaşi |
white |
3.1 ± 0.2h |
2.1 ± 0.6g |
| Traminer roz |
white |
7.3 ± 0.3g |
5.4 ± 0.24f |
| Riesling italian |
white |
8.8 ± 0.7f |
8.1 ± 0.4e |
| Feteasca regală |
white |
10.1 ± 0.18e |
12.2 ± 0.9d |
| Muscat Ottonel |
white |
16.0 ± 0.4c |
14.4 ± 0.2c |
| Timpuriu de Cluj |
white |
11.8 ± 1.12d |
15.73 ± 1.9c |
| Napoca |
blue-black |
32.12 ± 1.4a |
25.07 ± 1.3a |
| Chasselas doré |
white |
7.41 ± 0.3g |
2.8 ± 0.2g |
| Muscat Hamburg |
blue-black |
23.01 ± 0.1b |
22.77 ± 0.5b |
| LSD 0.05 | 0.86 | 1.73 |
Values are mean ± SD, n = 3; in each column, mean values with different letters are significantly different at P < 0.05.
ORAC - oxygen radical absorbance capacity
ORAC assay is probably the most widely used hydrogen atom transfer (HAT)-assay which indicates the free-radical scavenging ability of antioxidants against peroxyl radical. The values obtained (Table 4) were significantly different among samples, ranging from 16.8 to 43.5 μmol TE/g in organic grapes and from 14.7 to 40.4 μmol TE/g for conventional ones. These results showed 2.58 (organic) and 2.8 (conventional) - fold differences in antioxidant capacity among genotypes. The highest ORAC values were obtained for blue-black variety Napoca in both farming system (43.5 ± 0.95 μmol TE/g organic; 40.4 ± 0.5 μmol TE/g conventional) while Aromat de Iaşi showed the lowest values (16.8 ± 0.6 μmol TE/g organic; 14.7 ± 1.6 μmol TE/g conventional). The ORAC values obtained were higher than those reported by Cao, Sofic & Prior [32] in red grape samples (7.4 μmol TE/g) and lower than the values reported for 11 grape cultivars from India by Kedage et al. [33] (43.7-46.8 μmol TE/g). ORAC values in red wine grapes ranged from 37.2 mmol TE/kg to 135.8 5 mmol TE/kg in different genotypes [34]. The range of ORAC values obtained in this study was similar with the results of Hogan et al., 2009 [35] for three Virginia grown wine grape extract 22.9-26.7 μmol TE/g.
Table 4.
Antioxidant activity for grape skin using ORAC method
| Variety | Colour |
Organic |
Conventional |
|---|---|---|---|
|
Antioxidant activity |
Antioxidant activity |
||
| (μmol TE/g sample) | (μmol TE/g sample) | ||
| Aromat de Iaşi |
white |
16.8 ± 0.6g |
14.7 ± 1.6g |
| Traminer roz |
white |
17.4 ± 0.2g |
20.4 ± 1.4f |
| Riesling italian |
white |
25.6 ± 0.6e |
25.1 ± 1.4e |
| Feteasca regală |
white |
28.2 ± 0.9d |
28.4 ± 1.9d |
| Muscat Ottonel |
white |
30.1 ± 0.4c |
32.4 ± 0.2b |
| Timpuriu de Cluj |
white |
35.8 ± 1.2b |
30.4 ± 0.9c |
| Napoca |
blue-black |
43.5 ± 0.9a |
40.4 ± 0.5a |
| Chasselas doré |
white |
22.4 ± 1.2f |
19.8 ± 0.4f |
| Muscat Hamburg |
blue-black |
25.8 ± 1.8e |
25.2 ± 1.5e |
| LSD 0.05 | 0.92 | 1.14 |
Values are mean ± SD, n = 3; in each column, mean values with different letters are significantly different at P < 0.05.
Correlations
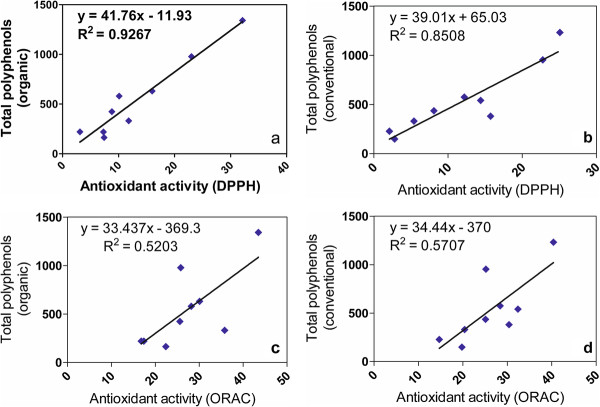
The relation between the polyphenol content and the antioxidant capacity was measured using different linear correlations. When the total polyphenol content was correlated with DPPH assay the coefficient value was high in both culture systems: R2 = 0.926 for organic (Figure 3a) and R2 = 0.850 for conventional system (Figure 3b). The high regression coefficients values obtained showed a strong correlation between antioxidant capacity and total polyphenol content [31].
Figure 3.
Linear simple regression analysis. Correlations between: a- DPPH and TPC for organic; b- DPPH and TPC for conventional; c- ORAC and TPC for organic; d- ORAC and TPC for conventional. (TPC: total phenolics content; DPPH: scavenging activity assay; ORAC: oxygen radical absorbance activity).
The regression coefficient values obtained for total polyphenol content and ORAC assay was lower compared with DPPH assay, but significant, in both systems, conventional (R2 = 0.570) and organic (R2 = 0.520) (Figure 3, c and d).
Conclusions
In the literature exists just one study regarding the antioxidant activity in organic and conventional grapes (red cultivar Monastrell-Vitis vinifera) [14]. Five wine and four table grape varieties cultivated in organic and conventional systems were compared for the carotenoids, total polyphenols content and antioxidant activity. The main carotenoids identified were lutein and ß-carotene. Muscat Ottonel variety has the highest ß-carotene content in both organic and conventional systems and Feteasca regală variety has the highest lutein content. The blue-black cultivars are characterized by high TPC and also high antioxidant activity (except for Muscat Hamburg-ORAC). On the other hand 5 organic grape varieties (Napoca, Muscat Hamburg, Muscat Ottonel, Fetească regală, Chasselas doré) recorded higher TPC compared with conventional and the most organic variants showed a higher antioxidant activity than conventional ones. The pytochemical concentration and antioxidant activity was higher in organically produced tomato juices compared with conventionally ones [36]. Also, apples grown in organic system showed significant differences in chlorogenic acid, flavanols and flavonols compared with conventional grown apples [37].The phenolic compound composition in grapes can be different depending on variety, season and environmental factors [38,39]. Organic agriculture does not use synthetic pesticides and fertilizers and plants being more susceptible to the pathogens action produce higher amount of phenolic compounds [40]. Vitis vinifera grape skin (from wine or table grape varieties) can be used as a potential source of natural antioxidants.
Experimental
Biological material and cultivation system
Five wine grape varieties (Aromat de Iaşi, Traminer roz, Riesling Italian, Fetească regală, Muscat Ottonel - whites) and four table grape varieties (Timpuriu de Cluj and Chasselas doré - whites, Napoca and Muscat Hamburg – blue-black) were tested in 2010, Cluj county, Romania, under two types of cultural practices: organic and conventional. Types of culture were differentiated by treatment for diseases, especially downy mildew of grapevine, caused by Plasmopara viticola (Berk. & Curt.) Berl. & de Toni which is one of the most serious diseases of grapevine worldwide [41] and by different fertilizing for organic practices and for the conventional. In the conventional system were used chemical fungicides: Ridomil Gold MZ 68 WP (metalaxyl-M 4% + mancozeb 64%), Melody Duo 66.8 WP (iprovalicarb 5.5% + propineb 61.3%), Curzate manox SC (cymoxanil 5% + copper 25% + mancozeb 18%), Quadris max SC (azoxystrobin 22.9%), Folpet 50 WP (folpet 50%) and Dithane M 45 (mancozeb 80%). For the organic treatments were applied ecological products: bordeaux mixture 0.5% + spraying with purine of greater nettle (Urtica dioica L.) fermented 1/20 dilution, copper sulphate 1%, Kocide 101 WP (copper hydroxide + metallic copper 50%), bordeaux mixture 1%, soluble sulphur 0.4% and biocontrol agent Trichoderma harzianum.
Grape samples
All samples of grapes were harvested 15 days before technological maturity from the vines grown at the experimental vineyards in Cluj county, Romania. The average yearly precipitation is 660 mm and the vineyards soil type was haplic luvisols. The research planting was organized in the 2.0 m × 1.1 m distance between rows and plants. All the vines were grafted on the same rootstock Selection Openheim nr.4 (SO-4) (V. berlandieri x V. riparia rootstock).
Grape berries were taken randomly from different parts of grape vines and different parts of clusters for each sample analyzed. Were collected every 100 berries (in three replicates) for each variety tested in organic and conventional systems. Grape berries were placed in polyethylene bags, transported under refrigerated conditions to the Department of Biochemistry from U. A. S.V. M. Cluj-Napoca within 1,5 h and processed in the same day into laboratory. The skins were manually separated from pulp, weighed and frozen at −20°C until analyzed.
Choosing neighboring plots give us the possibility to compare organic and conventional vineyards in the same soil and climate conditions. In the same spot there were placed two experimental plots: Plot 1-organic cultured and Plot 2-conventionally cultured.
Chemicals
Methanol, ethyl acetate, petroleum ether, diethyl ether, triethylamine, sodium chloride, anhydrous sodium sulphates were purchased from Sigma Chemical Co. (Madrid, Spain). The purity of carotenoid standards, ß-carotene and lutein, was estimated by registering their UV–Vis spectra and by an individual HPLC run. The ß-carotene and lutein were found to be 96% and 97.5% pure, respectively. All other chemicals were purchased from Merck (Darmstadt, Germany).
Carotenoids extraction and separation
Total carotenoids were extracted from 20 g fresh grape skin using a mixture of methanol/ethyl acetate/petroleum ether (1:1:1, v/v/v). After filtering the extract, the residue was re-extracted twice with the same mixture of solvents, following the procedure described by Breithaupt and Schwack [42]. The extracts were combined and partitioned in a separation funnel, successively with water, diethyl ether and saturated saline solution. The ether phase was evaporated to dryness under vacuum, using a rotary evaporator at 35°C. The samples were kept under nitrogen, at −20°C until further utilization. HPLC analyses for individual carotenoids were carried out on Shimadzu LC20 AT controller system with PDA detector SPD-M20A, using a reversed phase Hibar 250–4 Lichrosorb C18 column (250 × 4.6 mm), 5 μm. A dual gradient mobile phase was used and made of acetonitrile: water (9:1, v/v), with 0.25% triethylamine (solvent A) and ethyl acetate with 0.25% triethylamine (solvent B). The gradient started with 15% B to 50% B from 0 to 16 min and continued isocratically up to 30 min. The flow rate was 1 ml/min. All chromatograms were monitored at 450 nm. The HPLC peaks were identified by using parallel HPLC runs with carotenoid standards as well as by recording the UV–Vis spectra and their comparison with known carotenoid spectra [43]. Finally the results were expressed as micrograms carotenoid/kg FW (μg/kg sample).
Polyphenols extraction
For polyphenols extraction 10 g of grape skin, in three replicated each, was extracted by grinding the sample 1 min at 20,000 rpm in a blender (Ultra-Turrax Miccra D-9 KT Digitronic, Germany) with 10 ml of acidified methanol (85:15 v/v, MeOH:HCl). The homogenate was centrifuged at 3500 rpm for 10 min. The extract was separated and the residual tissue was re-extracted until the extraction solvents became colorless. The filtrates were combined in a total extract and dried using rotary evaporator at 40°C.
Total polyphenols
The amount of total polyphenols in grape skins was determined using modified Folin-Ciocâlteu colorimetric method [44]. Grape skin extracts (25 μl each) were dissolved in methanol and further dilution were performed to obtain readings within the standard curve made with gallic acid (R = 0.997). The extracts were oxidized by Folin-Ciocâlteu reagent (120 μl) and after 5 min, were added 340 μl Na2CO3 for neutralization. The samples were kept 90 min in the dark followed by the reading of the absorbance at 750 nm. The results were expressed as milligram of gallic acid/1 kg sample (mg GAE/1 kg sample).
Antioxidant activity
DPPH - scavenging activity assay
The DPPH scavenging activity assay was done according to a method reported by Brand-Williams, Cuvelier and Berset [45]. 80 μM of DPPH· solution was freshly prepared in 95% methanol. 250 μl of DPPH· solution was allowed to react with 35 μl sample and the absorbance was measured at 515 nm, for 60 min. The chemical kinetics of the extracts was recorded. The antioxidant activity was calculated as follows:
% DPPH· scavenging activity = (1-[Asample/Acontrol t=o]) 100 (1)
The results were expressed as microgram Trolox/g sample (μg TE/g sample).
ORAC - oxygen radical absorbance activity
The oxygen radical absorbance capacity (ORAC) measure the peroxyl radical scavenging activity using as standard 6-hydroxy-2,5,7,8-tetrametylchroman-2-carboxylic acid (Trolox) [46]. A fluorescein stock solution (4x10-3 μM) was made in phosphate buffer (75 mM, pH 7.4) and kept in the dark at 4°C. Before utilization, the fluorescein stock solution was diluted with the phosphate buffer. The fluorescein solution was added to each Trolox standard and grape sample (25 μl) made in phosphate buffer and incubated for 30 min, at 37°C. The reaction was initiated by adding 25 μl 2,2’-azobis-2-amidinopropane (AAPH) and the fluorescence was measured kinetically at excitation wavelength 485 nm and emission wavelength 535 nm, every minute using a fluorescence microplate reader BioTek (Synergy HT, BioTek Instruments, Winooski, VT). The ORAC values for each grape extract were calculated using the net area under the decay curves and were expressed as micromoles Trolox equivalents/g sample (μmol TE/g sample).
Statistical analysis
The design of the experiment was linear, bifactorial type (type of culture x cultivar). The data were expressed as mean ± standard deviation (SD) from three replicates for each sample. In order to determine the significant differences between values, analysis of variance (ANOVA) and Duncan multiple range tests (MRT) were performed. Significance of difference was defined at the 5% level (p < 0.05).
Abbreviations
TPC, Total phenolics content; DPPH, Scavenging activity assay; ORAC, Oxygen radical absorbance capacity; HAT, Hydrogen atom transfer; HPLC, High performance liquid chromatography; FW, Fresh weight; U. A. S.V. M., University of Agricultural Sciences and Veterinary Medicine.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CIB carried out the experimental design, analyses, interpretation of results and preparation of the paper. NP and ACB, contributed equally to the collection of data and development of the grapes sampling. CM and FD* contributed equally to the extraction procedure for carotenoids and polyphenol analysis, statistical analysis and preparation of the paper. AB contributed to the separation, identification and quantification of carotenoids and measuring the antioxidant activity. All authors read and approved the final form of the manuscript.
Contributor Information
Claudiu-Ioan Bunea, Email: claus_bunea@yahoo.com.
Nastasia Pop, Email: nastpop@yahoo.com.
Anca Cristina Babeş, Email: babesanca@yahoo.com.
Cristian Matea, Email: mateatcristian@gmail.com.
Francisc V Dulf, Email: francisc_dulf@yahoo.com.
Andrea Bunea, Email: andrea_bunea@yahoo.com.
Acknowledgements
This research was financially supported by Research Grant No 1215/20 (2012) of University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania. (Director grant: Lecturer Claudiu-Ioan Bunea Ph.D)
References
- Waterhouse AL. Wine phenolics. Annals of The New York Academy of Sciences. 2002;957:21–36. doi: 10.1111/j.1749-6632.2002.tb02903.x. [DOI] [PubMed] [Google Scholar]
- Walzem RL. Wine and health: state of proofs and research needs. Inflammopharmacology. 2008;16:265–271. doi: 10.1007/s10787-008-8027-6. [DOI] [PubMed] [Google Scholar]
- Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Buratti S, Pellegrini N, Brenna OV, Mannino S. Rapid electrochemical method for the evaluation of the antioxidant power of some lipophilic food extracts. J Agr Food Chem. 2001;49:5136–5141. doi: 10.1021/jf010731y. [DOI] [PubMed] [Google Scholar]
- Viljanen K, Sundberg S, Ohshima T, Heinonen M. Carotenoids as antioxidants to prevent photooxidation. Eur J Lipid Sci Tech. 2002;104:353–359. [Google Scholar]
- Giovanelli G, Brenna O. Evolution of some phenolic components, carotenoids and chlorophylls during ripening of three Italian grape varieties. Eur Food Res Technol. 2007;225:145–150. [Google Scholar]
- Guedes De Pinho P, Silva Ferreira AC, Mendes Pinto M, Gomez Benitez J, Hogg TA. Determination of carotenoid profiles in grapes, musts, and fortified wines from Douro varieties of Vitis vinifera. J Agr Food Chem. 2001;49:5484–5488. doi: 10.1021/jf010515p. [DOI] [PubMed] [Google Scholar]
- Oliveira C, Silva-Ferreira AC, Mendez-Pinto M, Hogg T, Alves F, Guedes-De-Pinho P. Carotenoid compounds in grapes and their relationship to plant water status. J Agr Food Chem. 2003;51:5967–5971. doi: 10.1021/jf034275k. [DOI] [PubMed] [Google Scholar]
- Briar SS, Grewal PS, Somasekhar N, Stinner D, Miller SA. Soil nematod community, organic matter, microbial biomass and nitrogen dynamics in field plots transitioning from conventional to organic management. Appl Soil Ecol. 2007;37:256–266. [Google Scholar]
- Winter CK, Davis SF. Organic foods. J Food Sci. 2006;71:117–124. [Google Scholar]
- Asami DK, Hong YJ, Barret DM, Mitchell AE. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic and sustainable agricultural practices. J Agr Food Chem. 2003;51:1237–1241. doi: 10.1021/jf020635c. [DOI] [PubMed] [Google Scholar]
- Lombardi-Boccia G, Lucarini M, Lanzi S, Aguzzi A, Capelloni M. Nutrients and antioxidant molecules in yellow plums (Prunus domestica L.) from conventional and organic productions: a comparative study. J Agr Food Chem. 2004;52:90–94. doi: 10.1021/jf0344690. [DOI] [PubMed] [Google Scholar]
- Dani C, Oliboni LS, Vanderlinde R, Bonatto D, Salvador M, Henriques JAP. Phenolic content and antioxidant activities of white and purple juices manufactured with organically- or conventionally- produced grapes. Food Chem Toxicol. 2007;45:2574–2580. doi: 10.1016/j.fct.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Mulero J, Pardo F, Zaffrilla P. Antioxidant activity and phenolic composition of organic and conventional grapes and wines. J Food Compos Anal. 2010;23:569–574. [Google Scholar]
- Vinkovic Vrcek I, Bojic M, Zuntar I, Mendas G, Medić-Saric M. Phenol content, antioxidant activity and metal composition of Croatian winesderiving from organically and conventionally grown grapes. Food Chem. 2011;124:354–361. [Google Scholar]
- Chassy AW, Bui L, Renaud ENC, Horn MV, Mitchell A. Three-year comparison of the content of antioxidant, microconstituents and several quality characteristics in organic and conventionally managed tomatoes and bell peppers. J Agr Food Chem. 2006;54:8244–8252. doi: 10.1021/jf060950p. [DOI] [PubMed] [Google Scholar]
- Dimbergh LH, Gissen C, Nilsson J. Phenolic compounds in oat grains (Avena sativa L.) grown in conventional and organic system. Ambio. 2005;34:331–337. doi: 10.1639/0044-7447(2005)034[0331:pcioga]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Luthria D, Singh AP, Wilson T, Vorsa N, Banuelos GS, Vinyard BT. Influence of conventional and organic agricultural practices on the phenolic content in eggplant pulp: Plant-to-plant variation. Food Chem. 2010;121:406–411. [Google Scholar]
- Mendes-Pinto MM, Silva-Ferreira AC, Beatriz M, Oliveira PP, Guedes De Pinho P. Evaluation of Some Carotenoids in grapes by Reversed- and Normal-Phase Liquid Chromatography: A qualitative Analysis. J Agr Food Chem. 2004;52(10):3182–3188. doi: 10.1021/jf0499469. [DOI] [PubMed] [Google Scholar]
- Wahlberg I, Eklund AM. In: Carotenoids: Biosynthesis and Metabolism. Britton G, Liaaen-Jensen S, Pfander Basel Boston, editor. Berlin: Birkäuser Verlag; 1998. Degraded carotenoids; pp. 195–216. [Google Scholar]
- Crupi P, Coletta A, Milella RA, Palmisano G, Baiano A, La Notte E, Antonacci D. Carotenoid and Chlorophyll-Derived Componds in Some Wine Grapes Grown in Apulian Region. J Food Sci. 2010;75(4):S191–S198. doi: 10.1111/j.1750-3841.2010.01564.x. [DOI] [PubMed] [Google Scholar]
- Oliveira C, Barbosa A, Silva Ferreira AC, Guerra J, De Pinho PG. Carotenoid Profile in Grapes Related to Aromatic Compounds in Wines from Douro Region. J Food Sci. 2006;71(1):S1–S7. [Google Scholar]
- Crupi P, Millela RA, Antonacci D. Simultaneous HPLC-DAD-MS (ESI+) determination of structural and geometrical isomers of carotenoids in mature grapes. J Mass Spectrom. 2010;45(Special Issue: Food Chemistry):971–980. doi: 10.1002/jms.1794. [DOI] [PubMed] [Google Scholar]
- Sabon I, De Revel G, Kosteridis Y, Bertrand A. Determination of volatile compounds in Grenache Wines in Relation with Different Terroirs in the Rhone Valley. J Agr Food Chem. 2002;50:6341–6345. doi: 10.1021/jf025611k. [DOI] [PubMed] [Google Scholar]
- Lee SH, Seo MJ, Cotta JP, Block DE, Dokoozlian NK, Ebeler S. Vine microclimate and norisoprenoid concentration in Cabernet Sauvignon grapes and wines. Am J Enol Viticult. 2007;58:291–301. [Google Scholar]
- Valls J, Lampreave M, Nadal MY, Arola L. Importancia de los compuestos fenólicos en la calidad de los vinos tintos de crianza, Alimentacion, equipos y tecnologia 2000, Unidad de Enología. pp. 119–124. Dpto. de Bioquímica y Biotecnología Universidad Rovira i Virgili (Tarragona Marzzo)
- Lutz M, Jorquera K, Cancino B, Ruby R, Henriquez C. Phenolics and Antioxidant Capacity of Table Grape (Vitis vinifera) Cultivars Grown in Chile. J Food Sci. 2011;76(7):1088–1093. doi: 10.1111/j.1750-3841.2011.02298.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Martison TE, Liu RH. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem. 2009;116:332–339. [Google Scholar]
- Katalinić V, Smole Mozina S, Skroza D, Generalic I, Abramovic H, Milos M, Liubenkov I, Piskernik S, Pezo I, Terpinic P, Boban M. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extract of 14 Vitis vinifera varieties grown in Dalmatia (Croatia) Food Chem. 2010;119:715–723. [Google Scholar]
- Cantos E, Espín JC, Tomás-Barberán FA. Varietal Differences among the Polyphenol Profiles of Seven Table Grape Cultivars Studied by LC − DAD − MS − MS. J Agr Food Chem. 2002;50(20):5691–5696. doi: 10.1021/jf0204102. [DOI] [PubMed] [Google Scholar]
- Anastasiadi M, Pratsinis H, Kletsas D, Skaltsounis A-L, Haroutounian SA. Bioactive non-coloured polyphenols content of grapes, wines and vinification by-products: Evaluation of the antioxidant activities of their extracts. Food Res Int. 2010;43:805–813. [Google Scholar]
- Cao G, Sofic E, Prior RL. Antioxidant capacity of tea and common vegetables. J Agr Food Chem. 1996;44(11):3426–3431. [Google Scholar]
- Kedage VV, Tilak JC, Dixit GB, Devasagayam TP, Mhatre M. Study of antioxidant properties of some varieties of grapes (Vitis vinifera L.) Crit Rev Food Sci Nutr. 2007;47(2):175–185. doi: 10.1080/10408390600634598. [DOI] [PubMed] [Google Scholar]
- Cho MJ, Howard LR, Prior RL, Clark JR. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high- performance liquid chromatography/mass spectrometry. J Sci Food Agri. 2004;84:1771–1782. [Google Scholar]
- Hogan S, Zhang L, Zoecklein B, Zhou K. Antioxidant properties and bioactive components of Norton (Vitis aestivalis) and Cabernet Franc (Vitis vinifera) winegrapes. LWT-Food Sci Technol. 2009;42(7):1269–1274. [Google Scholar]
- Vallverdú-Queralt A, Medina-Remón A, Casals-Ribes I, Lamuela- Raventos RM. Is there any difference between the phenolic content of organic and conventional tomato juices? Food Chem. 2012;130:222–227. [Google Scholar]
- Stracke B, Rufer C, Weibel F, Bub A, Watzl B. Three-year comparison of the polyphenol contents and antioxidant capacities in organically and conventionally produced apples (Malus domestica Bork. cultivar ‘Golden Delicious’) J Agr Food Chem. 2009;57:4598–4605. doi: 10.1021/jf803961f. [DOI] [PubMed] [Google Scholar]
- Ojeda H, Andary C, Creaba E, Carbonneau A, Deloire A. Influence of pre-and post verasion water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera var. Shiraz. Am J Enol Viticult. 2002;53(4):261–267. [Google Scholar]
- Du B, He PB, Shi PB, Li FY, Li J, Zhu FM. Phenolic content and antioxidant activity of wine grapes and table grapes. J Med Plants Res. 2012;6(17):3381–3387. [Google Scholar]
- Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: a molecules whose time has come? And gone? Clin Biochem. 1997;30:91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- Agrios GN. Plant pathology. fifth ed. London: Elsevier Academic Press; 2005. [Google Scholar]
- Breithaupt DE, Schwack W, Schwack W. Determination of free and bound carotenoids in paprika (Capsicum annuum L.) by LC/MS. Eur Food Res Technol. 2000;21(1):52–55. [Google Scholar]
- Britton G, Liaasen-Jensen S, Pfander H. Carotenoids: Spectroscopy. Basel Boston Berlin: Birkhäuser Verlag; 1994. [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM, Lester P. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method enzymol. 1999;299:152–178. [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. [Google Scholar]
- Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL. Highthroughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96- well format. J Agr Food Chem. 2002;50:4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]