Abstract
Purpose.
To design and develop a drug-delivery system containing a combination of poly(d,l-lactide-co-glycolide) (PLGA) microparticles and alginate hydrogel for sustained release of retinoids to treat retinal blinding diseases that result from an inadequate supply of retinol and generation of 11-cis-retinal.
Methods.
To study drug release in vivo, either the drug-loaded microparticle–hydrogel combination was injected subcutaneously or drug-loaded microparticles were injected intravitreally into Lrat−/− mice. Orally administered 9-cis-retinoids were used for comparison and drug concentrations in plasma were determined by HPLC. Electroretinography (ERG) and both chemical and histologic analyses were used to evaluate drug effects on visual function and morphology.
Results.
Lrat−/− mice demonstrated sustained drug release from the microparticle/hydrogel combination that lasted 4 weeks after subcutaneous injection. Drug concentrations in plasma of the control group treated with the same oral dose rose to higher levels for 6−7 hours but then dropped markedly by 24 hours. Significantly increased ERG responses and a markedly improved retinal pigmented epithelium (RPE)–rod outer segment (ROS) interface were observed after subcutaneous injection of the drug-loaded delivery combination. Intravitreal injection of just 2% of the systemic dose of drug-loaded microparticles provided comparable therapeutic efficacy.
Conclusions.
Sustained release of therapeutic levels of 9-cis-retinoids was achieved in Lrat−/− mice by subcutaneous injection in a microparticle/hydrogel drug-delivery system. Both subcutaneous and intravitreal injections of drug-loaded microparticles into Lrat−/− mice improved visual function and retinal structure.
A novel drug-delivery system was developed for sustained release of therapeutic levels of 9-cis-retinoids. A PLGA microsphere alginate hydrogel combination was used both in vitro and in vivo to evaluate its therapeutic efficacy in retinas of Lrat−/− mice.
Introduction
A continuous adequate supply of 11-cis-retinal is required to produce visual pigments, maintain vision, and preserve photoreceptor health.1–5 Production of this aldehyde involves several enzymatic steps, collectively named the retinoid or visual cycle.6–11 Mutations in genes encoding enzymatic proteins of the visual cycle can cause inadequate production of 11-cis-retinal.1,6,12 Lecithin:retinol acyl transferase (LRAT), which catalyzes the conversion of all-trans-retinol to all-trans-retinyl esters,13–18 and retinal pigment epithelium–specific 65 kDa (RPE65), retinoid isomerase,19–21 are critical enzymes in the retinoid cycle. Mutations in the LRAT or RPE65 genes can cause Leber congenital amaurosis (LCA), an autosomal recessive, early-onset, severe human retinal dystrophy that accounts for 5% of all such inherited disorders.12,22
Pharmacologic replacement of 11-cis-retinal provides great promise for the treatment of human retinal diseases with deficient chromophore biosynthesis.3,5,18,23,24 9-cis-Retinal can successfully replace 11-cis-retinal by bypassing certain biochemical defects in the visual cycle, such as the absence of LRAT activity.5 In fact, 9-cis-retinal is preferred over 11-cis-retinal for replacement therapy because the former is easier to synthesize and chemically more stable.24–26 9-cis-Retinyl esters, including 9-cis-retinyl acetate (9-cis-R-Ac), are prodrugs used for the metabolic generation of 9-cis-retinal in vivo.18,27
Our ultimate research goal was to design optimal drug-delivery systems for the sustained release of 9-cis-retinyl esters to treat animal models of human retinal diseases such as LCA. Our group previously reported that administration of 9-cis-R-Ac by oral gavage improves visual function and preserves retinal morphology in LCA mouse models with LRAT and RPE65 deficiency, that is, Lrat−/− and Rpe65−/− mice.18,23,28,29 We found that 9-cis-retinoid concentrations in plasma increased rapidly to high levels after gavage and then dropped markedly to low levels 5 hours after oral administration.27 Unless initial high drug blood levels are unfavorable, this pharmacokinetic property could be beneficial because it relates to the therapeutic window, especially for drugs targeting peripheral tissues such as the retina through protein-mediated and protein-independent transport systems. A bolus dose could also establish a reversible depot of the drug in other tissues, thereby reducing the need for frequent dosing. For example, we observed that a single dose of 9-cis-retinal to Rpe65−/− mice rescued visual function for months, even when animals were kept under laboratory lighting conditions.23
Nonetheless, sustained therapeutic dosing methods can hold numerous advantages over bolus dosing, including a lower risk of toxicity and an increased duration of therapeutic efficacy. In the case of retinoid supplementation therapy, it provides an additional depot for the drug. The retina poses unique challenges for sustained therapeutic dosing because of its anatomic isolation.30 Frequent injections of compounds directly into the vitreous cavity are associated with complications such as retinal detachment, hemorrhage, uveitis, endophthalmitis, and infections.31,32 Therefore, delivery of hydrophobic 9-cis-retinoids directly by this route presents an additional concern.
The Lrat−/− mouse provides an excellent model for human LCA because it exhibits early-onset, slowly progressive severe retinal degeneration. Because the RPE of Lrat−/− mice is devoid of all-trans-retinol and all-trans-retinyl esters, the photoreceptors lack functional rhodopsin, and electroretinographic (ERG) responses are dramatically attenuated. Lrat−/− mice have been used to test the efficacy of pharmacologic agents such as 9-cis-R-Ac and a standard gene replacement technique.16,18 Moreover, lack of LRAT in the liver and other tissues has no apparent deleterious effects.16,33–35 These mice constitute an important experimental model for retinoid metabolism and vitamin A deprivation as well as for human LCA.17,29,33–37
To achieve sustained release of 9-cis-retinoids, we designed a drug-delivery system that uses a combination of poly(d,l-lactic-co-glycolic) acid (PLGA) microparticles and alginate hydrogel.38 PLGA is a Food and Drug Administration–approved biodegradable polymer that undergoes hydrolytic degradation,39 and thus could be an ideal system to encapsulate highly hydrophobic 9-cis-R-Ac. Alginate is a biocompatible polysaccharide extensively studied as a matrix for cell and growth factor encapsulation due to its rapid ion-inducible gelation under mild conditions without the involvement of organic solvents40 (Fig. 1). The alginate hydrogel can be used to trap PLGA microparticles in an injectable microparticle/hydrogel combination.41 This drug-delivery system has several advantages. First, the alginate hydrogel retains microparticles at injection sites,42 which otherwise would rapidly disperse.43 Second, microspheres are susceptible to phagocytosis, and alginate hydrogel prevents phagocytosis of microparticles by macrophages.44 Third, the hydrogel slows drug diffusion from the microparticles and thus blunts an initial excessive drug release.45 Fourth, this drug-delivery system can maintain optimal therapeutic drug levels, reduce side effects by sustained drug release, and improve patient compliance by decreasing the frequency of drug administration in a minimally invasive manner.46
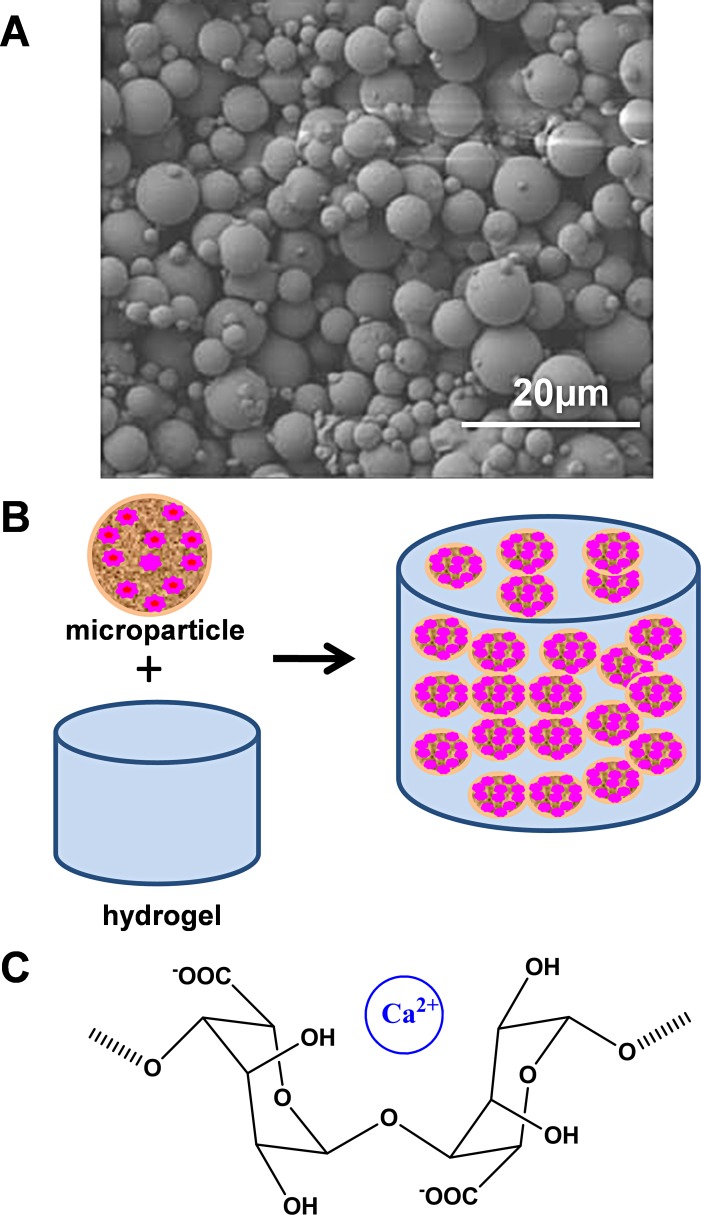
Figure 1. .
Schematic description of the microparticle/hydrogel combination delivery system. Scanning electron microscopic image of PLGA microparticles loaded with 9-cis-R-Ac. Schematic illustrating the preparation of a microparticle (brown) loaded with 9-cis-R-Ac (red) and combined with hydrogel. Chemical structure of the alginate monomer that condenses in the presence of Ca2+ to form the hydrogel.
To test our hypothesis that sustained retinoid release could be achieved by using a microsphere/hydrogel combination (Fig. 1) and rescue visual function, we prepared a PLGA microsphere alginate hydrogel combination, investigated 9-cis-retinoid release from this preparation both in vitro and in vivo, and evaluated its therapeutic efficacy in retinas of Lrat−/− mice.
Materials and Methods
Materials
The following materials (and suppliers) were used: poly(d,l-lactic-co-glycolic acid) (PLGA), Resomer RG 752 S (Boehringer Ingelheim Chemicals, Inc., Petersburg, VA); poloxamer-188 (Sigma 412325), ethyl acetate, alginate sodium, calcium chloride, dimethyl sulfoxide (DMSO), and soybean oil (Sigma-Aldrich, St. Louis, MO); all-trans-retinyl acetate (all-trans-R-Ac) and 9-cis-R-Ac (Toronto Research Chemicals, Inc., Toronto, Canada); vitamin A−free mouse diet (Research Diets, Inc., New Brunswick, NJ).
Animals
Lrat−/− mice were generated and genotyped as described previously,16 and 5-week-old animals of both sexes were used for the experiments described. All mice were housed in the animal facility at the School of Medicine, Case Western Reserve University, where they were maintained on a standard diet under a 12-hour light (<10 lux)/12-hour dark cycle. At least 24 hours before experiments were initiated, mice were placed and maintained in a dark environment. Procedures were performed under dim red light transmitted through a safelight filter (transmittance >560 nm; No. 1; Kodak, Rochester, NY). All animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committee and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanasia and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Preparation and Characterization of PLGA Microparticles and Alginate Hydrogel Loaded with Microparticles
9-cis-R-Ac−loaded microparticles were prepared by an oil-in-water emulsion method.47 Briefly, 90 mg of 9-cis-R-Ac and 360 mg of PLGA were dissolved in 1.2 mL ethyl acetate and 120 mg of poloxamer-188 were dissolved in 3 mL deionized water. The two solutions were combined and emulsified by vigorous mixing with a vortex. The resulting emulsion was stirred at room temperature for 3 hours to evaporate the organic solvent. Hydrogels loaded with the above microparticles were prepared by ionic cross-linking of alginate (2%, w/v) in a suspension of PLGA microspheres through addition of calcium chloride (1%, w/v) (Figs. 1A, 1B).
Scanning electron microscopy (SEC) (Field-Emission Gun Scanning Electron Microscope, Hitachi S4500; Hitachi Ltd., Tokyo, Japan) was used to determine the size and morphology of the microparticles. A suspension of microparticles in water solution was mounted on aluminum holders at room temperature, dried for 2 days, and then coated with palladium−gold. Microparticle images were captured and analyzed with ImageJ software (1.42q; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsbweb.nih.gov/ij/index.html) to obtain their mean diameters and standard deviations. To determine the content of 9-cis-R-Ac encapsulated in the microparticles, we dissolved PLGA microparticles in a small amount of ethyl acetate followed by dilution with ethyl alcohol. The concentration of 9-cis-R-Ac (or all-trans-R-Ac) was then determined by UV spectroscopy at 325 nm using ε = 49,528 M−1 cm−1. Loading efficiency is expressed as the ratio between the actual 9-cis-R-Ac or all-trans-R-Ac content of the microparticles and the amount initially added to the microparticles. Loading capacity is expressed as the ratio between the actual 9-cis-R-Ac or all-trans-R-Ac content of the microparticles and the total amount of microparticles.
Retinoid Administration
All retinoids were administrated to Lrat−/− mice under dim red light. Hydrogel-loaded microparticles were subcutaneously injected into the dorsal region of mice as a single dose containing 6 mg 9-cis-R-Ac or all-trans-R-Ac/mouse in a volume of 200 μL. For oral administration, retinyl acetate was first dissolved in ethanol (5%, v/v) and then diluted with soybean oil (95%, v/v) to a final concentration of 20 mg/mL. The soybean oil preparation (300 μL) was gavaged into mice as a single dose of 6 mg of retinyl acetate/mouse. For intravitreal injection, a PBS suspension of microparticles was injected into a mouse eye as a single dose containing 0.06 mg of retinyl esters in 1 μL of volume. The intravitreous injection was performed as previously described.48
Retinoid Analyses
Retinoid extraction and analyses were conducted as previously published.23,24,37,49 All experimental procedures were performed under dim red light provided by a safelight filter (transmittance 560 nm; Eastman Kodak). Retinoids in plasma were extracted with hexane and analyzed by HPLC (Agilent 1100; Agilent Technologies, Palo Alto, CA). A normal-phase column (Zorbax SIL 4.6 × 250 mm, 5 μm; Agilent Technologies) was used to separate retinoids with a mobile phase of 10% ethyl acetate and 90% hexane at a flow rate of 1.4 mL/min with detection at a wavelength of 325 nm. Identification and quantification of retinoids in eluted fractions were achieved by comparing their retention times and UV absorption at 325 nm with those of pure retinoid standards.
Electroretinography (ERG) Recording
ERG recordings were performed as described previously.49–52 Lrat−/− mice were dark-adapted prior to the procedure and then anesthetized by intraperitoneal injection of a mixture containing ketamine (80 mg/kg body weight) and xylazine (20 mg/kg body weight) in 10 mM sodium phosphate, pH 7.2, with 100 mM NaCl. Pupils were dilated with 1 drop of 1% tropicamide ophthalmic solution. ERG responses were measured with a universal testing and electrophysiologic system (UTAS with BigShot; LKC Technologies, Inc., Gaithersburg, MD). To stabilize body temperature, mice were placed on a heated pad (37°C) in a Ganzfeld chamber, where positioning of the contact lens electrodes was adjusted. Then two needle electrodes were placed under the skin of the forehead and tail as a reference and ground electrode, respectively, and two contact lens electrodes were placed on the eyes. Recording commenced when the lenses were well contacted and the baseline was stable. Single-flash ERGs were recorded simultaneously from both eyes with scotopic flash intensities (from −3.7 to 1.6 log cd·s·m−2), and two to five recordings were made at sufficient intervals between flash stimuli (from 10–60 seconds) to allow mice time to recover. Amplitudes were measured by a commercial software package (EM software for Windows; LKC Technologies, Inc.) from baseline to the negative peak for a-waves and from the trough to the highest peak for b-waves.
Spectral Domain–Optical Coherence Tomography (SD-OCT)
Ultra-high resolution SD-OCT (Bioptigen Inc., Durham, NC) was used for in vivo imaging of mouse retinas as described previously.49 Mice were anesthetized by intraperitoneal injection of a mixture containing ketamine (80 mg/kg body weight) and xylazine (20 mg/kg body weight) in 10 mM sodium phosphate, pH 7.2, with 100 mM NaCl. Pupils were dilated with 1% tropicamide ophthalmic solution. Four pictures acquired in the B-scan mode were used to construct each final averaged SD-OCT image. The superior retina, inferior retina, and optical nerve head regions were imaged in each eye.
Histologic Evaluation
Retinal histologic analyses were described previously.49,53 Briefly, mouse eyecups were prepared immediately after euthanasia by removal of the cornea and lens. Eyecups were fixed in 2% glutaraldehyde, 4% paraformaldehyde and processed for embedding in Epon. Sections for routine histology were cut at 1 μm and stained with toluidine blue.
Statistical Analyses
All data are presented as means ± SD values, with the number of experiments indicated. ImageJ image-analysis software (1.42q; National Institutes of Health) was used for quantification. The two-tailed Student's t-test was used for statistical analyses with a value of P < 0.05 considered statistically significant.
Results
Production of Retinoid-Loaded Microparticles
Initially we characterized microparticle preparations by their morphologic appearance and chemical content. Examined by SEC (Fig. 1A), the microparticles were essentially spherical with relatively nonporous surfaces. Particle sizes varied from 1 to 9 μm with an average of 5 ± 3.1 μm. Analysis of microparticle retinoid content by UV spectroscopy indicated that retinoids were encapsulated within microparticles with a loading efficiency close to 100% and a loading capacity of 19 ± 2% (see Materials and Methods section). These preparations were highly reproducible from one batch to the next. Then we used a calcium method to embed the microparticles into alginate hydrogel41 (Figs. 1B, 1C).
Retinoid Concentrations in Lrat−/− Mouse Plasma after a Single Dose of Retinoids Delivered by Oral Gavage or by Microparticle Subcutaneous Injection
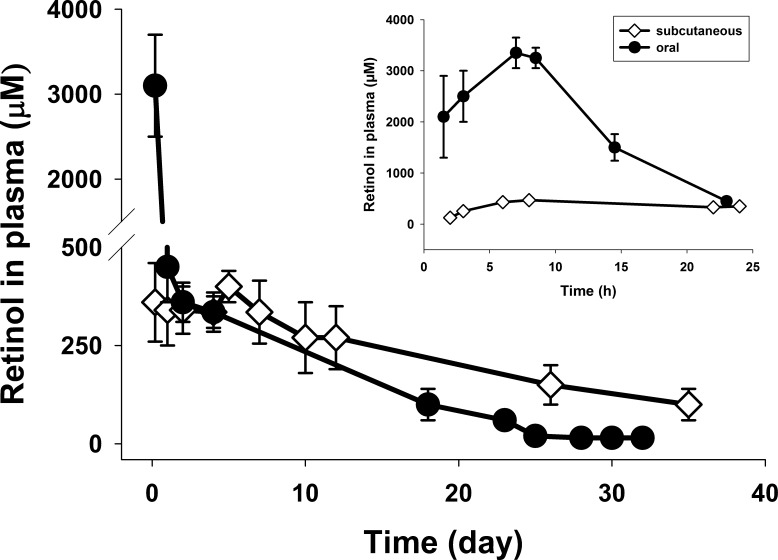
Vitamin A (all-trans-retinol) is esterified by LRAT and stored in liver and eye. Knockout of LRAT markedly diminishes these retinyl ester stores and makes mice highly susceptible to vitamin A deficiency,16,33,34 such that only trace amounts of all-trans-retinyl esters are found in the liver and blood of Lrat−/− mice.16,18 Due to lack of vitamin A stores, nearly all the retinol in the bodies of Lrat−/− mice is derived from the food supply.16,18 Feeding Lrat−/− mice a diet lacking vitamin A results in rapid retinol deficiency.34 Our experimental data demonstrated that the retinol concentration in plasma of Lrat−/− mice decreased from 270 ± 30 to 50 ± 20 μM in plasma 1 month after they were placed on a vitamin A−free diet. However, retinyl esters are readily metabolized to retinol in the body by hydrolysis.27 First, to test all-trans-R-Ac release in Lrat−/− mice, we maintained them on a diet lacking vitamin A for 1 month. Then all-trans-R-Ac in the microparticle/hydrogel delivery system was subcutaneously injected in a single dose of 6 mg/mouse. Mice remained on the vitamin A−free diet until euthanized at scheduled time points when their plasma was collected for HPLC analysis. The results are shown in Figure 2. Retinol concentrations (μM) in plasma postsubcutaneous injection were approximately 160 μM at 2 hours, 450 μM at 7 hours, 330 μM at 24 hours (Fig. 2, inset, extended scale), then remained nearly constant at approximately 330 μM for 2 weeks, and still could be detected at 140 μM after 1 month (Fig. 2). In control experiments, all-trans-R-Ac in soybean oil was delivered by gastric gavage to Lrat−/− mice in a single dose of 6 mg/mouse. Retinol concentrations in plasma after oral administration dramatically differed from those after subcutaneous injection. Drug concentrations rapidly increased after oral administration, reaching a peak 7 hours postgavage, and then decreased to a much lower level 24 hours postgavage. Concentrations (μM) in plasma post−oral gavage were approximately 2500 μM at 2 hours, 3400 μM at 7 hours, decreasing to 450 μM at 24 hours and then kept on decreasing until they were barely detectable 1 month later (Fig. 2). Lrat−/− mice tolerated subcutaneous injection and oral gavage well; no weight loss or gross changes in behavior were observed.
Figure 2. .
Retinol concentrations in the plasma of 5-week-old Lrat−/− mice after a single dose of all-trans-R-Ac. Before administration of all-trans-R-Ac, 5-week-old Lrat−/− mice were maintained for 1 month on a diet lacking vitamin A. For subcutaneous injection, all-trans-R-Ac in the microparticle/hydrogel delivery system was subcutaneously injected into Lrat−/− mice in a single dose of 6 mg/mouse. For oral administration, all-trans-R-Ac in soybean oil was delivered by gastric gavage to Lrat−/− mice at the same single dose of 6 mg/mouse. Inset: an expanded initial time course.
Improved ERG Responses in Lrat−/− Mice 1 Month after Subcutaneous Injection of 9-cis-R-Ac in the Microparticle/Hydrogel Delivery System
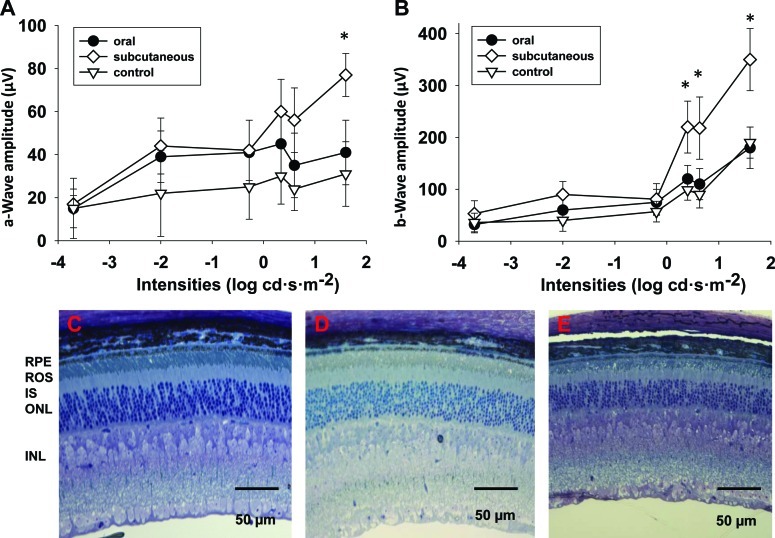
Rod and cone functions of Lrat−/− mice become dramatically attenuated because of chromophore deficiency and resulting absence of visual pigments. Rods of Lrat−/− mice are approximately 2000-fold less sensitive to light stimuli than WT rods.18 Delivering the artificial 9-cis-retinoid chromophore to the retina leads to generation of the visual pigment, isorhodopsin, in Lrat−/− mice, thereby restoring visual function. We recorded ERG responses in Lrat−/− mice treated with 9-cis-R-Ac to monitor their visual function. All experiments were initiated with 5-week-old Lrat−/− mice. 9-cis-R-Ac in the microparticle/hydrogel delivery system was subcutaneously injected into Lrat−/− mice in a single dose of 6 mg/mouse (n = 9). In the oral administration group, 9-cis-R-Ac in soybean oil was delivered by gavage in a single dose of 6 mg/mouse (n = 9) and in the control group, no drug was administrated (n = 5). All mice were maintained under a regular 12-hour light (<10 lux)/12-hour dark cycle. Three weeks after drug administration, mice were transferred to a darkroom for 1 week, and then single-flash scotopic ERGs were recorded. Delivery of 9-cis-retinoid in the microparticle/hydrogel produced significant increases in both a-wave and b-wave amplitudes, starting at a stimulus intensity of 1.6 log cd·s·m−2 for a-waves and 0.4 log cd·s·m−2 for b-waves. However, ERG responses of Lrat−/− mice orally gavaged with 9-cis-retinoid were comparable to baseline responses in untreated Lrat−/− mice (Fig. 3). Thus, the increase of both ERG a-wave and b-wave amplitudes suggested that release of 9-cis-R-Ac from microparticle/hydrogel, and perhaps buffered by retinosomes,17,37,54–57 was sustained continuously as indicated in the retinoid-releasing profile (Fig. 2). This maintained release of 9-cis-R-Ac caused an improvement of retinal function in Lrat−/− mice at even 4 weeks after administration, which was not attainable by single dose oral treatment.
Figure 3. .
Improved ERG and retinal histology 1 month after 5-week-old Lrat−/− mice received subcutaneous injection of 9-cis-R-Ac in the microparticle/hydrogel delivery system. For the subcutaneous injection group, 9-cis-R-Ac in the microparticle/hydrogel suspension was subcutaneously injected into 5-week-old Lrat−/− mice in a single dose of 6 mg/mouse (n = 9). For the oral administration group, 9-cis-R-Ac in soybean oil was delivered by gavage to Lrat−/− mice in a single dose of 6 mg/mouse (n = 9). No drug was given to Lrat−/− mice in the control group (n = 5). All mice were housed in a regular 12-hour light (<10 lux)/12-hour dark environment. (A, B) Illustration of a-wave and b-wave amplitudes, respectively. Data are presented as means ± SDs. (C) The rod outer segment (ROS) layer of the subcutaneous injection group was thicker than that of the untreated control group, and more tightly packed than ROS layers in both the orally gavaged and untreated control groups 1 month after subcutaneous injection of 9-cis-R-Ac in microparticle/hydrogel. Panels from left to right present cross-sections of retinas from representative Lrat−/− mice after 9-cis-R-Ac subcutaneous injection (C), oral administration (D), and no treatment (control) (E), respectively. Labeled layers of the retina are as follows: ONL, outer nuclear layer; IS, inner segment; OS, outer segment; RPE, retinal pigmented epithelium.
Retinal Histology of 9-cis-R-Ac−Treated Lrat−/− Mice
Functional rhodopsin (or isorhodopsin) is required to maintain normal ROS morphology.16,18,58 Therefore, if treatment of Lrat−/− mice with 9-cis-retinoids can restore visual function, it should also preserve ROS structure. Histologic analyses revealed that at the age of 6−8 weeks, ROS lengths of Lrat−/− mice were reduced to 65% of those observed in WT control mice.18 EM analysis of retinas demonstrated that ROS layers of Lrat−/− mice were shorter, thinner, and less tightly packed than those of WT mice. It was also reported that, after gavaging dark room−reared Lrat−/− mice with a high dose of 9-cis-R-Ac (total dose of 19.68 mg in 6 gavages of 3.28 mg each at 3-day intervals), the ROS layer became substantially thicker, increasing from 10.7 ± 0.16 to 14.2 ± 2.2 μm, and the RPE−ROS interface displayed closer apposition than that seen in untreated control Lrat−/− mice.18 In this study, we observed histologic retention of ROS structures 1 month after subcutaneous injection of 9-cis-R-Ac in the microparticle/hydrogel (Fig. 3, bottom panels). The ROS layer of the subcutaneous injection group was thicker than that of the untreated control group, and more tightly packed than ROS layers in both the orally gavaged and untreated control groups.
Improved ERG Responses in Lrat−/− Mice 10 Days after Intravitreal Injection of 9-cis-R-Ac in PLGA Microparticles at 2% of the Dose Used for Systemic Administration, Subcutaneous Injection, and Oral Administration
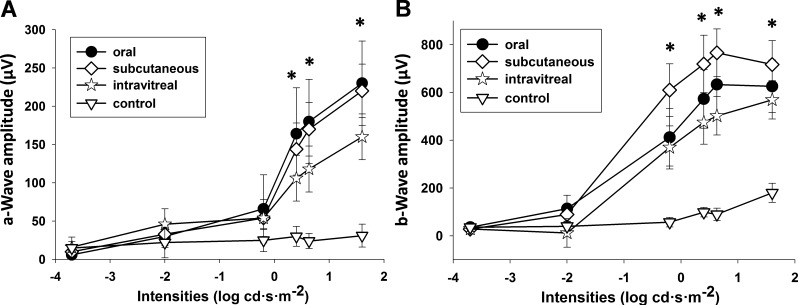
Local therapy can maximize a therapeutic response by increasing drug levels in the target tissue while minimizing adverse side effects by reducing systemic drug concentrations.59–61 We recorded ERG responses to evaluate retinal function after intravitreal injection given as a local therapy. All experiments were initiated in 5-week-old dark-reared Lrat−/− mice divided into four groups. Mice were maintained in the dark after treatment to minimize light-dependent bleaching of iso-rhodopsin and the release of isomerized chromophore as all-trans-retinal in treated Lrat−/− mice. For the intravitreal injection group, 9-cis-R-Ac in microparticles was injected into both mouse eyes as a single dose of 0.06 mg/eye (n = 9). For the subcutaneous injection group, 9-cis-R-Ac in microparticle/hydrogel was subcutaneously injected into the Lrat−/− mice in a single dose of 6 mg/mouse (n = 9). 9-cis-R-Ac in soybean oil was gavaged into Lrat−/− mice in a single dose of 6 mg/mouse for the oral administration group (n = 9). No drug was administrated to Lrat−/− mice in the control group (n = 5). Single-flash scotopic ERGs were recorded 10 days after drug administration. ERG responses were significantly improved by 9-cis-R-Ac therapy. Both a-wave and b-wave amplitudes were significantly increased beginning at a stimulus intensity of 0.4 log cd·s·m−2 for a-waves and −0.2 log cd·s·m−2 for b-waves in all three treatment groups (intravitreal injection, oral, and subcutaneous injection) as compared with the untreated control group (Fig. 4). There was no significant difference in both a-wave and b-wave amplitudes between the intravitreal injection group and the other treated groups. However, the dose used for intravitreal injection was only 2% that used for the other two groups treated by systemic 9-cis-R-Ac administration. Presumably local treatment required a much lower dose to achieve a comparable effect.
Figure 4. .
Improved ERG responses in 5-week-old Lrat−/− mice 10 days after intravitreal injection of 9-cis-R-Ac in PLGA microparticles at 2% of the 6 mg per mouse dose used for subcutaneous injection and oral administration. ERG responses at high illumination intensities improved in the intravitreal group as compared with the control group and were comparable to those of the other 9-cis-R-Ac−treated groups. For the intravitreal injection group, 9-cis-R-Ac in microparticles was injected into mouse eye at a single dose of 0.06 mg/eye (n = 9). For the subcutaneous injection group, 9-cis-R-Ac in microparticle/hydrogel was subcutaneously injected into the Lrat−/− mice in a single dose of 6 mg/mouse (n = 9). For the oral administration group, 9-cis-R-Ac in soybean oil was gavaged into Lrat−/− mice in a single dose of 6 mg/mouse (n = 9). No drug was given to the control untreated group (n = 5). Lrat−/− mice were housed in a darkroom after these treatments. (A, B) Quantification of a-wave and b-wave amplitudes, respectively. Data are presented as means ± SD.
Retinal Morphology of Lrat−/− Mice after Intravitreal Injection of 9-cis-R-Ac in PLGA Microparticles
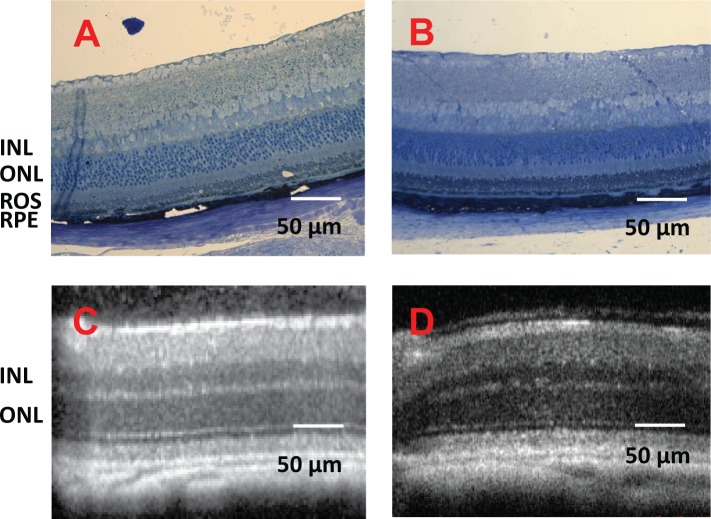
To assess the impact of intraocular administration of the microparticle formulation, high-resolution SD-OCT images were obtained at 10, 20, and 30 days after Lrat−/− mice received an intravitreal injection of 9-cis-R-Ac in PLGA microparticles. SD-OCT provides the capability of imaging the retina in vivo and tracking changes in the same eye over time. The SD-OCT imaging revealed no apparent changes in retinal structures over 30 days after intravitreal microparticle injection (Fig. 5). Thus, intravitreal injection of these microparticles caused no apparent damage to the retina.
Figure 5. .
Retinal morphology 1 month after 5-week-old Lrat−/− mice received an intravitreal injection of 9-cis-R-Ac in PLGA microparticles. No retinal damage was detected 1 month after intravitreal injection of these microparticles. (A, B) Representative histologic cross-sections of retinas from the intravitreal injected and uninjected control groups, respectively. (C, D) OCT images of representative retinas from the same two groups. Labeled layers of the retina are as follows: ONL, outer nuclear layer; OS, outer segment; RPE, retinal pigmented epithelium.
Improved ERG Responses in Lrat−/− Mice 1 Month after Intravitreal Injection of 9-cis-R-Ac in PLGA Microparticles
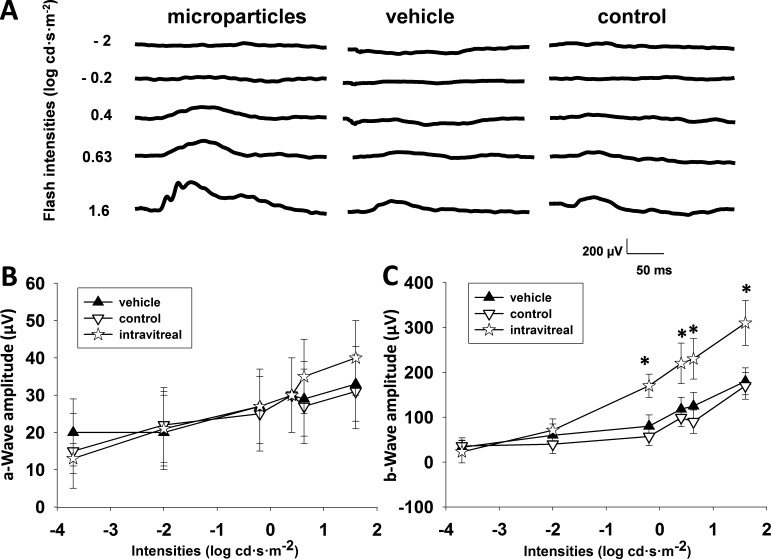
9-cis-Retinoid can be released from PLGA microparticles loaded with 9-cis-R-Ac by hydrolytic degradation of the polymer after intravitreal injection. To assess the duration of drug release, ERG responses of Lrat−/− mice were recorded 1 month after intravitreal injection of 9-cis-R-Ac in PLGA microparticles. All mice were maintained under a regular 12-hour light (<10 lux)/12-hour dark cycle. Three weeks after intravitreal injection, mice were transferred to a dark environment for 1 week and then single-flash scotopic ERGs were recorded. Five-week-old mice were divided into three groups when the experiments were initiated. For the intravitreal injection group, 9-cis-R-Ac in microparticles was injected into Lrat−/− mouse eyes in a single dose of 0.06 mg/eye (n = 9). For the vehicle group, PLGA microparticles in PBS lacking retinoids were intravitreally injected into Lrat−/− mouse eyes (n = 5). For the other control group, no injection was given to Lrat−/− mice (n = 5). Release of 9-cis-retinoid from microparticles improved the ERG response; b-wave amplitudes increased significantly beginning at a stimulus intensity of −0.2 log cd·s·m−2, as compared with similar but smaller amplitudes exhibited by the control vehicle and untreated groups (Fig. 6). No significant differences among all these groups were noted in a-wave amplitudes. Light exposure caused bleaching of visual pigment by releasing isomerized chromophore as all-trans-retinol in treated Lrat−/− mice in proportion to the period of bleaching light exposure. Sustained uptake of 9-cis-retinoid (released from microparticles) by opsin can restore retinal function efficiently. Presumably such sustained release of 9-cis-retinoid from microparticles lasted for nearly 1 month to improve visual function, thereby substantially increasing b-wave amplitudes relative to those in either the vehicle-treated or noninjected control groups. At 1 month after administration, retinoid contents in the eyes and blood were analyzed by HPLC. However, only trace levels of 9-cis-retinoid were detected in these samples.
Figure 6. .
Improved ERG responses 1 month after 5-week-old Lrat−/− mice received an intravitreal injection of 9-cis-R-Ac in PLGA microparticles. For the intravitreal injection group, 9-cis-R-Ac in microparticles was injected into Lrat−/− mouse eyes as a single dose of 0.06 mg per each eye (n = 9). For the vehicle group, PLGA microparticles lacking retinoids were intravitreally injected into each Lrat−/− mouse eye instead (n = 5). (A) Representative ERG traces from 9-cis-R-Ac/microparticle−treated, vehicle-treated, and uninjected control animals. Data are presented as means ± SDs. The control group of Lrat−/− mice received no injections (n = 5). (B, C) a- and b-wave amplitudes, respectively.
Discussion
In this study we demonstrate that a drug-delivery system containing a combination of PLGA microparticles and alginate hydrogel for sustained release of 9-cis-R-Ac is effective in treating mice with a dysfunctional retinoid cycle that provides an inadequate supply of 11-cis-retinal, the chromophore essential for vision.4,8 Two methods of 9-cis-R-Ac delivery were tested. First, we injected the drug-loaded microparticle/hydrogel combination subcutaneously into Lrat−/− mice. Second, local intravitreal injection of drug-loaded microparticles was performed to compare its therapeutic efficacy with that derived from subcutaneous injection and oral administration. Oral administration of 9-cis-retinoids in soybean oil along with determination of drug-derived metabolites in plasma were used to compare the present experimental findings with those of previous studies.18 Our in vivo studies in Lrat−/− mice demonstrated sustained drug release from the microparticle/hydrogel combination that lasted 4 weeks after subcutaneous injection. In comparison, drug concentrations in the plasma of the control group treated orally with the same dose had significantly higher peak levels initially, but drug levels had decreased markedly by 24 hours and were similar to those after subcutaneous injection. Significantly increased ERG responses and a markedly improved RPE−ROS interface were observed after subcutaneous injection of the delivery system loaded with drug. Intravitreal injection of 2% of the systemic dose of drug-loaded microparticles provided comparable therapeutic efficacy. We conclude that sustained release of therapeutic levels of 9-cis-retinoids was achieved in Lrat−/− mice by subcutaneous injection of this microparticle/hydrogel drug-delivery system. Moreover, subcutaneous injection of the combination into Lrat−/− mice proved as efficacious as oral administration of 9-cis-retinoid. Intravitreal injection of the preparation as local therapy was just as efficacious as both systemic treatments, but at a much lower dose. Thus, sustained delivery of 9-cis-retinal to photoreceptors by this drug-delivery system offers a potential alternative to maintain and even restore vision in humans with certain forms of hereditary blindness. These findings support previous work carried out by the Crouch laboratory that used a different matrix for retinoid delivery.62
To overcome metabolic blockade in the visual cycle, two pharmacologic interventions are currently under development. Conceptually, the simplest strategy is to use gene therapy to replace defective genes. This strategy has been used successfully in LCA animal models (mice and dogs), and its positive effect was sustained for several years after a single treatment in RPE65-null dogs.63,64 Recently, the first LCA patients were enrolled for gene therapy. Initial results revealed that this treatment partially restored vision in retinal areas of gene transfer for patients with blindness attributed to RPE65 mutations65–67 (for review see Cideciyan1). The advantage of this approach is its intellectual simplicity and applicability to many different mutations in single or several genes. However, pharmacologic replacement of a missing chromophore is also applicable to diseases resulting from deficient chromophore biosynthesis. Examples include LCA arising from mutations in either the LRAT or RPE65 genes. Initial experiments aimed at bypassing the biochemical defect caused by absence of RPE65 were performed by oral gavage of Rpe65−/− mice with 9-cis-retinal.23 9-cis-Retinal, which combines with opsin to form light-sensitive iso-rhodopsin,23 was initially selected because it is chemically more stable than 11-cis-retinal. Moreover, iso-rhodopsin has an absorbance maximum of 494 nm versus 502 nm for rhodopsin, permitting experimental identification of reconstituted iso-rhodopsin.68 Further extensive testing then identified 9-cis-R-Ac as a useful drug.18,27 Dietary supplementation of Rpe65−/− mice with 9-cis-retinal restored light sensitivity to levels found in WT animals, as assessed by both single-cell and scotopic ERG recordings. Pharmacologic intervention also has the advantage that several options for drug delivery are now feasible.
Delivering therapeutics to the eye is challenging because several barriers prevent or limit foreign materials from entering this isolated specialized structure. Topical administration of eyedrops69 is simple and offers good patient compliance, but suffers from low drug uptake (<5% by the eye) and usually is limited to treatment of diseases of the anterior segment. Systemic injection/infusion70 is also convenient, but requires high doses that can cause systemic side effects and toxicity, to achieve and maintain therapeutic concentrations in the eye. Subconjunctival injection70 has been used to increase drug concentrations in the uvea, but drug penetration to the retina is often limited by the sclera and choroid.70 Intravitreal injection of the drug directly as an implant59 is the most effective but also the most invasive approach for treating retinal diseases. Indeed, in a proof-of-principle study, 9-cis-retinal was used successfully for pharmacologic trials in RPE65−/− dogs.71 In most treated dogs, 9-cis-retinal injected intravitreally resulted in increased rod electroretinogram responses and improved vision. However, injected therapeutics often have short retention times in the vitreous as well, requiring frequent drug administration to maintain therapeutic levels with the attendant risk of serious complications.32,72 To reduce injection frequency, various intraocular drug delivery systems, including nanoparticles and implants, have been developed to maintain therapeutic drug concentrations in the vitreous for prolonged periods.73
9-cis-Retinoids are high-potential drug candidates for several retinal degenerative diseases.3,5,27,28,74 These molecules can bypass defects in the visual cycle and regenerate visual pigments in photoreceptor cells, preserving visual function and ameliorating progressive retinal degeneration. We injected the PLGA system loaded with 9-cis-retinoids subdermally into Lrat−/− mice (a model for human LCA that cannot regenerate 11-cis-retinal) and then compared the recovery of visual function after a bright-light stimulus and 9-cis-retinal levels in the eye with the same parameters in mice gavaged with 9-cis-retinoids. Four weeks after drug administration, visual function of PLGA-treated mice was better than that of mice receiving 9-cis-retinoids by oral gavage. PLGA is a promising polymer for drug delivery but it has not yet been designed or optimized for delivery of retinoid drugs that can be used in clinical studies. The first study involving sustainable delivery of retinoids was carried by the Crouch laboratory with a biodegradable gelatinous protein mixture secreted by mouse tumor cells62 (Matrigel; BD Biosciences, Franklin Lakes, NJ) that was loaded with 9-cis-retinal and tested in Rpe65−/− mice with favorable results,62 suggesting that this drug strategy could be viable; however, the proteinous composition of secreted mixture will likely limit its use in humans.
Selection of an appropriate delivery system is critical for drug efficacy and safety. Oral delivery is preferable, but rapid time to peak blood concentrations after absorption combined with rapid clearance mechanisms can limit its applications, especially when high concentrations are toxic and low levels fail to achieve therapeutic efficacy. The search for an appropriate system with favorable pharmacokinetics to achieve and maintain therapeutic levels of a drug in the eye is an essential requirement for optimal treatment of ocular diseases. Present polymers suffer limitations such as low ratios of active compounds to carrier, complications from either intraocular or subcutaneous injections that carry their own inherent risks, and poor control of drug levels at their sites of action. These challenges should be mitigated by the next generation of improved ocular drug-delivery systems.
Acknowledgments
The authors thank Zhiqian Dong for expert handling of mice, Satsumi Roos for block preparation and plastic sectioning, Melissa Matosky for retinoid analyses; Leslie T. Webster Jr, Johannes von Lintig, and members of Palczewski's laboratory for critical comments on the manuscript.
Footnotes
Supported in part by National Eye Institute/National Institutes of Health Grants EY009339, EY021126, EY019880, P30 EY11373, and T32EY007157 (S-QG); the Research to Prevent Blindness Foundation; Foundation Fighting Blindness; Fight for Sight and the Ohio Lions Eye Research Foundation. KP is John H. Hord Professor of Pharmacology.
Disclosure: S.-Q. Gao, None; T. Maeda, QLT, Inc. (C); K. Okano, None; K. Palczewski, QLT, Inc. (C), P
References
- 1.Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29:398–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu SM, Thompson DA, Srikumari CR, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197 [DOI] [PubMed] [Google Scholar]
- 3.Palczewski K. Blind dogs that can see: pharmacological treatment of Leber congenital amaurosis caused by a defective visual cycle. Arch Ophthalmol. 2010;128:1483–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palczewski K. Retinoids for treatment of retinal diseases. Trends Pharmacol Sci. 2010;31:284–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20:469–529 [DOI] [PubMed] [Google Scholar]
- 8.von Lintig J, Kiser PD, Golczak M, Palczewski K. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem Sci. 2010;35:400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiser PD, Golczak M, Maeda A, Palczewski K. Key enzymes of the retinoid (visual) cycle in vertebrate retina. Biochim Biophys Acta. 2012;1821:137–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker RO, Crouch RK. Retinol dehydrogenases (RDHs) in the visual cycle. Exp Eye Res. 2010;91:788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb TD, Pugh EN Jr. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380 [DOI] [PubMed] [Google Scholar]
- 12.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419 [DOI] [PubMed] [Google Scholar]
- 13.Saari JC, Bredberg DL, Farrell DF. Retinol esterification in bovine retinal pigment epithelium: reversibility of lecithin: retinol acyltransferase. Biochem J. 1993;291:697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald PN, Ong DE. Evidence for a lecithin-retinol acyltransferase activity in the rat small intestine. J Biol Chem. 1988;263:12478–12482 [PubMed] [Google Scholar]
- 15.Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, Bok D. Molecular and biochemical characterization of lecithin retinol acyltransferase. J Biol Chem. 1999;274:3834–3841 [DOI] [PubMed] [Google Scholar]
- 16.Batten ML, Imanishi Y, Maeda T, et al. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J Cell Biol. 2004;164:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batten ML, Imanishi Y, Tu DC, et al. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005;2:e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102:12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102:13658–13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson DA, Li Y, McHenry CL, et al. Mutations in the gene encoding lecithin retinol acyltransferase are associated with early-onset severe retinal dystrophy. Nat Genet. 2001;28:123–124 [DOI] [PubMed] [Google Scholar]
- 23.Van Hooser JP, Liang Y, Maeda T, et al. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J Biol Chem. 2002;277:19173–19182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Hooser JP, Aleman TS, He YG, et al. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc Natl Acad Sci U S A. 2000;97:8623–8628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wald G, Brown PK, Hubbard R, Oroshnik W. Hindered cis isomers of vitamin A and retinene: the structure of the neo-B isomer. Proc Natl Acad Sci U S A. 1955;41:438–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeGrip WJ, Liu RS, Ramamurthy V, Asato A. Rhodopsin analogues from highly hindered 7-cis isomers of retinal. Nature. 1976;262:416–418 [DOI] [PubMed] [Google Scholar]
- 27.Maeda T, Maeda A, Casadesus G, Palczewski K, Margaron P. Evaluation of 9-cis-retinyl acetate therapy in Rpe65−/− mice. Invest Ophthalmol Vis Sci. 2009;50:4368–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda T, Perusek L, Amengual J, Babino D, Palczewski K, von Lintig J. Dietary 9-cis-beta, beta-carotene fails to rescue vision in mouse models of leber congenital amaurosis. Mol Pharmacol. 2011;80:943–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda T, Cideciyan AV, Maeda A, et al. Loss of cone photoreceptors caused by chromophore depletion is partially prevented by the artificial chromophore pro-drug, 9-cis-retinyl acetate. Hum Mol Genet. 2009;18:2277–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babizhayev MA, Kasus-Jacobi A. State of the art clinical efficacy and safety evaluation of N-acetylcarnosine dipeptide ophthalmic prodrug. Principles for the delivery, self-bioactivation, molecular targets and interaction with a highly evolved histidyl-hydrazide structure in the treatment and therapeutic management of a group of sight-threatening eye diseases. Curr Clin Pharmacol. 2009;4:4–37 [DOI] [PubMed] [Google Scholar]
- 31.Davis J, Olsen TW, Stewart M, Sternberg P Jr. How the comparison of age-related macular degeneration treatments trial results will impact clinical care. Am J Ophthalmol. 2011;152:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intravitreous injection: a comprehensive review. Retina. 2004;24:676–698 [DOI] [PubMed] [Google Scholar]
- 33.O'Byrne SM, Kako Y, Deckelbaum RJ, et al. Multiple pathways ensure retinoid delivery to milk: studies in genetically modified mice. Am J Physiol Endocrinol Metab. 2010;298:E862–E870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wongsiriroj N, Piantedosi R, Palczewski K, et al. The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem. 2008;283:13510–13519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YK, Wassef L, Hamberger L, et al. Retinyl ester formation by lecithin: retinol acyltransferase is a key regulator of retinoid homeostasis in mouse embryogenesis. J Biol Chem. 2008;283:5611–5621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Byrne SM, Wongsiriroj N, Libien J, et al. Retinoid absorption and storage is impaired in mice lacking lecithin: retinol acyltransferase (LRAT). J Biol Chem. 2005;280:35647–35657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palczewska G, Maeda T, Imanishi Y, et al. Noninvasive multiphoton fluorescence microscopy resolves retinol and retinal condensation products in mouse eyes. Nat Med. 2010;16:1444–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mata E, Igartua M, Patarroyo ME, Pedraz JL, Hernandez RM. Enhancing immunogenicity to PLGA microparticulate systems by incorporation of alginate and RGD-modified alginate. Eur J Pharm Sci. 2011;44:32–40 [DOI] [PubMed] [Google Scholar]
- 39.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nature Materials. 2009;8:526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee A, Arha M, Choudhary S, et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30:4695–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J, Tan CY, Lee SK, Kim YH, Lee KY. Controlled delivery of heat shock protein using an injectable microsphere/hydrogel combination system for the treatment of myocardial infarction. J Control Release. 2009;137:196–202 [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Bhang SH, Park H, Kim BS, Lee KY. Active blood vessel formation in the ischemic hindlimb mouse model using a microsphere/hydrogel combination system. Pharm Res. 2010;27:767–774 [DOI] [PubMed] [Google Scholar]
- 43.Lee J, Lee KY. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm Res. 2009;26:1739–1744 [DOI] [PubMed] [Google Scholar]
- 44.Ateh DD, Leinster VH, Lambert SR, et al. The intracellular uptake of CD95 modified paclitaxel-loaded poly(lactic-co-glycolic acid) microparticles. Biomaterials. 2011;32:8538–8547 [DOI] [PubMed] [Google Scholar]
- 45.Allison SD. Analysis of initial burst in PLGA microparticles. Expert Opin Drug Deliv. 2008;5:615–628 [DOI] [PubMed] [Google Scholar]
- 46.Krebs MD, Jeon O, Alsberg E. Localized and sustained delivery of silencing RNA from macroscopic biopolymer hydrogels. J Am Chem Soc. 2009;131:9204–9206 [DOI] [PubMed] [Google Scholar]
- 47.Rosca ID, Watari F, Uo M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J Control Release. 2004;99:271–280 [DOI] [PubMed] [Google Scholar]
- 48.Shiose S, Chen Y, Okano K, et al. Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice. J Biol Chem. 2011;286:15543–15555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maeda A, Golczak M, Chen Y, et al. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat Chem Biol. 2012;8:170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maeda A, Maeda T, Golczak M, et al. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009;284:15173–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maeda A, Maeda T, Golczak M, et al. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Mol Pharmacol. 2006;70:1220–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakami S, Maeda T, Bereta G, et al. Probing mechanisms of photoreceptor degeneration in a new mouse model of the common form of autosomal dominant retinitis pigmentosa due to P23H opsin mutations. J Biol Chem. 2011;286:10551–10567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golczak M, Imanishi Y, Kuksa V, Maeda T, Kubota R, Palczewski K. Lecithin: retinol acyltransferase is responsible for amidation of retinylamine, a potent inhibitor of the retinoid cycle. J Biol Chem. 2005;280:42263–42273 [DOI] [PubMed] [Google Scholar]
- 55.Imanishi Y, Gerke V, Palczewski K. Retinosomes: new insights into intracellular managing of hydrophobic substances in lipid bodies. J Cell Biol. 2004;166:447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maeda A, Maeda T, Imanishi Y, Golczak M, Moise AR, Palczewski K. Aberrant metabolites in mouse models of congenital blinding diseases: formation and storage of retinyl esters. Biochemistry. 2006;45:4210–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orban T, Palczewska G, Palczewski K. Retinyl ester storage particles (retinosomes) from the retinal pigmented epithelium resemble lipid droplets in other tissues. J Biol Chem. 2011;286:17248–17258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351 [DOI] [PubMed] [Google Scholar]
- 59.Choonara YE, Pillay V, Danckwerts MP, Carmichael TR, du Toit LC. A review of implantable intravitreal drug delivery technologies for the treatment of posterior segment eye diseases. J Pharm Sci. 2010;99:2219–2239 [DOI] [PubMed] [Google Scholar]
- 60.Baranano DE, Kim SJ, Edelhauser HF, Durairaj C, Kompella UB, Handa JT. Efficacy and pharmacokinetics of intravitreal non-steroidal anti-inflammatory drugs for intraocular inflammation. Br J Ophthalmol. 2009;93:1387–1390 [DOI] [PubMed] [Google Scholar]
- 61.Guidetti B, Azema J, Malet-Martino M, Martino R. Delivery systems for the treatment of proliferative vitreoretinopathy: materials, devices and colloidal carriers. Curr Drug Deliv. 2008;5:7–19 [DOI] [PubMed] [Google Scholar]
- 62.Tang PH, Fan J, Goletz PW, Wheless L, Crouch RK. Effective and sustained delivery of hydrophobic retinoids to photoreceptors. Invest Ophthalmol Vis Sci. 2010;51:5958–5964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95 [DOI] [PubMed] [Google Scholar]
- 65.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239 [DOI] [PubMed] [Google Scholar]
- 66.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105:15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takezaki M, Kito Y. Circular dichroism of rhodopsin and isorhodopsin. Nature. 1967;215:1197–1199 [DOI] [PubMed] [Google Scholar]
- 69.Wilson CG. Topical drug delivery in the eye. Exp Eye Res. 2004;78:737–743 [DOI] [PubMed] [Google Scholar]
- 70.Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol. 2005;57:1555–1563 [DOI] [PubMed] [Google Scholar]
- 71.Gearhart PM, Gearhart C, Thompson DA, Petersen-Jones SM. Improvement of visual performance with intravitreal administration of 9-cis-retinal in Rpe65-mutant dogs. Arch Ophthalmol. 2010;128:1442–1448 [DOI] [PubMed] [Google Scholar]
- 72.Myles ME, Neumann DM, Hill JM. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv Drug Deliv Rev. 2005;57:2063–2079 [DOI] [PubMed] [Google Scholar]
- 73.Yasukawa T, Ogura Y, Tabata Y, Kimura H, Wiedemann P, Honda Y. Drug delivery systems for vitreoretinal diseases. Prog Retin Eye Res. 2004;23:253–281 [DOI] [PubMed] [Google Scholar]
- 74.Maeda A, Maeda T, Palczewski K. Improvement in rod and cone function in mouse model of Fundus albipunctatus after pharmacologic treatment with 9-cis-retinal. Invest Ophthalmol Vis Sci. 2006;47:4540–4546 [DOI] [PMC free article] [PubMed] [Google Scholar]