Abstract
Context
To improve patient care, athletic training clinicians and researchers should work together to translate research findings into clinical practice. Problems with patient care observed in clinical practice should be translated into research frameworks, where they can be studied. Practice-based research networks (PBRNs) provide a compelling model for linking clinicians and researchers so they can conduct translational research to improve patient care.
Objective
To describe (1) the translational research model, (2) practice-based research as a mechanism for translating research findings into clinical practice, (3) the PBRN model and infrastructure, (4) the research potential using the PBRN model, and (5) protection of human participants in PBRN research.
Description
Translational research is the process of transforming research findings into health behavior that ultimately serves the public and attempts to bridge the gap between research and clinical practice. Practice-based research represents the final step in the translational research continuum and describes research conducted by providers in clinical practices. The PBRNs are characterized by an organizational framework that transcends a single site or study and serves as the clinical research “laboratory” for conducting comparative-effectiveness studies using patient-oriented measures. The PBRN approach to research has many benefits, including enhanced generalizability of results, pooling of resources, rapid patient recruitment, and collaborative opportunities. However, multisite research also brings challenges related to the protection of human participants and institutional review board oversight.
Clinical and Research Advantages
Athletic training studies frequently include relatively few participants and, consequently, are able to detect only large effects. The incidence of injury at a single site is sufficiently low that gathering enough data to adequately power a treatment study may take many years. Collaborative efforts across diverse clinical practice environments can yield larger patient samples to overcome the limitations inherent in single-site research efforts.
Key Words: translational research, comparative effectiveness, clinical outcomes, HIPAA, FERPA, institutional review board
Key Points
To assess the effectiveness of preventive and health care services athletic trainers provide, clinicians and scholars should collaborate in translational research to guide clinical decision making.
Practice-based research networks bring together groups of clinicians and academic centers, eliminating the problems posed by single-site practice-based research.
Such networks must meet requirements for infrastructure, protection of human participants, and compliance with federal guidelines, but they offer numerous advantages, including generalizability of results, pooling of resources, and rapid patient recruitment.
Historically, athletic training research has been hampered by many of the same issues that plague medical research. The majority of research is conducted in association with academic centers,1 where the minority of patients receive health care services. Further, athletic training research has predominantly been conducted using small samples of nonpatient participants (ie, healthy individuals) under controlled conditions that may not apply to the clinical environment.2 The preponderance of research published in the Journal of Athletic Training is lower-level evidence that lacks the inclusion of important patient-oriented measurements in the assessment of clinical outcomes.3 Furthermore, it has been reported that only 14% of research findings in medicine are translated into clinical practice and that it takes an average of 17 years before they are actually incorporated.2 These numbers are alarming, and it is unlikely that the athletic training profession is experiencing significantly better translation of research into practice than medicine.
The definition of clinical translational research is less clear than traditional definitions of clinical and basic science research.4 Rubio et al4(p471) offered the following definition: “Translational research fosters the multidirectional integration of basic research, patient-oriented research, and population-based research, with the long-term aim of improving the health of the public.” The traditional model of translational research often stops after the initial translation of bench or laboratory research findings into human studies of clinical efficacy. However, a second translational step from “bedside” efficacy research (ie, works with patients in the laboratory setting) to clinical effectiveness (ie, works with patients in the clinical practice setting) is required to change clinician performance and improve patient care.5 Research in athletic training is frequently conducted in laboratories on nonpatient participants. Unfortunately, in many instances, laboratory findings are not translated into either efficacy or effectiveness studies. Therefore, a great deal of the laboratory-based research disseminated in athletic training may never actually be translated into clinical practice within a clinical research framework that leads to improved patient care.
The observed disconnect between research and clinical practice is attributed to 2 major factors: research is not translated quickly into clinical practice, and it fails to address the patient care problems encountered in common clinical practice.2 Creating a link between clinicians and researchers is critical if athletic training research is to provide high-quality patient-oriented evidence that can be readily implemented in clinical practice to improve the quality of patient care.6–8 The National Institutes of Health (NIH) have identified practice-based research networks (PBRNs) as fundamentally necessary to support the translation of research into clinical practice.9 The PBRNs provide the constructs and infrastructure to bring clinicians and researchers together to conduct point-of-care research that serves as the transitional link between the laboratory and usual clinical practice. These PBRNs are clinical “laboratories” in which the effectiveness of patient care interventions can be compared or compared with no treatment at all to determine the best practices in clinical care.
The purpose of this 2-part series is to define and discuss the value of PBRNs for engaging in multisite studies that bring clinicians and scientists together with the goal of improving the patient care provided by athletic trainers and to describe an athletic training PBRN. The specific aims of part I of this series are to (1) describe the translational research model, (2) describe practice-based research for translating research findings into clinical practice, (3) describe the PBRN model and infrastructure, (4) discuss the research potential of using the PBRN model, and (5) briefly discuss human-participant protection within PBRN research. The aim of the companion paper10 following this manuscript is to provide an overview of the Athletic Training PBRN (AT-PBRN).
TRANSLATIONAL RESEARCH MODEL
Translational research refers to the process of translating research knowledge and findings to health behavior that eventually serves the public.11 It attempts to bridge the gap between research and clinical practice. The NIH defined translational research as having 2 distinct areas of translation:
One is the process of applying discoveries generated during research in the laboratory, and in preclinical studies, to the development of trials and studies in humans. The second area of translation concerns research aimed at enhancing the adoption of best practices in the community.12
These 2 translational areas aim to bring bench or laboratory-based research into a more practical light by studying patients in clinical practice. Recently, the NIH has called for the development of new degree-granting programs in clinical and translational sciences (CTS) and has identified the specific competencies associated with CTS.13
Translational Continuum
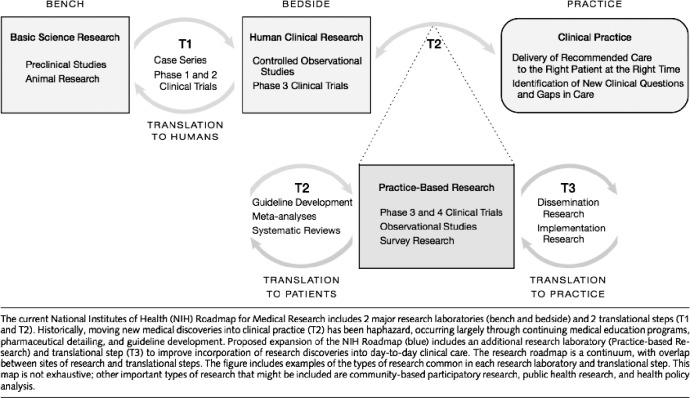
Translational research is an “iterative, bidirectional”14 process that has traditionally been described as consisting of 3 phases (Figure) that coexist at any point in time.5 The first phase is traditional bench, or basic science, research. Bench research is often conducted using animal models to examine hypotheses involving theoretical relationships, such as drug interactions. In athletic training, examples of very traditional bench research can be found in the literature, such as animal studies examining collagen expression in the anterior cruciate ligament.15
Figure.
The translational research continuum. Copyright © (2007) American Medical Association. All rights reserved.5
Promising bench research findings should first be translated into bedside studies that involve actual patient populations. Within the context of athletic training research, the term bedside research is equivalent to the majority of patient-based therapeutic studies that are conducted within research laboratories. Unfortunately, these studies often fail to address the primary goals of phase II (controlled studies using actual patients) bedside research. A primary goal of bedside research is to examine the safety of a particular intervention, such as a new drug or other therapy, when the intervention introduces potential risk to patients' health. In addition to safety concerns, the cost of a new intervention may also be an important consideration. From a cost perspective, it may be beneficial to first demonstrate efficacy before implementation into a real-world clinical practice study. For these and other reasons, bedside research is a critical step in the introduction of new therapies and one that must continue in athletic training research. The term bedside, while common in medicine, is somewhat confusing within the athletic training context. Consistent with the goals of safety and efficacy studies, bedside research in athletic training is most likely to occur in controlled laboratory studies in patients with the condition of interest (eg, functional ankle instability). These initial studies in patients are usually highly controlled and exhibit a great degree of internal validity. In athletic training, instead of studying a patient population, sometimes it may be more important to study a population of interest, such as female soccer players, if the research question is one of prevention.
In the United States, bedside research is usually conducted in academic medical centers, where fewer than 1% of all patients actually receive medical care.1 This has been considered a major limitation because the providers, patients, and controlled environments of bedside efficacy studies often fail to accurately reflect the state of medicine in the usual clinical practice environment. Bedside studies are more tightly controlled and lack external validity because patients in real clinical practices often have comorbidities or other important physiologic or psychological conditions that differ from those of patients in a controlled bedside study. In athletic training, we often study samples of convenience (eg, healthy college students), who may not mirror either our patient population or our population of interest. In addition, significant differences may be present in the provider and practice characteristics at academic medical centers versus those in the usual clinical practice providers and settings. These differences can greatly limit the applicability of research findings when hospitals, clinics, or individual providers attempt to implement practice recommendations based on bedside efficacy research. Therefore, although this type of research is necessary to explore alternative approaches to patient care outside the laboratory, bedside research is often of limited value for the widespread improvement of patient care in the usual clinical environment, where most patients receive their health care services.
To overcome the limitations of bedside research, the third phase of translational research should be conducted in the usual clinical practice environment (Figure). This clinical research is conducted in a broad range of settings beyond the traditional academic medical center and examines interventions provided by typical providers to typical patients under typical circumstances. The movement from bedside to clinical practice research represents the second major translation in the research continuum. This is where research meets reality and the effectiveness of health care services is evaluated. Clinical research during this third phase of translational research is far less controlled, thereby increasing the external validity of the findings. For example, community implementation of anterior cruciate ligament injury-prevention programs typifies the translation of laboratory-based research findings into the usual clinical practice environment on a population of interest, as opposed to a patient population.16
The type of research (bench, bedside, or clinical) is directly linked to evidence-based practice (EBP) through the assignment of levels of evidence to individual studies. Expert opinion based upon bench research is given a level 5 (the lowest level of evidence).17 Higher levels of evidence are assigned to studies that evaluate patient-oriented outcomes, such as health-related quality of life.17,18 So, to produce the highest levels of evidence in athletic training to support clinical decision making within the framework of EBP, it is important to translate research from the laboratory into patient care settings, use patient-oriented outcomes, and evaluate quality improvement in athletic training services.19,20
In athletic training, very little has been published regarding the translation of research findings into clinical practice. A probable explanation is that a great deal of the published research in athletic training is conducted in laboratories on healthy participants from samples of convenience.3 It is rare for phase I laboratory (mechanistic) studies conducted by athletic training scholars to ever be translated into controlled studies using actual patients (ie, phase II: bedside research). Therefore, the athletic training literature is largely void of the vital second phase of translational research, when the efficacy of athletic training interventions is evaluated. Fortunately, very few interventions in athletic training carry a high degree of risk (ie, safety concerns) or significant cost. These studies carry relatively few negative consequences when compared with drug studies in medicine, aside from potentially providing unnecessary or ineffective care. When significant risks or costs are associated with a new intervention, such as diagnostic tests for ruling in or ruling out a life-threatening condition, the importance of conducting phase II bedside research before less controlled clinical studies becomes much more apparent.
Simply moving to phase II bedside studies in patient populations and populations of interest for preventive research would elevate the overall quality of athletic training research. However, to achieve widespread improvements in athletic training patient care, basic science (ie, laboratory, mechanistic) studies must make the second translational step, which transitions highly controlled patient studies (phase II) into less tightly controlled effectiveness studies in the usual clinical practice environment (phase III studies; Figure).
Comparative Effectiveness Research
Comparative effectiveness research is currently a high priority in health care. The Agency for Health care Research and Quality (AHRQ) defines comparative effectiveness research (CER) as
. . . a type of health care research that compares the results of one approach for managing a disease to the results of other approaches. Comparative effectiveness usually compares two or more types of treatment, such as different drugs, for the same disease.21
According to the US Department of Health and Human Services (HHS),
The purpose of comparative effectiveness research (CER) is to provide information that helps clinicians and patients choose which option best fits an individual patient's needs and preferences.22
Funding for CER in the amount of $1.1 billion was allocated in the American Recovery and Reinvestment Act of 2009.22 These funds were distributed to the AHRQ ($300 million), NIH ($400 million), and Office of the Secretary of HHS ($400 million). The Act created the Federal Coordinating Council for Comparative Effectiveness Research to coordinate CER that will compare interventions for improving patients' health and the broader health of our nation. According to HHS,22
These funds are to support research assessing the comparative effectiveness of health care treatments and strategies, through efforts that:
-
1.
Conduct, support, or synthesize research that compares the clinical outcomes, effectiveness, and appropriateness of items, services, and procedures that are used to prevent, diagnose, or treat diseases, disorders, and other health conditions.
-
2.
Encourage the development and use of clinical registries, clinical data networks, and other forms of electronic health data that can be used to generate or obtain outcomes data.
Interestingly, the term comparative effectiveness research is being replaced by AHRQ because it was negatively affiliated with the fictional “death panels” depicted in the media during the health care reform debates. In its place, AHRQ is now using the term patient-oriented outcomes research.23
In an environment of increasing regulation and accountability, it is incumbent upon the health professions to demonstrate the effectiveness of their interventions. Specifically, CER should be used to compare the relative effectiveness of a specific intervention, such as patellar mobilizations for increasing knee extension after anterior cruciate ligament reconstruction, with other interventions (eg, mobilization with movement, traditional passive range-of-motion stretching) or no intervention at all. The goal of CER is not to compare the effectiveness of one profession or provider type with another but instead to focus on the effectiveness of different interventions. Recognition is widespread that the patient perspective is vital to determining the outcome of any intervention.24 Therefore, the incorporation of patient-oriented outcome assessments is vital for determining the effectiveness of health care interventions and EBP.19
The final step of translating research into clinical practice requires the collection of high-quality patient-oriented evidence that can be easily incorporated into clinical decision making.7 This type of evidence is obtained through investigations of clinical outcomes and specifically with those studies that assess patient-oriented evidence.19,25 By definition, clinical outcomes is the study of the end result of health care, focusing on an examination of the patient's values and experiences with his or her care and the effectiveness of the health care services delivered.26,27 Central to the delivery of quality athletic training patient care are the assessment of the clinical outcomes achieved and the effectiveness of the care provided for improving patient outcomes.19,20,28,29 A number of authors 6,19,29–32 acknowledge the current lack of information regarding the effectiveness of athletic training interventions and the potential professional and patient care issues that may arise in the absence of this requisite data.
Collecting and assessing clinical outcomes data for each patient is an important aspect of clinical care and quality assurance efforts. Participation in continuous quality improvement activities in athletic training is actually included in the Board of Certification Standards of Professional Practice.33 However, quality improvement patient care efforts in athletic training practice have received little formal attention to date. To enhance patient care, the effectiveness of a particular patient care intervention or treatment must be established through clinical research, and these findings must then be disseminated and translated back into clinical practice. Ultimately, this process of assessing patient-oriented outcomes for the purpose of determining treatment effectiveness and then translating these findings into practice for clinical decision making serves as the cornerstone of EBP. Many clinicians simply lack the time, training, and centralized support services to aggregate their individual patient outcomes into a broader context that would serve to inform how they should provide care to future patients. Therefore, partnerships between scientists and clinicians to conduct practice-based CER are necessary. However, obtaining high-quality patient-oriented comparative effectiveness data takes time, training, resources, and extensive collaboration, endeavors that may be difficult for individual practitioners to undertake.
PRACTICE-BASED RESEARCH
Practice-based research describes research conducted by providers in clinical practice, typically occurring in individual or small group practices.5 Practice-based research represents the final step in the translational research continuum and may include clinical trials, observational studies, and survey research. This step considers the translation of research findings to include both the study of patients and the dissemination and implementation of research recommendation practices to overcome potential problems by providers who attempt to implement research findings into their own clinical practices.5 The primary goal of practice-based research is to study the “delivery of recommended care to the right patient at the right time” and is critical for the “identification of new clinical questions and gaps in care.”5
Isolated practice-based research takes place at one clinical practice site or within a single health system. The evidence gained from isolated practice-based research conducted by a single clinician or within a single health system is often used only by the practice site at which the research was conducted, and important clinical findings may not be widely disseminated among other providers. Accumulating a sufficient number of patients is extremely difficult in a single-practice research study. Many important research questions cannot be addressed effectively within an isolated practice-based research framework. Failure to translate isolated practice-based research findings into more widespread clinical practice and the inability to answer many important research questions are major limitations and serve as barriers to improving patient care and patient outcomes.
PRACTICE-BASED RESEARCH NETWORKS
To overcome the limitations inherent when practice-based research occurs in isolation, PBRNs have evolved. According to the AHRQ, PBRNs are
. . . groups of primary care clinicians and practices working together to answer community-based health care questions and translate research findings into practice. PBRNs engage clinicians in quality improvement activities and an evidence-based culture in primary care practice to improve the health of all Americans.34
The United States Congress charged the AHRQ to support PBRNs as a means to
. . . address the full continuum of care and outcomes research, to link research to practice improvement, and to speed the dissemination of research findings to community practice settings . . .35
Furthermore, NIH has identified PBRNs as fundamentally necessary to support the translation of research into clinical practice.9
The first US PBRNs were regional primary care networks that emerged in the 1970s.36 Although most AHRQ-registered PBRNs focus on primary care, a wide variety of PBRNs focus on areas including dentistry, pharmacy, and chiropractic.37 In addition to their different foci, PBRNs vary widely in their size, geographic reach (local versus national), and the sophistication of the research in which they are engaged (observational studies to randomized controlled trials). Even the smallest and most focused PBRNs require appropriate infrastructure to manage the complexities of engaging in clinical research.
PBRN Infrastructure
The PBRN is characterized by an organizational framework that transcends a single site or study. It serves as the clinical research “laboratory” for conducting comparative effectiveness studies using patient-oriented measures. Infrastructures of PBRNs vary widely; however, the AHRQ has established specific criteria that must be in place for a PBRN to qualify for federal grant funding.38 Networks that meet these criteria can apply to become registered with the AHRQ. Registry approval requires the completion of an electronic application in which applicants must document their ability to meet the specific infrastructure criteria. Registered PBRNs are then listed on the AHRQ Web site.37
For PBRNs that intend to conduct research beyond simple observational studies, needs include more sophisticated technology infrastructure, training programs for clinical practice site personnel, and formal partnerships with academic centers where research methodology and biostatistical expertise are available.38 These partnerships between academic centers and community-based practice sites can also facilitate compliance with human-participant protection39 and Health Insurance Portability and Accountability Act (HIPAA)40 standards, both of which are discussed in more detail later.
The use of electronic data collection in geographically diverse PBRNs is considered optimal for ensuring data quality (completeness, accuracy, and eliminating secondary data entry), improving the collection process (timely transmission, reduced burden on participants), and decreasing costs.41 Numerous electronic data-collection options are available and may include paper-and-pencil data collection transferred to desktop computers, the use of personal digital assistants, locally networked computers, or Web-based systems accessed via mobile computers. Experts suggest that small mobile computers (ie, netbooks) running Web-based electronic medical records systems are easier to implement and have the potential to revolutionize data-collection capabilities in athletic training.41
CONDUCTING RESEARCH WITHIN A PBRN
Studies in the athletic training literature frequently include relatively few participants and, consequently, are able to detect only large effects. If the investigators conscientiously perform a prospective power analysis, the effect size estimates are often inappropriately optimistic.42 Executing sound research is a complicated and difficult task. Research in athletic training is made more difficult by the slow accrual of patients who experience 1 particular injury or who are treated using 1 intervention rather than another. The incidence of injuries at 1 site is sufficiently low that gathering enough data to adequately power a treatment study may take many years. Therefore, it is imperative that the research and clinical communities combine efforts across many sites so that we can engage in CER to determine best practices for patient care in athletic training. Collaborative efforts across diverse clinical practice environments can yield larger patient samples to overcome the limitations (eg, low statistical power, slow accumulation of patient-participants) inherent in single-site research efforts.
Designing and conducting high-quality (eg, appropriate care, randomization, blinding) practice-based research studies requires the synergistic, yet often unique, expertise of both clinicians and scientists. Clinicians should provide input into the patient care problems that need to be studied based on their clinical practice patterns, and scientists should develop the appropriate study designs and data analyses to answer those questions important to clinicians.
In a typical research setting, investigators often recruit clinicians to participate solely to obtain access to patients and data, while the researchers are primarily responsible for developing the research questions, analyzing the data, and disseminating the findings.43 In this model, clinicians may receive a stipend for participation but are not often consulted regarding the study questions, nor is there regular communication from those conducting the research, resulting in a lack of translation of research findings into regular clinical practice.43
In contrast, research conducted within a PBRN is a collaborative effort between clinicians and researchers. Clinical observations can be shared with the wider PBRN and studies developed to answer questions related to those observations.44 This model is advantageous because it promotes mutual understanding between clinicians and researchers, with clinicians gaining a better understanding of the benefits of participating in practice-based research and researchers gaining respect for the time and effort clinicians devote to assisting with research.43 Moreover, allowing clinicians to drive the research agenda with questions that matter to their practice provides a greater sense of ownership and contribution to the research efforts, which should lead to the acquisition of better-quality data and the implementation of new research findings into clinical practice.
Important to this collaborative effort is the appropriate training of the practicing clinician as a clinical researcher. Clinicians involved in PBRNs need, at minimum, a basic understanding of the process for conducting and interpreting research,43 which may promote the implementation of research findings into their own practices and increase their willingness to contribute to practice-based research efforts.45
HUMAN-PARTICIPANT PROTECTION AND PBRN RESEARCH
Multisite research involves many challenges associated with managing and coordinating issues related to the protection of human participants and the necessary institutional review board (IRB) oversight.46 Federal regulations govern research involving human participants. Institutions engaged in human-participant research that is subject to the Department of HHS regulations must have an Office for Human Research Protections-approved assurance of compliance and certify that the research has been approved by an IRB before any research commences.47 These requirements apply to all research involving human participants. Further, most peer-reviewed journals and many conferences require an avowal that research has been approved by a human-participant committee.
A number of authors, most writing from the family practice physician perspective, have discussed complexities inherent in human-participant research in a PBRN setting.46,48–53 Many of these issues translate, and some are magnified, in research on the student-athletes that athletic trainers frequently provide care for in the secondary and postsecondary settings. Foremost among these is the necessity of ensuring compliance with federal regulations governing human-participant research across multiple sites. Athletic trainers in clinical practice may not be familiar with regulatory research requirements and may not have access to an IRB for purposes of protocol review. Local IRBs, if available, vary substantially in submission requirements and procedures, application formats, application questions, and review procedures.39
Obtaining consent from research participants in a school setting can be challenging because potential participants who are not of legal age (typically 18 years) cannot provide consent. Parents must consent to their child's participation, and the underage athlete usually provides assent. The successful transport of a consent form by the adolescent athlete home to the parents, where it is signed and then returned to the school requires sophisticated logistics, reminders, and multiple attempts. Further, underage athletes belong to a class of “vulnerable” participants and are afforded special consideration under the Code of Federal Regulations governing human-participant research (45 CFR 46).54
Student-athletes' medical records may be protected from disclosure by 2 federal privacy laws. Medical data collected by secondary schools are typically classified as part of the education record and thus are protected by the US Department of Education and the Family Educational Rights and Privacy Act (FERPA).55 Medical data collected in a postsecondary school setting may, in some circumstances, be subject to the US Health Insurance Portability and Accountability Act (HIPAA).56
One way of ensuring the confidentiality of protected health information under HIPAA is by removing 18 explicitly named direct and indirect unique identifiers (eg, date of birth, Social Security number, phone number) from the record. Release of deidentified information is allowed by FERPA; however, the act does not list all of the information that must be removed in order to satisfy deidentification requirements.
If all personal identifying information collected as part of a medical record is removed from participant data in a manner such that an individual cannot be identified based on his or her record set and provided that the data may not be relinked to a participant, an IRB may waive the requirement for consent and assent. A safe harbor certificate—an agreement between the researcher and the entity storing the data—is developed to guarantee complete anonymity of the data. Yet PBRNs may wish to incorporate data from athletic trainers who are subject to local IRBs. In this case, the local IRB must have the final say on the specific elements required for approval of a research project. Some may require consent and assent, whereas others may waive this requirement due to deidentification of the data.
Graham et al,46 of the American Academy of Family Physicians National Research Network, have suggested the adoption of a centralized IRB review model to address the challenges associated with multisite studies conducted through a PBRN. Briefly, the American Academy of Family Physicians National Research Network has developed procedures for members who do not report to a local IRB. Those sites without a local IRB are required to sign an Unaffiliated Investigator Agreement, developed by a central IRB, stating that they will complete the appropriate human-participant protection training and comply with the procedural standards of the US Department of HHS regulations for the protection of human participants.54 The central IRB then becomes the IRB of record for these members. For members who report to a local IRB, a centralized tracking process is recommended for submitting and tracking the various local IRB applications. As a result, the study may be monitored by multiple IRBs.
CONCLUSIONS
Athletic training clinicians and scholars should partner to assess the effectiveness of preventive and health care services provided by athletic trainers.6 The underlying principles necessary to ensure safe and effective patient care are embedded within the principles of EBP and assessment of patient-oriented outcomes.7,19,20 The 5th edition of the Athletic Training Educational Competencies57 requires that the contemporary practice of athletic training include a minimum understanding of EBP and assessment of patient-oriented outcomes. To produce the kind of evidence required to guide clinical decision making, athletic training clinicians and scientists should partner to engage in translational research.7 Research at the point of care is where important bench and bedside findings are translated into clinical practice and problems encountered in clinical practice are translated into research studies aimed at producing effective patient care solutions.
Practice-based research is conducted at the point of care and seeks to examine the effectiveness of care provided by the usual providers to the usual patients. Limitations of single-site practice-based research led to the development of PBRNs to bring together larger groups of clinicians and academic centers to answer clinically important patient care questions. These PBRNs are the laboratories for conducting many types of patient-oriented research, including comparative effectiveness studies, cost-effectiveness analyses, and randomized controlled trials. Numerous infrastructure requirements must be met to develop and maintain a successful PBRN, but the centralized aggregation of large sets of patient-oriented data has the potential to revolutionize research and clinical practice in athletic training. A multitude of factors regarding the protection of human participants must be considered when PBRNs seek to engage in multisite clinical studies. With collaboration and due diligence, the appropriate steps to ensure compliance with federal guidelines can be assured.
REFERENCES
- 1.Green LA, Fryer GE, Jr, Yawn BP, Lanier D, Dovey SM. The ecology of medical care revisited. N Engl J Med. 2001;344(26):2021–2025. doi: 10.1056/NEJM200106283442611. [DOI] [PubMed] [Google Scholar]
- 2.Tierney WM, Oppenheimer CC, Hudson BL, et al. A national survey of primary care practice-based research networks. Ann Fam Med. 2007;5(3):242–250. doi: 10.1370/afm.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons JT, Snyder AR, Fried A, Evidence-based Wade R. and critical appraisal characteristics of the Journal of Athletic Training: 2002–2006. J Athl Train. 2009;44((suppl 3)):S98. [Google Scholar]
- 4.Rubio DM, Schoenbaum EE, Lee LS, et al. Defining translational research: implications for training. Acad Med. 2010;85(3):470–475. doi: 10.1097/ACM.0b013e3181ccd618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westfall JM, Mold J, Fagnan L. Practice-based research—“Blue Highways” on the NIH roadmap. JAMA. 2007;297(4):403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 6.Sauers EL. Establishing an evidence-based practice culture: our patients deserve it. Athl Train Sports Health. 2009;1(6):244–247. [Google Scholar]
- 7.Sauers EL, Snyder AR. A team approach: demonstrating sport rehabilitation's effectiveness and enhancing patient care through clinical outcomes assessment. J Sport Rehabil. 2011;20(1):3–7. doi: 10.1123/jsr.20.1.3. [DOI] [PubMed] [Google Scholar]
- 8.Hertel J. Research training for clinicians: the crucial link between evidence-based practice and third-party reimbursement. J Athl Train. 2005;40(2):69–70. [PMC free article] [PubMed] [Google Scholar]
- 9.National Institutes of Health. National Institutes of Health Roadmap for Medical Research. 2009 http://nihroadmap.nih.gov/. Accessed March 10. [Google Scholar]
- 10.Valovich McLeod TC, Lam KC, Bay RC, Sauers EL, Snyder AR. Practice-based research networks (PBRNs), part II: a descriptive analysis of the athletic training practice-based research network in the secondary school setting. J Athl Train. 2012;47(5):xx–xx. doi: 10.4085/1062-6050-47.5.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simons-Morton BG, Winston FK. Translational research in child and adolescent transportation safety. Eval Health Prof. 2006;29(1):33–64. doi: 10.1177/0163278705284442. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health. Institutional Clinical and Translational Science Award (U54). RFA-RM-07-007. 2011 http://grants.nih.gov/grants/guide/rfa-files/RFA-RM-06-002.html. Accessed December 1. [Google Scholar]
- 13.National Center for Research Resources. Strategic plan 2009–2013: translating research from basic discovery to improved patient care. 2012 http://www.ncrr.nih.gov/strategic_plan/online_version/contents.asp. Accessed April 11. [Google Scholar]
- 14.Ginexi EM, Hilton TF. What's next for translational research? Eval Health Prof. 2006;29(3):334–347. doi: 10.1177/0163278706290409. [DOI] [PubMed] [Google Scholar]
- 15.Romani WA, Langenberg P, Belkoff SM. Sex, collagen expression, and anterior cruciate ligament strength in rats. J Athl Train. 2010;45(1):22–28. doi: 10.4085/1062-6050-45.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003–1010. doi: 10.1177/0363546504272261. [DOI] [PubMed] [Google Scholar]
- 17.Howick J, Chalmers I, Glasziou P. The Oxford 2011 levels of evidence. Oxford Centre for Evidence-Based Medicine; 2012. OCEBM Levels of Evidence Working Group. http://www.cebm.net/index.aspx?o=5653. Accessed April 11. [Google Scholar]
- 18.Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Am Board Fam Pract. 2004;17(1):59–67. doi: 10.3122/jabfm.17.1.59. [DOI] [PubMed] [Google Scholar]
- 19.Snyder AR, Parsons JT. Valovich McLeod TC, Bay RC, Michener LA, Sauers EL. Utilizing disablement models and clinical outcomes assessment to enable evidence-based athletic training practice, part I: disablement models. J Athl Train. 2008;43(4):428–436. doi: 10.4085/1062-6050-43.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valovich McLeod TC, Snyder AR, Parsons JT, Bay RC, Michener LA, Sauers EL. Utilizing disablement models and clinical outcomes assessment to enable evidence-based athletic training practice, part II: clinical outcomes assessment. J Ath Train. 2008;43(4):437–445. doi: 10.4085/1062-6050-43.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality. Effective health care program: glossary of terms 2011 . http://www.effectivehealth care.ahrq.gov/index.cfm/glossary-of-terms/?termid=118&pageaction=showterm. Accessed December 1. [Google Scholar]
- 22.U.S. Department of Health & Human Services. Report to the President and the Congress on Comparative Effectiveness Research. 2011 http://www.hhs.gov/recovery/programs/cer/execsummary.html. Accessed December 1. [Google Scholar]
- 23.Slutsky J. Association of Schools of Allied Health Professions Annual Conference. Charlotte, NC.: 2010. Patient-centered outcomes research: closing the gaps between research, practice and policy: Agency for Health care Research and Quality. Paper presented at: October 20–22. [Google Scholar]
- 24.Washington AE, Lipstein SH. The Patient-Centered Outcomes Research Institute—promoting better information, decisions, and health. N Engl J Med. 2011;365(15) doi: 10.1056/NEJMp1109407. e31. [DOI] [PubMed] [Google Scholar]
- 25.Valovich McLeod TC, Bay RC, Parsons JT, Sauers EL, Snyder AR. Health-related quality of life is affected by recent injury in adolescent athletes. J Athl Train. 2009;44(6):603–610. doi: 10.4085/1062-6050-44.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82((10 suppl)):S26–S31. doi: 10.1097/01.PHM.0000086996.89383.A1. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz SR, Slawson D, Shaughnessy A. Orthopaedic information mastery: applying evidence-based information tools to improve patient outcomes while saving orthopaedists' time. J Bone Joint Surg Am. 2000;82(6):888–894. doi: 10.2106/00004623-200006000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Snyder AR. Valovich McLeod TC, Sauers EL. Defining, valuing, and teaching clinical outcomes assessment in professional and post-professional athletic training education programs. Athl Train Educ J. 2007;2(2):1–11. [Google Scholar]
- 29.Steves R, Hootman JM. Evidence-based medicine: what is it and how does it apply to athletic training? J Athl Train. 2004;39(1):83–87. [PMC free article] [PubMed] [Google Scholar]
- 30.APTA breaks off relationship with NATA. NATA News. 2005 Nov;16 [Google Scholar]
- 31.Kirkland M. What value an athletic trainer? NATA News. 2006 Oct;40 [Google Scholar]
- 32.National Athletic Trainers' Association. Outcomes research summit coming in May. 2007 http://www.nata.org/members1/documents/eblast/011907.cfm. Accessed January 17. [Google Scholar]
- 33.Board of Certification. Standards of Professional Practice. Omaha, NE: 2006. [Google Scholar]
- 34.Agency for Health care Research and Quality. Practice-based research networks (PBRNs) 2009 http://pbrn.ahrq.gov/portal/server.pt. Accessed April 10, [Google Scholar]
- 35.Health care Research and Quality Act, S 580, 106th Cong. (1999) [Google Scholar]
- 36.Green LA, Hickner J. A short history of primary care practice-based research networks: from concept to essential research laboratories. J Am Board Fam Med. 2006;19(1):1–10. doi: 10.3122/jabfm.19.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Agency for Health care Research and Quality. PBRN Registry. 2011 http://pbrn.ahrq.gov/portal/server.pt?open=512&objID=855&mode=2&cached=true#affiliate_networks. Accessed December 1. [Google Scholar]
- 38.Green LA, White LL, Barry HC, Nease DE, Jr, Hudson BL. Infrastructure requirements for practice-based research networks. Ann Fam Med. 2005;3((suppl 1)):S5–S11. doi: 10.1370/afm.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolor RJ, Smith PC, Neale AV. Institutional review board training for community practices: advice from the Agency for Health Care Research and Quality Practice-Based Research Network listserv. J Am Board Fam Med. 2008;21(4):345–352. doi: 10.3122/jabfm.2008.04.080088. [DOI] [PubMed] [Google Scholar]
- 40.Pace WD, Staton EW, Holcomb S. Practice-based research network studies in the age of HIPAA. Ann Fam Med. 2005;3((suppl 1)):S38–S45. doi: 10.1370/afm.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pace WD, Staton EW. Electronic data collection options for practice-based research networks. Ann Fam Med. 2005;3((suppl 1)):S21–S29. doi: 10.1370/afm.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2(8) doi: 10.1371/journal.pmed.0020124. e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genel M, Dobs A. Translating clinical research into practice: practice-based research networks—a promising solution. J Investig Med. 2003;51(2):64–71. doi: 10.1136/jim-51-02-07. [DOI] [PubMed] [Google Scholar]
- 44.Sears SF, Kirian K. Shock and patient-centered outcomes research: is an ICD shock still a critical event? Pacing Clin Electrophysiol. 2010;33(12):1437–1441. doi: 10.1111/j.1540-8159.2010.02872.x. [DOI] [PubMed] [Google Scholar]
- 45.Guyatt G, Drummond R, Meade MO, Cook DJ. Users' Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice. 2nd Ed. New York, NY: McGraw Hill Medical: 2008. [Google Scholar]
- 46.Graham DG, Spano MS, Manning B. The IRB challenge for practice-based research: strategies of the American Academy of Family Physicians National Research Network (AAFP NRN) J Am Board Fam Med. 2007;20(2):181–187. doi: 10.3122/jabfm.2007.02.060110. [DOI] [PubMed] [Google Scholar]
- 47.US Department of Health and Human Services. Office for Human Research Protections (homepage) 2010 http://www.hhs.gov/ohrp. Accessed February 22. [Google Scholar]
- 48.Burman WJ, Reves RR, Cohn DL, Schooley RT. Breaking the camel's back: multicenter clinical trials and local institutional review boards. Ann Intern Med. 2001;134(2):152–157. doi: 10.7326/0003-4819-134-2-200101160-00016. [DOI] [PubMed] [Google Scholar]
- 49.McWilliams R, Hoover-Fong J, Hamosh A, Beck S, Beaty T, Cutting G. Problematic variation in local institutional review of a multicenter genetic epidemiology study. JAMA. 2003;290(3):360–366. doi: 10.1001/jama.290.3.360. [DOI] [PubMed] [Google Scholar]
- 50.Gold JL, Dewa CS. Institutional review boards and multisite studies in health services research: is there a better way? Health Serv Res. 2005;40(1):291–307. doi: 10.1111/j.1475-6773.2005.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green LA, Lowery JC, Kowalski CP, Wyszewianski L. Impact of institutional review board practice variation on observational health services research. Health Serv Res. 2006;41(1):214–230. doi: 10.1111/j.1475-6773.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolf LE, Croughan M, Lo B. The challenges of IRB review and human subjects protections in practice-based research. Med Care. 2002;40(6):521–529. doi: 10.1097/00005650-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Wolf LE, Walden JF, Lo B. Human subjects issues and IRB review in practice-based research. Ann Fam Med. 2005;3((suppl 1)):S30–S37. doi: 10.1370/afm.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Protection of Human Subjects. 45 CFR §46. (2005) [Google Scholar]
- 55.Family Educational Rights and Privacy Act (FERPA), USC §1232g. (1974) doi: 10.1525/jer.2007.2.1.101. [DOI] [PubMed] [Google Scholar]
- 56.Health Insurance Portability and Accountability Act (HIPPA), Pub L 104–191, 104th Cong. (1996) [Google Scholar]
- 57.National Athletic Trainers' Association. Athletic Training Educational Competencies. 5th ed. 2011 Dallas, TX. [Google Scholar]