Abstract
This NATA position statement was developed by the NATA Research & Education Foundation.
Objective
This manuscript summarizes the best available scholarly evidence related to anabolic-androgenic steroids (AAS) as a reference for health care professionals, including athletic trainers, educators, and interested others.
Background
Health care professionals associated with sports or exercise should understand and be prepared to educate others about AAS. These synthetic, testosterone-based derivatives are widely abused by athletes and nonathletes to gain athletic performance advantages, develop their physiques, and improve their body image. Although AAS can be ergogenic, their abuse may lead to numerous negative health effects.
Recommendations
Abusers of AAS often rely on questionable information sources. Sports medicine professionals can therefore serve an important role by providing accurate, reliable information. The recommendations provide health care professionals with a current and accurate synopsis of the AAS-related research.
Key Words: testosterone, androgen, ergogenic aids, drugs, abuse, doping, sport, athletes
Anabolic-androgenic steroids (AAS) are synthetic testosterone analogs1,2 legally classified as Schedule III controlled substances.1,3 These hormones increase lean muscle mass and can improve athletic performance.1,2,4,5 Although AAS have valid medicinal uses, nontherapeutic abuse also occurs.1,4,5 Recent increases in androgen prescriptions are evident.6,7 Those involved in organized athletics and nonathletes both abuse AAS.1,2 Nontherapeutic users often obtain AAS information and products from a variety of dubious sources and make questionable health decisions.8,9 Health care professionals, including athletic trainers and educators, may interact with individuals who abuse or intend to abuse AAS. Therefore, it is imperative that such professionals understand these substances so that they can educate others using the most current and accurate evidence.
This position statement is a summation of the best available scholarly evidence with regard to AAS and integrates a Strength of Recommendation Taxonomy (SORT) criterion scale from the American Academy of Family Physicians (Table 1).10 Recommendations are ranked as A (good-quality evidence), B (inconsistent or limited-quality or limited-quantity evidence), or C (recommendations based on consensus, usual practice, opinion, or case series). Although other frequently abused pharmaceuticals (eg, human growth hormone, insulin growth factor 1, and selective androgen receptor modulators) and nutritionals (eg, creatine, amino acids, protein powders) purport to promote anabolism, this position statement solely addresses AAS. For logistical and ethical reasons, few prospective, outcome-based, scholarly studies duplicated the typical nontherapeutic AAS abuse patterns often used.11–13
Table 1.
Strength of Recommendations Taxonomy (SORT)a,10
| Strength of Recommendation |
Definition |
| A | Recommendation based upon consistent and good-quality patient-oriented evidence (morbidity, mortality, symptom improvement, cost reduction, and quality of life)b |
| B | Recommendation based on inconsistent or limited-quality patient-oriented evidenceb |
| C | Recommendation based on consensus, usual practice, opinion, disease-oriented evidence (measures of intermediate, physiologic, or surrogate end points that may or may not reflect improvements in patient outcomes), or case series for studies of diagnosis, treatment, prevention, or screening |
Reprinted with permission from “Strength of Recommendation Taxonomy (SORT): A Patient-Centered Approach to Grading Evidence in the Medical Literature,” February 1, 2004, Vol 69, No 3, American Family Physician. Copyright© 2004 American Academy of Family Physicians. All Rights Reserved.
Patient-oriented evidence measures outcomes that matter to patients: morbidity, mortality, symptom improvement, cost reduction, quality of life. Disease-oriented evidence measures intermediate, physiologic, or surrogate endpoints that may or may not reflect improvements in patient outcomes (ie, blood pressure, blood chemistry, physiological function, and pathological findings).
Health care professionals owe their patients evidence-based knowledge to help in their health care decisions. Identification of the AAS abuser (or potential abuser) by a health care professional is critical to help prevent any negative consequences. Proper direction, guidance, support, and possible referral are essential in assisting AAS abusers and potential abusers.
RECOMMENDATIONS
Basic Science
-
1.
Health care professionals and educators should recall that the endocrine system synthesizes hormones that help regulate the body's physiology. Primarily synthesized in the gonads and adrenal glands, steroid hormones are a particular class of chemical messengers that affect body tissues and have anti-inflammatory, salt-retaining, and feminizing or masculinizing properties, depending on the substance.1,4,7,14–16 Evidence Category: A
-
2.
Health care professionals and educators should understand that testosterone, the key androgen, promotes both androgenic (masculinizing) and anabolic (tissue-building) effects.1,4,7,14–16 Normal testosterone levels vary with age, sex, and health status, and levels in males are significantly greater than levels in females.7,17–19 In males, testosterone levels normally peak during early adulthood and then decrease.7,18–20 Abnormal endogenous testosterone levels in adulthood appear to be associated with specific disorders or diseases.7,18–20 Evidence Category: A
-
3.
Health care professionals and educators should appreciate that AAS are synthetic substances related to the primary male hormone, testosterone.1,2,4,7,14 The United States classifies the many available AAS as Schedule III controlled substances.1,13–15 Pharmaceutical companies and clandestine laboratories may develop various AAS to maximize anabolic effects, minimize androgenic effects, improve pharmacokinetics, increase receptor affinity, and, in some cases, avoid detection.14,15 The AAS are available as oral, injectable, and buccal (intraoral, next to cheek) agents, subcutaneous pellets, and transdermal patches, creams, and gels.14,15 Evidence Category: A
-
4.
Health care professionals and educators should know that the mechanisms of action for AAS are complex and variable.7,14,15 The AAS appear to promote protein synthesis through increased transcription while also acting as glucocorticoid antagonists, limiting catabolism.14,15 They also induce various potentially ergogenic psychotropic actions, altering neurochemistry,11,14,21,22 and may stimulate human growth hormone and insulin growth factor 1 synthesis,12 as well as downregulate myostatin.7,23,24 Evidence Category: C
Therapeutic AAS Use and Nontherapeutic AAS Abuse
-
5.
Health care professionals and educators should recognize the possible therapeutic uses for AAS, which include therapy for male hypogonadism, certain rare anemias, and other medical conditions; anticatabolism for those with chronic wasting syndromes; and preserving or restoring bone health.6,7,18–20,24–33 Although some suggest AAS may act as “antiaging” substances, the evidence remains questionable.6,17,18,33 Evidence Category: C
-
6.
Health care professionals and educators should appreciate that although AAS prevalence studies have limitations, the current evidence suggests that nontherapeutic AAS use is a worldwide phenomenon.3,8,9,34–49 Abuse of AAS occurs for performance improvement, physique development, and body-image enhancement.8,9,34–44,46,49 Males abuse AAS at greater frequency than do females.8,35,36,45,46 Those who use AAS for nontherapeutic reasons often do not participate in organized sports.9,41,43,45,46,49 Abusers of AAS include adolescents, collegians, professional and Olympic athletes, body builders, and recreational athletes, among others.8,34–37,39–47,49 The nonmedicinal abuse of AAS often begins in adolescence, sometimes as early as middle school.35–37,41–43,45–47 Evidence Category: C
-
7.
Health care professionals and educators should realize that AAS abusers choose from many possible agents,11,50 including “designer steroids” unapproved by the Food and Drug Administration and veterinary-quality and black-market substances.1,3,13,41,49 Although AAS abusers may obtain androgens from physicians, their supplies more commonly come from other sources, such as the Internet, training partners, gymnasium owners or instructors, teammates, and coaches.8,9,36,37,40–42,49 Counterfeit AAS are common, lending to product quality concerns: impurities, contaminants, and inaccurate product labels.51–53 Evidence Category: C
-
8.
Health care professionals and educators should understand that doses taken by AAS abusers are often much greater than therapeutic replacement levels.13,14,51,53,54 Nontherapeutic AAS users frequently “stack,” or simultaneously abuse, multiple AAS, with differences in half lives and solubilities.3,9,36,41,44,45 Abuse of AAS often occurs in repeated cycles of 6 to 12 weeks, followed by periods of nonuse.1,3,8,9,36,41 Evidence Category: B
AAS Efficacy
-
9.
Health care professionals and educators should respect the limitations of high-quality research designs in determining AAS effectiveness and side effects.11–14,22,51,55,56 Ethical issues disallow using the typically extreme dosing schedules in research studies due to the federally controlled status of AAS, as well as the reported risk profile of androgen use at nontherapeutic doses.11,14,51,55,56 Evidence Category: C
-
10.
Health care professionals and educators should realize that the efficacy of AAS as anabolic agents suggests a dose-related potential for increased relative lean body mass.57–61 These substances can generally act as ergogenic agents when the measure involves strength- or power-related performances.6,59–66 Evidence Category: A
AAS Abuse Health Effects
Health care professionals and educators should be aware of the following possible AAS abuse side effects on various biologic systems and organs.
-
11.
Supraphysiologic AAS dosing may occasionally be associated with hypomanic or manic syndromes that are often characterized by irritable or aggressive behavior.21,54,67–78 Episodes of major depression may be associated with AAS withdrawal.79–81 Abusers of AAS may develop a dependence syndrome related to both myoactive and psychoactive effects82–89 and may exhibit other forms of drug dependence, such as opioids.83,90–93 Evidence Category: B
-
12.
The cardiovascular effects of therapeutic AAS remain unclear. Substantial research findings now suggest that AAS abuse negatively influences the cardiovascular system.25,67,94–103 The best evidence indicates that nontherapeutic AAS-related conditions include cardiomyopathy96,97,99,100 and the potential for atherosclerotic vascular disease caused by detrimental lipid changes, which may adversely affect one's risk for coronary artery disease.25,102,103 Evidence Category: B
-
13.
Although rare, hepatic maladies including cholestatic jaundice and peliosis hepatis might occur with the nontherapeutic abuse of AAS, especially when the oral C17α-alkylated group of AAS is involved.7,11–14,22,24,51,55,104 Evidence Category: A
-
14.
Exogenous AAS abuse inhibits the hypothalamic-pituitary-gonadal axis, reducing the production of endogenous testosterone, luteinizing hormone, and follicle-stimulating hormone.7,11–14,22,51 It can also alter thyroid function11,12,22 and negatively affect glucose tolerance.12,22,105 Evidence Category: B
-
15.
Abuse of AAS directly affects the male reproductive system, with possible side effects including hypogonadism, decreased spermatogenesis, decreased sperm motility, erectile dysfunction, impotence, gynecomastia, and male-pattern baldness.7,11–14,22,51,55 Many of these conditions are reversible with cessation of AAS, although breast tissue changes and hair loss often require additional treatments, including surgery.7,11–13,22,51,55 Evidence Category: B
-
16.
Reproductive changes to females who abuse AAS generally involve virilization, including voice deepening, hirsutism, clitoral hypertrophy, breast reduction, libido changes, menstrual dysfunction, male-pattern baldness, and acne.11–14,22,51 Unlike the side effects in males, many of these changes are permanent in females.12,14,22 Evidence Category: B
-
17.
Skeletally immature AAS abusers might experience premature epiphyseal closure of the long bones, resulting in shortened stature.11,12,14,22,51 Other negative effects of AAS abuse on the musculoskeletal system may include tendinopathies (including possible rupture).7,11–13,22,51 Little evidence supports the therapeutic use of AAS for musculotendinous injury recovery.7,106 Evidence Category: C
-
18.
A possible immunosuppressant effect of AAS abuse may exist in humans.11,22,107 Abusers risk local and systemic infections (including hepatitis and human immunodeficiency virus) with unsterile syringe usage.11,12,14,22 Evidence Category: B
-
19.
High-dose AAS abuse often leads to a number of dermatologic conditions, most commonly some form of acne.11,12,14,22,108,109 Kidney structure and function may be at risk with supraphysiologic doses of AAS, especially when combined with use of nonsteroidal anti-inflammatory drugs, high-protein diets, certain nutritional supplements, and dehydration.11,22,51,55,110,111 Gingival and other oral tissues may also be affected.52,112 Evidence Category: C
AAS Abuse Prevention
-
20.
Health care professionals and educators should recognize the great variance in drug testing programs.113 Due to the small number of high-quality studies, whether such screenings significantly deter AAS abuse remains unclear.114,115 A need exists for prospective randomized trials to investigate the deterrent efficacy of AAS screening. Evidence Category: C
-
21.
Health care professionals and educators should realize that although AAS abuse education requires further study, well-designed programs might effectively inhibit such abuse in adolescents.114–124 Consideration for the early integration of prevention models into educational curricula and sports programs is important. Evidence Category: C
AAS Abuse Identification and Intervention
-
22.
Health care professionals and educators should be aware of the dynamic, social process of AAS abuse. Active monitoring for AAS abuse and maintaining an open, honest, and evidence-based dialogue with all stakeholders, including athletes, coaches, administrators, parents, advisory groups, and others, is vital.125,126 Athletic trainers should develop relationships with and call on other qualified health care professionals as referral resources. Evidence Category: C
BACKGROUND AND LITERATURE REVIEW
History of Anabolic-Androgenic Steroids
Testosterone Isolation and Development
More than 6000 years ago, herders recognized numerous changes in castrated animals.1,16 Centuries later, crude studies investigating the testes' biologic role involved their transfer from castrated roosters into the abdominal cavity of hens to observe changes in the animals.1,16 In the mid-1800s, the effects of castration were directly related to a secreted testicular substance.1,16 Charles Brown-Séquard, a founder of endocrinology, published results suggesting remarkable rejuvenation effects from his self-experimentation using guinea pig and canine testicular extract.1,2,16 Testosterone was eventually chemically isolated1,2,5 and then synthetically developed.1,2,16 In 1935, Kockakian suggested testosterone might help stimulate anabolism and benefit certain wasting conditions.2 Thereafter, sports physiology researchers began investigating the effects of testicular extracts on physical performance.1,2,5
AAS Ergogenics
The 1940s brought the first published reports on the ergogenic effects of testosterone and its derivatives.2 The 1945 book The Male Hormone may have increased athletic AAS abuse.1,2,7 Anecdotal reports described West Coast body builders experimenting with AAS in the late 1940s.1,2,16 The 1952 Helsinki Olympic Games brought about the seemingly valid US claim of hormonal manipulation by the successful Soviet weightlifters.4,5 Soviet physicians purportedly admitted that their athletes used synthetic hormones at the 1954 World Weightlifting Championships.5 This led to the development and 1958 release of the first commercially available AAS in the United States: methandrostenolone (Dianabol; Ciba Specialty Chemicals, Basel, Switzerland).1,4,5,7
Abuse of AAS increased through the 1960s and 1970s,4,5 with evidence for abuse at every Olympic competition since 1960.4 The abuse spread to professional and collegiate sports,5 and typical dosages increased well beyond therapeutic levels.14 This era also involved large-scale, top-secret, government-supported, systemic hormonal manipulation in some Eastern bloc countries.4 Increased AAS abuse led to the 1967 establishment of the International Olympic Committee's Medical Commission, with the primary role of doping oversight.4 Urine AAS tests were first conducted at the 1974 Commonwealth Games, where more than 16% of the sampled athletes tested positive.5 The 1976 Montreal Games marked the first Olympic AAS tests.4,5 During this time, most other sport governing bodies, including colleges and professional leagues, generally did little to address AAS abuse.5
Although AAS abusers learned about the efficacy of these substances through trial and error, many medical professionals and scientists erroneously suggested that these drugs were ineffective based on laboratory studies that failed to duplicate the doses and training conditions experienced by actual AAS abusers in the field.4,5,14,51 This discrepancy led to distrust of the science and those reporting it.5,51 Drug testing and the knowledge gap pushed AAS abuse further underground.4,5 Many trade publications promoting AAS abuse became available in the 1970s and 1980s.4,5 Ben Johnson's apparent gold medal and world record at the 1988 Seoul Olympics highlighted the enormity of AAS abuse.4 The 1990 Anabolic Steroid Control Act reclassified many AAS as Schedule III controlled substances.1 A follow-up statute—the Anabolic Steroid Act of 1994—added more AAS and some testosterone precursors to the Schedule III drug list.1
So-called designer steroids became known4 when a contaminated needle was anonymously sent to the US Anti-Doping Agency in 2003. The substance was a new AAS not approved by the US Food and Drug Administration and since named tetrahydrogestrinone.4 These findings led to the Bay Area Laboratory Cooperative (BALCO) scandal that involved many high-profile athletes.4,127 The US Congress held hearings concerning AAS abuse, leading to the 2004 Anabolic Steroid Control Act, which reclassified many anabolic-related pharmaceuticals as Schedule III controlled substances.53,107
In 2004, the World Anti-Doping Agency began AAS oversight for all international competitions involving Olympic sports.4 Many collegiate sport organizations, as well as US professional sport leagues, now include AAS testing, although the rigor of these programs varies greatly.113 Some modern competitors continue to seek increasingly sophisticated, illegal, and unethical ways to maximize their performance or physique (or both) with AAS.4 The most common source of positive doping tests by the World Anti-Doping Agency is AAS.4,51
Epidemiology of AAS Abuse
Although AAS have legal therapeutic uses for specific medical disorders, healthy persons also abuse them to enhance physical performance or physique (or both).1,2,4,5,7,14 Researchers have most frequently concentrated on the prevalence of AAS abuse but have also investigated commonly abused AAS, abuser characteristics, patterns of abuse, and AAS sources.3,8,9,35–46 These abuser profiles are important to understand before educational and preventive initiatives are devised.
Prevalence of Nontherapeutic AAS Abuse
Most authors of prevalence studies used anonymous, direct survey methods, typically within specific populations, such as adolescents, collegians, elite athletes, or gym attendees. Many published reports with various research designs detailed AAS prevalence rates (Table 2).3,8,9,34–47,49,128 Anonymous survey research, although valuable, involves limitations, and interpreting results requires caution. For example, respondents may falsely indicate they used steroids when they actually used only corticosteroids or dietary supplements.129 Alternatively, those who complete such questionnaires may be less likely to abuse drugs. Given these possibilities, the research results presented here generally include those studies with the strongest sampling techniques, sample sizes, survey administrations, and reporting methods, as well as documentation of instrument validity and reliability.3,8,9,34–47
Table 2.
Anabolic-Androgenic Steroid Abuse Prevalence Studies Implementing the Highest-Quality Research Techniques
| AAS Abusers |
Author(s) (Year) |
Total Participants, N |
Males, n |
Females, n |
Sampling Technique |
Group Description |
Age/ Grade |
Location |
Total Abusers (n) |
Total Abusers (%) |
Male Abusers (n) |
Male Abusers (%) |
Female Abusers (n) |
Female Abusers (%) |
| Adolescents | Buckley et al (1988)41 | 3403 | 3403 | 0 | Stratified random cluster | Students at 46 high schools | Grade 12 | United States | 226 | 6.6 | 226 | 6.6 | 0 | 0.0 |
| Dodge and Jaccard (2006)46 | 14 322 | 6759 | 7563 | Representative sample | Students at junior high and high schools | Grades 7–12 | United States | 183 | 1.3* | 153 | 2.7 | 30 | 0.4 | |
| European School Survey Project on Alcohol and Other Drugs (2007)47 | 104 828 | 51249 | 53 579 | Populations or representative clusters | Students (1991 births) | Average, 15.8 y | Europe (35 countries) | NA | NA | NA | 2.0 | NA | 1.0 | |
| Hoffman et al (2007)53 | 3248 | 1559 | 1689 | NA | Students | Grades 8–12 | United States (12 states) | NA | 1.6 | NA | 2.4 | NA | 0.8 | |
| Kokkevi et al (2008)36 | 18 430 | NA | NA | Cluster | Students at high schools | Average, 16 y | Europe (6 countries) | 388 | 2.1 | NA | NA | NA | NA | |
| Stilger and Yesalis (1999)42 | 873 | 873 | 0 | Random cluster | Football athletes at 347 high schools | NA | Indiana | 55 | 6.3 | 55 | 6.3 | 0 | 0.0 | |
| vandenBerg et al (2007)43 | 2516 | 1130 | 1386 | Selected | Students at 31 junior high and high schools | Grades 7–12 | St Paul, MN | 38* | 1.5* | 19 | 1.7 | 19 | 1.4 | |
| Wroble et al (2002)34 | 1553 | 1079 | 474 | Cluster | Athletes in youth sports | Range, 10–15 years | United States (34 states) | 11* | 0.7 | 10* | 0.9 | 1* | 0.2 | |
| Centers for Disease Control and Prevention (2009)35 | 16 410 | NA | NA | 2-Stage cluster sample | Students at high schools | Grades 9–12 | United States | NA | 3.3 | NA | 4.3 | NA | 2.2 | |
| Collegians | Kersey (1996)8 | 1185 | 833 | 352 | Stratified random cluster | Athletes at ten 2-year colleges | Average, 19.6 y | California | 38 | 3.3 | 34 | 4.2 | 4 | 1.2 |
| McCabe et al (2007)3 | 54 567 | NA | NA | Random cluster | Students at 119 4-year colleges | About 21 y | United States | NA | 1.0 | NA | NA | NA | NA | |
| National Collegiate Athletic Association (2012)37 | 20 474 | NA | NA | NA | Collegiate athletes | NA | United States | NA | 0.8 | NA | NA | NA | NA | |
| Elite athletes | Horn et al (2009)39 | 2552 | 2552 | 0 | Population | Retired National Football League players | Average, 53.8 y | United States | 233 | 9.1 | 233 | 9.1 | 0 | 0.0 |
| Tricker et al (1989)38 | 176 | 108 | 68 | Population | National Physique Committee registered bodybuilders | Average, 26.7 y | Kansas and Missouri | 66 | 37.5 | 59 | 54.6 | 7 | 10.3 | |
| Wagman et al (1995)40 | 15 | 15 | 0 | Population | Elite powerlifters | Range, 20–45 y | United States | 10 | 66.7 | 10 | 66.7 | NA | NA | |
| Gym attendees | Baker and Graham (2006)45 | 146 | 136 | 10 | Convenience | Clients at “hardcore” gyms | Average, 33.6 y | South Wales, UK | 102 | 69.9 | NA | NA | NA | NA |
| Bolding et al (2002)44 | 772 | 772 | 0 | Convenience | Gay male attendees at 6 gyms | Median, 35 y | London, UK | 117 | 15.2 | 117 | 15.2 | 0 | 0.0 | |
| Kanayama et al (2001)48 | 511 | 334 | 177 | Convenience | Clients at 5 gyms | Range, 14–70 y | Boston, MA | 18 | 3.5 | 18 | 5.4 | 0 | 0.0 | |
| Perry et al (2005)9 | 260 | NA | NA | Self-selected via Internet | Weight lifters and bodybuilders | NA | United States | 207 | 79.6 | NA | NA | NA | NA | |
| Santos et al (2010)49 | 123 | 123 | 0 | Convenience | Bodybuilders | Range, 18–50 years | Brazil | 41 | 33.3 | 41 | 33.3 | 0 | 0.0 |
Abbreviations: AAS, anabolic-androgenic steroids; *, numbers were calculated by present authors, not directly stated by original authors; NA, not available.
Adolescent AAS Abuse
Adolescents are the most studied population for the prevalence of AAS abuse (Table 2). The first national high school survey (1988) suggested AAS abuse by 6.6% of male high school seniors.41 A survey of male Indiana high school athletes in 1999 indicated a 6.3% lifetime prevalence of AAS abuse.42 In 2002, others34 suggested that 0.7% of sport participants aged 10 to 15 years from 34 states abused AAS. A 2006 US study of junior high and high school students reflected an AAS abuse prevalence of 1.3%, including 2.7% of males and 0.4% of females.46 Results from a 2007 study of Minnesota students in grades 7 through 12 indicated AAS abuse rates of 1.7% and 1.4% in male and female adolescents, respectively.43 The 2009 Centers for Disease Control and Prevention Youth Risk Behavior Surveillance System35 reported that national AAS abuse rates were 4.3% and 2.2% for males and females, respectively (3.3% overall). Results from the 2007 European School Survey Project on Alcohol and Other Drugs involving 35 European nations noted an AAS abuse prevalence of 2% for males and 1% for females.47 A 2008 six-country European study reflected an overall AAS abuse prevalence of 2.1% among high school students.36
Collegiate AAS Abuse
The National Collegiate Athletic Association (NCAA), among others, studied AAS abuse among collegiate student-athletes every 4 years since 1985. The most recent (2009) findings (Table 2) indicated an overall lifetime AAS abuse prevalence of 0.8%.37 A summation of 4 independent studies at 119 US universities indicated that 0.9% of all college students abused AAS.3 Only one author8 investigated AAS abuse among community college student-athletes and reported a prevalence of 3.3% (4.2% of men, 1.2% of women).
Elite Athlete AAS Abuse
The prevalence of AAS abuse by elite athletes has been examined in relatively few high-quality studies (Table 2).38–40 In a 1989 study,38 37.5% of competitive body builders admitted AAS abuse, whereas a 1995 investigation revealed that 66.7% of competitive power lifters abused AAS.40 A 2009 paper39 indicated that 9.1% of retired National Football League athletes admitted using AAS during their professional careers. Prevalence was highest among offensive (16.3%) and defensive (14.8%) linemen.
Gym Attendee AAS Abuse
A few well-designed studies described AAS abuse by gym attendees (Table 2). A 5.4% reported lifetime prevalence of AAS abuse was demonstrated among 334 men in 5 Boston-area gyms, but no cases of AAS use among 177 women were noted.48 In a high-quality British study,44 AAS abuse was found in 15.2% of gay male gym attendees. In body-building gyms, AAS abuse prevalence rates were 33.3% in Brazil49 and 69.9% in the United Kingdom.45
Commonly Abused AAS
Options for AAS abusers are many,13–15 including pharmaceutical-grade, veterinary-quality, and unapproved AAS.1,4,14,49,127 Abuse of “designer” AAS, such as tetrahydrogestrinone and others, developed outside the normal US Food and Drug Administration approval process and is a recent and apparently growing phenomenon.1,4,14,127 The most common administration routes are oral and parenteral, although topical and transdermal routes are also options.1,4,14,127 Examples of commonly abused AAS are shown in Table 3.8,9,38,45,49
Table 3.
Available Anabolic-Androgenic Steroids, Including Veterinary Pharmaceuticals
| Generic Name |
Trade Names |
Formula |
Administration |
| Testosterone | |||
| Gel | AndroGel, Axiron, Testim, Fortigel | C19H28O2 | Transdermal |
| Patches | Testoderm, Androderm | C19H28O2 | Transdermal |
| Pellets | Testopel | C19H28O2 | Subcutaneous |
| Buccal system | Striant | C19H28O2 | Buccal |
| Suspension | Testosus, Aquaviron, Univet Uni-test | C19H28O2 | Injection |
| Testosterone propionate | Testrex, Androgeston, Testogen | C22H32O3 | Injection |
| Testosterone enanthate | Delatestryl, Primotestone, Testinon | C26H40O3 | Injection |
| Testosterone cypionate | Depo-Testosterone, Durandro | C27H40O3 | Injection |
| Testosterone undecanoate | Andriol, Undestor, Nebido | C30H48O3 | Injection |
| Nandrolone decanoate | Deca-Durabolin, Anabolin | C28H44O3 | Injection |
| Boldenone undecylenate | Equipoise, Ganadol, Equigon | C30H44O3 | Injection |
| Methenolone enanthate | Primobolan depot, Delapromor | C27H42O3 | Injection |
| Oxandrolone | Anavar, Oxandrin, Vasorome | C19H30O3 | Oral |
| Methandrostenolone | Dianabol, Anabol, Refovit | C20H28O2 | Oral |
| Fluoxymesterone | Halotestin, Fluotestin, Ora-Testryl | C20H29FO3 | Oral |
| Oxymetholone | Anadrol, Roboral, Anasteron | C21H32O3 | Oral |
| Methyltestosterone | Android, Testred, Methitest | C20H30O2 | Oral, buccal, sublingual |
| Trenbolone acetate | Finaplix H, Finaplix S, Parabolan | C20H24O3 | Oral, injection, pellets |
| Stanozolol | Winstrol, Stromba, Winstrol V | C21H32N2O | Oral/injection |
Patterns of AAS Abuse
Unique patterns of AAS abuse evolved through anecdotal evidence, as well as trial and error. Research14,54,127 suggests that most AAS abusers administer doses well beyond medicinal levels. These doses vary depending on the desired outcomes, but weekly totals in excess of 1000 mg are not uncommon127; typical medicinal doses for hypogonadal males are 35 to 70 mg per week.7 Additionally, AAS abusers frequently “stack” (simultaneously administer) multiple AAS.1,4,8,9,41,45 Abuse often occurs in cycles, or periods of use followed by nonuse, typically lasting 6 to 12 weeks.1,4,8,9,41 The efficacy of these abuse patterns is unconfirmed by research, but extreme athletic performances and physiques may indicate that practice is ahead of science.1,127
AAS Abuser Characteristics
Many AAS prevalence studies also gathered related demographic information that helped to define the AAS abuser.3,8,35–37,46 A 2009 national Centers for Disease Control and Prevention study of adolescents found that male teens (4.3%) were more likely to abuse AAS than female teens (2.2%).35 Hispanics, whites, and blacks were the most likely AAS abusers.35 In another investigation,46 an association was suggested between sport participation and AAS abuse, especially with males. Other authors36 reported a similar association between AAS abuse and physical exercise in European adolescents. Some researchers46 found a strong association between the use of legal performance-enhancing substances and AAS, whereas others36 suggested a similar association for recent alcohol abuse and lifetime abuse of sedatives or cannabis.
The most recent NCAA study indicated collegians typically begin their AAS abuse under age 20, with some declaring their first-time abuse occurring between ages 12 and 13.37 Most collegians claimed to abuse AAS to improve athletic performance and for reasons unrelated to sports, with fewer using AAS to treat injury.37 Males were more likely than females to utilize nontherapeutic AAS within the past 12 months.37 Other findings3 suggested that collegiate AAS abusers tended to be married, male, student-athletes, and older than 23 years of age. Although students at commuter colleges had a higher prevalence of AAS abuse, geographic region, NCAA division, institutional status (private versus public), and size were not associated with the nontherapeutic use of AAS.3 These authors reported a significant association between AAS abuse and cigarette smoking, binge drinking, illicit drug abuse (marijuana and cocaine), and other risky behaviors.3
Sources of Nontherapeutic AAS
The United States classifies AAS as Schedule III controlled substances, which require a prescription.1,3 Research indicates that AAS abusers obtain their drugs through many venues.8,37,40,41,49 Among male high school senior AAS abusers, 60.5% obtained their drugs via black-market sources, with about 20% obtained from physicians, pharmacists, and veterinarians.41 Similarly, 59% of community college abusers obtained their AAS through black-market sources.8 Physicians who provided AAS with a prescription (26.5%) and without a prescription (8.8%) were the next most common sources.8 Only 10% of AAS-abusing power lifters obtained their drugs from physicians or pharmacists.40 The 2009 NCAA study revealed the most common AAS source for collegiate abusers was a teammate, friend, or family member.37 About 14% acquired their drugs via the Internet, whereas physicians, coaches, and athletic trainers were the declared sources for 13%, 7%, and 6%, respectively.37 With many of these drugs coming from nonmedicinal sources, one must always consider the likelihood of contamination, as well as the accuracy of the actual drug amount.52,127
AAS Pharmacology
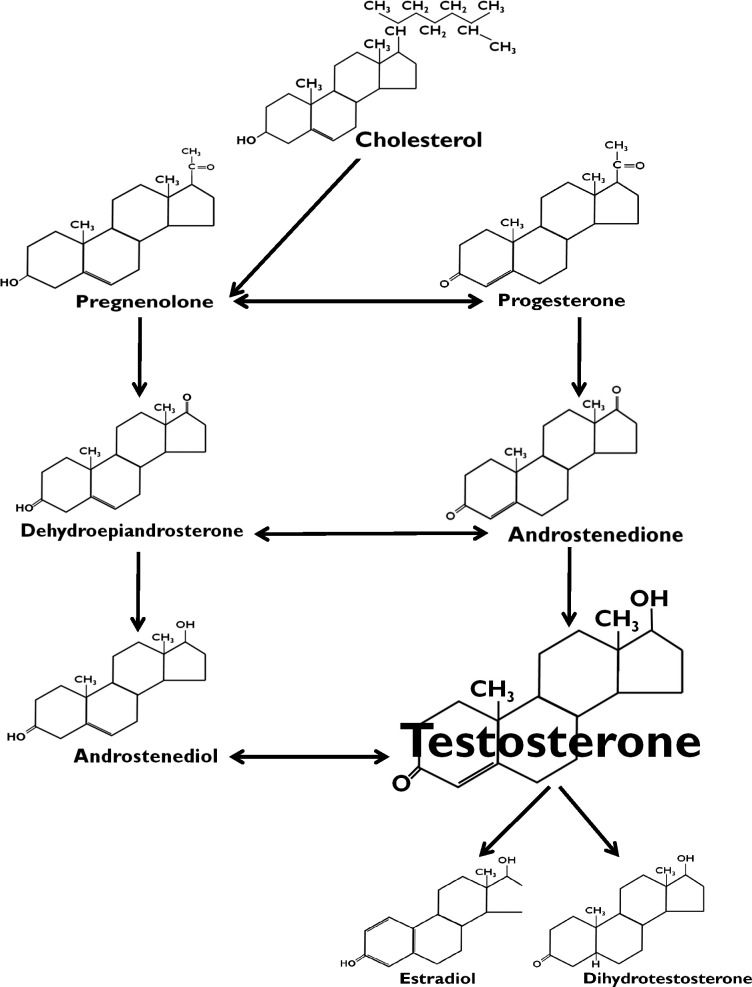
Hormones are compounds produced by the endocrine system that chemically regulate specific body functions. Steroid hormones have a biochemically unique 4-ring structure, including three 6-carbon rings and one 5-carbon ring (Figure 1).14 Androgens are steroid hormones synthesized primarily in the gonads and adrenal glands.1,4,7,14–16,51,127 Dihydrotestosterone, dehydroepiandrosterone, androstenedione, androstenediol, and testosterone are the human androgens (Figure 2).15 Testosterone (Figure 3) is the key androgen promoting masculine (androgenic) characteristics, as well as maintaining nitrogen balance and facilitating protein synthesis (anabolism).14 Males synthesize 2.5 to 11 mg of testosterone per day, whereas females produce about 0.25 mg daily.7 Although both males and females synthesize testosterone, serum concentrations in males (300 to 1000 ng/dL) are significantly greater than in females (15 to 65 ng/dL).7 In addition to individual differences and sex variability, testosterone levels generally decline in older men and vary with certain disease states (eg, adrenal gland disorders in males and females, as well as polycystic ovary syndrome and rare ovarian cancers in women).7,17–20
Figure 1.
Chemical structure of steroid hormones with three 6-carbon rings and one 5-carbon ring.
Figure 2.
Biochemical pathways of the human androgens: androstenediol, androstenedione, dehydroepiandrosterone, dihydrotestosterone, and testosterone.
Figure 3.
Chemical structure of testosterone.
Throughout history, pharmaceutical companies and clandestine laboratories developed scores of unique derivatives of the endogenous androgenic hormones, including testosterone (Table 3).13–15 New AAS innovations seek to enhance or minimize certain effects, improve administration or absorption, improve receptor affinity, and, more recently, avoid detection.14,15 Frequently, efforts are focused on facilitating anabolism and inhibiting androgenesis, although to date no AAS are solely anabolic.7,14
Pharmacokinetics
Unaltered oral testosterone is readily metabolized by the liver, so pharmaceutical companies modify the molecule or the delivery system to enhance its bioavailability, change its rate of absorption, and enhance or diminish its various characteristics.14 Traditionally, AAS administration routes included oral or parenteral routes and subcutaneous pellets, but buccal and transdermal methods are now also available.14,15 Testosterone and its derivatives are often alkylated at the 17β-hydroxy position to decrease first-pass liver metabolism when administered orally.14,15 Esterification at the 17β-hydroxy site makes AAS molecules less polar and more soluble as an oil-based injectable.14,15
Once absorbed into the blood, AAS pass throughout the circulatory system, generally bound to either sex-hormone-binding globulin or more loosely to the plasma protein albumin.7,14,15 Less than 2% of testosterone remains unbound and freely available.7,9 Commonly metabolized by the liver, AAS molecules convert to androsterone and etiocholanolone.7,15 These biologically inert compounds undergo glucuronidation or sulfation and are primarily excreted through the urinary system.14
Pharmacodynamics
The AAS are exogenous synthetic derivatives of, and generally function as agonists for, the androgen hormones.1,2,4,7,14 The AAS mechanism of action occurs at the cellular level and requires a variety of specific enzymes in concert with the many androgen receptors.14,15 The best current evidence suggests that AAS pharmacodynamics mechanisms on muscle tissue are numerous and varied.7,14,15
The primary AAS mechanism of action involves the stimulus for DNA transcription.14,15 Once they are available at the cellular level, the lipid-soluble AAS molecules diffuse across the cell membrane into the cytoplasm, where they bind with the intracellular androgen receptors.14,15 These receptor-bound compounds enter the cell nucleus to bind with DNA, which promotes transcription, thus stimulating protein synthesis.14,15 The AAS also may work as glucocorticoid antagonists and displace these catabolic compounds from their receptor sites, thereby limiting the possible effects of cortisol and related compounds.14 Some authors14,22 also suggest a possible ergogenic effect of AAS through psychotropic actions. Preliminary research indicates that other possible ergogenic actions of AAS require further study,14 including the downregulation of myostatin,7,23,24 and the possible stimulus to synthesize human growth hormone and insulin growth factor 1.12
Therapeutic Uses of AAS
Certain medicinal uses for AAS are accepted; other possible health benefits are not currently acknowledged or accepted by the medical community.1,2,6,7,14,17–19,24,26–33 Pharmacologic uses of AAS for the treatment of diseases and disorders continue to evolve.1,2,7,14,18,19 Although any potential therapeutic benefits must not be exaggerated, it is just as important to not overstate the health risks.14,56
Reproductive Health
Testosterone and its synthetic analogs have long been considered possible treatment agents for various male reproductive disorders.1,2,7,16–19,26 Two meta-analyses18,19 suggested that AAS treatment may be associated with benefits for male sexual function but cautioned about the somewhat weak results and cited a need for more well-designed, long-term studies. Additionally, the authors26 of a systematic review reported that no AAS-based male contraceptive was ready for therapeutic use but encouraged continued research.
Anticatabolism
The AAS have been clinically used to treat catabolic conditions since the 1940s.7 Generally, the strongest evidence for anticatabolic AAS efficacy involves the treatment of severe burn patients.7,24 High-quality systematic reviews and meta-analyses that addressed chronic catabolism with AIDS tend to show positive outcomes for AAS therapy.7,24,27,28 Equivocal results were demonstrated for AAS use in other wasting conditions, including chronic obstructive pulmonary disease,7,30,32 wound healing and postoperative recovery,15 and alcohol-related liver disease.7,24,31 To solidify the efficacy and safety of therapeutic AAS use for anticatabolism, more well-designed studies are needed.7,24,28–30
Bone Health
Based on a 2006 systematic review and meta-analysis20 on testosterone and bone health, intramuscular AAS injections moderately improved lumbar bone density, but the effects on femoral-neck density were inconclusive. The clinical efficacy of testosterone to prevent or treat osteoporosis was weak, although the authors encouraged further study of fractures and testosterone.
Anti-Aging
With advancing age, male endogenous testosterone production declines,7,18–20 which has been suggested by some to underlie many health maladies.17,18 Therapeutic AAS use has increased in middle-aged to older men as an anti-aging therapy while spurring reviews of efficacy and safety.6,17,18 Additionally, recent concerns about female hormone replacement therapy (HRT) contribute to the controversial use of AAS for “andropause.”6 A number of systematic reviews and meta-analyses6,17,18,33 indicate possible benefits, but the findings remain inconclusive. Lifestyle, including increased activity, may limit some age-related negative hormonal changes without the negative pharmacologic side effects.62 Further study is required before definitive statements can be made.18
Other Medicinal Purposes
Although treatment efficacy remains uncertain, AAS also have been considered and used in the therapeutic treatment of some cancers (eg, certain breast cancers) and chronic renal failure, among other conditions.2,7,16
Ergogenic Effects of AAS
A number of high-quality scientific papers57–66,130–132 addressed the efficacy of AAS on mass, body composition, and physical performance (Table 4). Many issues confound AAS efficacy study designs, thus yielding diverse results.11,13,22,51,52,55,56 Ethical issues aside, institutional review boards generally do not allow researchers to duplicate real-world supraphysiologic, polypharmaceutical dosing regimes.11–13,51,52,55,56 Furthermore, the effectiveness of participant blinding when studying AAS is questionable.59 Efficacy studies involve many different research factors, including numerous AAS, varied dosages, assorted training protocols, dissimilar periods, genetically diverse participants, dietary variances, and numerous physical performance measures.11–13,20,51,55,59 The complexity of AAS efficacy studies makes comparisons and conclusions difficult.11,20,22,51,55,56,59 Well-designed studies are prospective, placebo controlled, and double blind in nature.20,51 Protocols also must involve random assignment and control diet and training.20,51 Sample size, participant background, performance measures, and a crossover design also affect a study's overall quality.20,51 After evaluating scores of AAS efficacy studies according to these criteria, we provide the following examples of high-quality studies.
Table 4.
Selected High-Quality Studies on Anabolic-Androgenic Steroid Efficacy
| Author(s) (Year) |
Diet Controlled? |
Participants, N |
Age, y |
Training |
Study Groups |
Outcomes |
| Baume et al (2006)132 | No | 25 | 20–30 | 7 d/wk × 4 wk (endurance) | 300 mg/wk 19-nor-4-androstenedione240 mg/wk testosterone undecanoate900 mg/wk placebo | Ø Serum: AST, creatine kinase, ureaØ Urine: adrenaline, noradrenaline, dopamineØ Treadmill running: maximum, submaximum |
| Bhasin et al (2005)58 | Yes | 52 | 60–75 | 20 wk | 25 mg/wk testosterone enanthate50 mg/wk testosterone enanthate125 mg/wk testosterone enanthate300 mg/wk testosterone enanthate600 mg/wk testosterone enanthate | ↑ Leg press (1RM)↑ Lean mass, triceps and quadriceps size ↑ Testosterone (free, total)↓ FSH, LH |
| Bhasin et al (1996)60 | Yes | 40 | 19–40 | 3 d/wk × 10 wk | 600 mg/wk placebo w/o exercise600 mg/wk placebo w/exercise600 mg/wk testosterone enanthate w/o exercise600 mg/wk testosterone enanthate w/exercise | ↑ Bench press, squat (1RM)↑ Fat-free mass, triceps and quadriceps size↑ Testosterone (free) ↓ FSH, LH, SHBG |
| Fahey and Brown (1973)130 | No | 28 | College | 3 d/wk × 8 wk | 1 mg/kg × 3 wk nandrolone decanoate1 mg/kg × 3 wk placebo | Ø Body composition, anthropometricsØ Bench press, dead lift (1RM)Ø Maximal aerobic capacity, peak work |
| Giorgi et al (1999)61 | No | 21 | 19–45 | 4 d/wk × 12 wk | 3.5 mg/kg/wk testosterone enanthate3.5 mg/kg/wk placebo | ↑ Bench press (1RM), mass, lean mass↑ Arm girth, rectus femoris circumference↓ Abdominal skinfold thickness |
| Hervey et al (1981)65 | No | 7 | 25–38 | Variable for 6 wk | 100 mg/d methandienone100 mg/d placebo | ↑ Mass, lean mass, limb circumferences, muscle widths↑ Total potassium, total nitrogen, leg strengthØ Arm or hand strength, fat and subcutaneous fat |
| Johnson et al (1972)64 | Protein intake only | 31 | 18–38 | 3 d/wk × 3 wk | 10 mg/d methandrostenolone10 mg/d placebocontrol | ↑ Dynamic and static strength, mass, limb sizeØ V˙o2max, sperm count, subcutaneous fat |
| Lichtenbelt et al (2004)57 | Yes | 16 | 19–44 | 8 wk | 200 mg/wk nandrolone decanoate200 mg/wk placebo | ↑ Mass (total, fat free), body mass index |
| O'Shea (1971)63 | Protein intake only | 20 | 19–26 | 3 d/wk × 4 wk | 10 mg/d methandrostenolone10 mg/d placebo | ↑ Mass, squat, bench press (1RM) |
| Rogerson et al (2007)59 | Yes | 16 | 21–35 | 2–3 d/wk × 6 wk | 3.5 mg/kg/wk testosterone enanthate3.5 mg/kg/wk placebo | ↑ Mass, leg press, bench press (1RM)↑ Cycle sprint (peak power, total work) |
| Storer et al (2003)66 | Yes | 54 | 18–35 | None | 25 mg/wk testosterone enanthate50 mg/wk testosterone enanthate125 mg/wk testosterone enanthate300 mg/wk testosterone enanthate600 mg/wk testosterone enanthate | ↑ Dose-dependent leg press, leg power (1RM)Ø Muscle fatigability, specific tension |
| Stromme et al (1974)131 | Protein intake only | 21 | 21–27 | 3 d/wk × 8 wk | 75 mg/d × 4 wk to 150 mg/d × 4 wk mesterolone75 mg/d × 4 wk to 150mg/d × 4 wk placebo | ↑ Thigh circumferenceØ Maximal strength, mass, V˙o2max, free testosterone↓ Testosterone (plasma-bound, total) |
Abbreviations: Ø, no changes; ↑ increase; ↓, decrease; 1RM, 1-repetition maximum; AST, aspartate aminotransferase; FSH, follicle-stimulating hormone; LH, leutinizing hormone; SHGB, sex-hormone-binding globulin; w/o, without.
AAS Effects on Body Composition
In one early study130 involving 28 moderately active college-aged males, participants were randomly assigned to the group receiving 1.0 mg/kg of nandrolone decanoate in weeks 2, 5, and 8 or a placebo group. After 9 weeks of progressive strength training without dietary controls, no changes were noted in body mass or composition. Another group131 completed a double-blind, placebo-controlled, random-assignment project in which participants received either a placebo or 75 mg/d (increased to 150 mg/d after 4 weeks) of mesterolone. All 21 healthy young male participants completed a high-intensity, progressive resistive training program throughout the 8-week study. No changes were seen in body mass for either group, although thigh circumference increased for the AAS group.131 In a 4-week, double-blind, placebo-controlled 1971 study,63 20 collegiate weightlifters were randomly assigned to ingest 10 mg/d of methandrostenolone or a placebo. A significant increase in total body mass occurred, with caliper measurements suggesting an increase in lean tissue. Others64 reported that healthy males given daily 5-mg doses of methandrostenolone increased muscle size (circumference) and total weight and decreased subcutaneous adipose tissue compared with the placebo group. Authors of a 1981 study65 reported the effects of 100 mg/d of methandienone on 7 male weightlifters. The double-blind, placebo-controlled study involved a crossover design, including two 6-week periods with a 6-week washout. Participants gained weight during the AAS periods, although body fat did not vary.
A well-designed and well-controlled 1996 study60 investigated the effects of a 600-mg weekly dose of testosterone enanthate or placebo on body mass and composition. A total of 40 young, healthy participants completed the 30-week study, which consisted of 4 weeks of a control period, 10 weeks of treatment, and 16 weeks of recovery. This double-blind study involved 4 randomly determined groups: placebo without training, placebo with training, AAS without training, and AAS with training. The researchers also controlled protein and total caloric intake. Mean weight in the placebo groups did not change but increased in both AAS groups; the AAS-with-training group gained significantly more weight than the AAS-without-training group. Additionally, except for the placebo-without-training group, all groups gained lean mass. Finally, muscle cross-sectional area increased for both AAS groups; the AAS-with-training group achieved the greatest gains.60
A 2004 double-blind, randomized study57 of 16 experienced bodybuilders involved weekly injections of nandrolone decanoate or a placebo (200 mg) for 8 weeks. Participants continued training at their normal levels. The AAS group increased total body mass and relative lean tissue compared with the placebo group.
Authors of a 1999 study61 reported weight and size (ie, girth) increases for strength-training participants given testosterone enanthate compared with a placebo. After random assignment, 21 young, healthy males completed 12 weeks of double-blind drug or placebo (weekly doses of 3.5 mg/kg) and strength-training intervention. Weight and size increases reverted to normal 12 weeks after the drug intervention.61
One 2005 paper58 detailed a 36-week prospective study of testosterone enanthate and gonadotropin-releasing hormone agonists on 60 healthy, older (60–75 years) men. During the 20-week treatment period, participants received weekly injections of doses ranging from 25 to 600 mg, depending on their random group assignment. Participants did not strength train or complete moderate to intense aerobic exercise during the study. A positive, dose-related response with regard to lean mass increases and an inverse dose-response relationship to fat mass were observed.
Finally, 16 healthy collegiate men enrolled in a 2007 placebo-controlled, double-blind study59 to investigate, in part, the effects of AAS on body mass and composition. Researchers randomly assigned participants to receive weekly injections of either testosterone enanthate or placebo. All trained for muscle hypertrophy during the trial. The AAS group gained more mass than the placebo group.59
Overall, the best science related to the effects of exogenous, nontherapeutic abuse of AAS on body composition suggests a dose-related potential for increased relative lean body mass.57–61
AAS Effects on Physical Performance
Even though evidence suggests possible increased lean mass with AAS abuse,57–61,63–65 more muscle does not always equal improved performance. In some endeavors, such as marathons and triathlons, increased mass (lean or otherwise) may lead to a performance decrease. Considering the known association between muscle mass and force development, a relationship between AAS abusers and strength or power activities, although not necessarily causal, is not unexpected.
The authors of an early study63 investigated methandrostenolone use at 10 mg/d and resistance training by university students over 4 weeks. All participants ingested a high-protein supplement. The protocol involved a randomized, double-blind, placebo-controlled design. Training with AAS increased performance in the 1-repetition maximum (1RM) bench press and squat. Others64 studied the use of 10 mg/d of methandrostenolone and a protein supplement by college students. This randomized, double-blind, placebo-controlled study lasted 7 weeks, including the 3-week treatment phase. Participants trained 3 times per week at increasing intensities. The authors reported increases in 1RM bench press and squats by the treatment group over both the placebo and control groups.
A 1973 paper involved a randomized, double-blind, placebo-controlled study on AAS efficacy in 28 healthy males.130 Throughout the 9-week study, participants strength trained 3 days per week using progressive resistance. Participants received 1.0 mg of nandrolone decanoate or saline solution per kilogram of body weight during weeks 2, 5, and 8. This study did not control for diet, nor did it supplement with additional protein. No significant gains were seen in the AAS group over the placebo group for any performance indicators, including 1RM bench press and dead lift, as well as all isokinetic peak torques for elbow, shoulder, and knee flexion-extension movements.
Other investigators131 designed a double-blind, placebo-controlled prospective study involving 21 healthy college students. Random assignment was to either the mesterolone or placebo group. Drug dosage increased from 75 mg/d to 150 mg/d midway through the 8-week study. The authors controlled a progressively increasing, high-intensity training program. Dietary protein (30 g/d) was supplied to both groups throughout the study. Performance measures included maximal isometric force for 4 motions (elbow flexion, trunk flexion, trunk extension, and knee flexion-extension). No differences were seen between the AAS and placebo groups.
A 1981 prospective, randomized, double-blinded, placebo-controlled, and crossover study consisted of 7 healthy, young weight lifters.65 Two treatment periods (100 mg/d of methandrostenolone) of 6 weeks were separated by 6 weeks of drug washout. Progressive resistance training occurred over the full 18 weeks. Gains were observed in leg strength (isometric dynamometry similar to the dead lift) but not with the isometric shoulder press or hand grip.
More recently, a few well-designed AAS efficacy studies were conducted in healthy, eugonadal participants.59–61,66,132 One group60 examined the effects of 600 mg/wk of testosterone enanthate on 40 experienced male weight lifters, ages 19 to 41 years. Participants were assigned to 1 of 4 groups: testosterone with exercise, testosterone without exercise, placebo with exercise, and placebo without exercise. The authors controlled diets, and all participants consumed 1.5 g of protein per kilogram of body weight. Training consisted of supervised, moderate-intensity weight training 3 days per week for 10 weeks. Participants in the testosterone-with-exercise group experienced the greatest performance gains, as measured by 1RM bench press and squat. The testosterone-without-exercise and placebo-with-exercise groups also gained strength when compared with the placebo-without-exercise group.60
A 1999 study61 prospectively investigated the effects of 3.5 mg/kg of testosterone enanthate over 12 weeks of strength training by 21 healthy, active males in a double-blind trial. After random assignment, participants completed a 4-d/wk, periodized, whole-body strength-training program determined by their individual baselines. Gains occurred in the 1RM bench press for those receiving the testosterone injections compared with the placebo group. Twelve weeks of follow-up training without injections resulted in a return of the measure to that of the placebo group.61
In a prospective, 20-week 2003 study,66 61 young, healthy males with prior resistance training experience were randomly assigned to 1 of 5 treatment groups receiving weekly testosterone enanthate injections: 25 mg, 50 mg, 150 mg, 300 mg, or 600 mg. They did not train during the treatment period. Daily energy intake involved 150 kJ·kg−1·d−1, including 1.3 g/kg of protein. A dose-dependent increase in leg-press strength and power was observed.
Authors59 of a 2007 paper reported findings of a 6-week, randomized, double-blind, placebo-controlled investigation of 16 young, healthy males. Participants received 3.5 mg of testosterone enanthate or saline per kilogram of body weight each week for 6 weeks and performed a structured heavy-resistance training protocol throughout the trial. Living conditions were also controlled, and participants consumed standardized meals that included protein supplementation. The experimental group's 1RM bench press and total work values during a 10-second cycle sprint were improved at 3 and 6 weeks.59
A well-designed project132 looked at the efficacy of AAS as related to endurance performance. Twenty-four young, active, healthy male volunteers participated. After initial testing to determine running velocity at individual anaerobic thresholds, they completed personalized 4-week training protocols designed to improve this measure and during that time ingested 300 mg/wk of 19-nor-4-androstenedione, 240 mg/wk of testosterone undecanoate, or 300 mg/wk of placebo. The study was randomized and double blinded. Anaerobic threshold velocities did not vary either before or after treatments. Although all groups improved their running velocity at anaerobic threshold after 4 weeks of AAS use and training, neither of the experimental groups (19-nor-4-androstenedione or testosterone) improved more than the placebo group.
Last, many recent scientific investigations involved the use of AAS for HRT among older adults or hypogonadal participants. Dozens of published papers examined the effects of HRT on a variety of variables, including muscular performance.6,62 The HRT findings were summarized in a meta-analysis6 and a systematic review.62 In their 2006 meta-analysis6 of 11 prospective studies, the authors suggested a “moderate increase in muscle strength” with HRT in elderly men. In a systematic review62 of various sarcopenia treatments, the authors concluded that male HRT produced moderate strength increases, although not all protocols were successful.
Health Effects of AAS
Some56 have suggested that AAS abuse risks are exaggerated, but others72,73,133,134 proposed that supraphysiologic AAS abuse negatively affects human health, even to the point of premature death. Therapeutic AAS use helps to resolve specific health maladies,6,7,13,18–20,24,26–33 but like all medicinal agents, AAS also have side effects (Table 5). Making definitive claims concerning the effects of long-term, high-dose, multidrug AAS abuse is difficult because researchers cannot ethically administer such protocols.11–13,51,52,55,56 These scenarios have never been and may never be duplicated in a controlled research setting.22,51,52,55,56 With AAS abuse, as with those seeking improved performances or physiques, the negative effects appear to increase, thus presenting health risks.
Table 5.
Possible Anabolic-Androgenic Steroid Abuse Health Effects
| System |
Possible Adverse Effects |
| Cardiovascular | Cardiac dysfunction: ↓ left ventricle peak ejection fraction, systolic strain, early diastolic peak velocities, E/A ratio, right ventricle function |
| Central nervous | Major mood disorders, dependence, progression to other forms of substance dependence |
| Hematologic | ↑ total cholesterol, low-density lipoproteins; ↓ high-density lipoproteins |
| Hepatic | Cholestatic hepatis, cholestatic jaundice, peliosis hepatis, adenomas, carcinomas |
| Immune | General immunosuppression: ↓ antibody synthesis, B- and T lymphocyte maturation, natural killer cell activity; systemic infection (from needle, syringe misuse) |
| Integumentary | Sebaceous gland hypertrophy, ↑ sebum and skin lipid excretion, ↑ acne, seborrhea, striae distensae, hypertrichosis, hirsutism, rosacea, folliculitis, keloids, furunculosis |
| Neuroendocrine | |
| Male | Hypothalamic-pituitary-testicular axis suppression, hypogonadism, testicular atrophy, sexual dysfunction, infertility, gynecomastia, male-pattern baldness, libido changes, prostate hypertrophy, cancer |
| Female | General virilization, hirsutism, voice deepening, male-pattern baldness, clitoral hypertrophy, breast reduction, menstrual dysfunction, libido changes |
| Musculoskeletal | Premature epiphyseal closure, tendinopathies |
| Nephritic | Kidney stones, cholestasis, tubular necrosis, hyperbilirubinemia, proteinuria, focal segmental glomerulosclerosis, renal cell cancer, Wilms tumor |
| Oral | Gingival hypertrophy, ↑ temporomandibular dysfunction |
Abbreviations: ↑, increase; ↓, decrease; E/A ratio, the ratio of passive ventricle filling (early [E] wave) and active filling with atrial systole (atrial [A] wave).
Effects of AAS Abuse on the Central Nervous System
Supraphysiologic doses of AAS are associated with a variety of neuropsychological effects. The best established of these effects are AAS-associated mood disorders, AAS dependence syndromes, and progression from AAS to other forms of substance abuse and dependence.
AAS-Associated Mood Disorders
A growing body of literature indicates that markedly supraphysiologic doses of AAS may cause hypomanic or manic syndromes in some individuals.21,54,67–78 These syndromes involve euphoric or irritable mood, exaggerated self-confidence (which may occasionally result in grandiose delusions), decreased need for sleep, hyperactivity (including sexual hyperactivity), and reckless or sometimes violent behavior.67 However, these syndromes are idiosyncratic. Most AAS abusers, even when using high doses, do not display major mood disturbances, although a minority may exhibit dramatic changes, with behavior completely different from their baseline personalities.21 We have no clear way to predict who will and will not develop these syndromes. Hypomanic syndromes may occur in individuals without a history of mood disorder or personality disturbance.21
Some evidence suggests an association between AAS dose and the risk of manic or hypomanic episodes. For example, laboratory studies using only modestly supraphysiologic doses of testosterone or equivalent AAS (up to 300 mg/wk, representing 4 to 6 times normal male endogenous testosterone production) have rarely found clinically significant mood disorders among participants.21 Similarly, among illicit AAS abusers, mood disorders rarely appeared in individuals taking the equivalent of 300 mg/wk of testosterone135 and are uncommon even in those taking the equivalent of 300 to 1000 mg/wk.21,68 However, these disorders occur at increased frequency among men taking doses of more than 1000 mg per week.68 In women, AAS abuse is rare, and indeed, in only 1 study69 in the past 15 years have investigators successfully recruited and interviewed female AAS abusers. Among the 25 AAS-abusing participants, none met the diagnostic criteria for hypomanic or manic syndrome, although some described feelings of euphoria, irritability, or increased libido.69
The lay press commonly refers to AAS-induced mood syndromes by the popular term roid rage, implying that aggressive or violent behavior is a central feature. Although a colorful term, roid rage has no agreed scientific meaning. Nevertheless, AAS-induced hypomanic and manic syndromes often are associated with irritable or aggressive behavior.21 In some, this behavior may rise to the level of outright violence, and the victims are often women.70 Several case reports21 have described individuals who committed violent crimes while using AAS. Some of them had no history of violence, no criminal records, and no evidence of psychiatric disorder before AAS exposure, suggesting that the observed behavior was attributable to a biological AAS effect. The authors71 of a literature review on AAS-induced aggression suggested that this phenomenon is sufficiently well documented to meet the so-called Daubert criteria, as a scientifically accepted event admissible in court testimony.
Various forensic studies suggest an association between AAS abuse and violent or criminal behavior.72–76 These include case series, describing unusual violence among young AAS abusers74; epidemiologic data suggesting both a possible link between AAS abuse and death by homicide or suicide72,73 and a relationship between AAS abuse and conviction for weapons offenses.76 It is important to note the direction of causality cannot be determined in such studies; it may be that AAS abuse predisposes individuals to violent behavior, that violent individuals are more predisposed to AAS abuse, or both.
This issue of causality raises the more general question of whether AAS-associated manic syndromes are attributable to a biological effect of AAS themselves, as opposed to underlying personality attributes of the abuser or psychosocial factors surrounding AAS abuse.21,71,136 One might argue that individuals who abuse AAS are inherently more prone to aggression or, alternatively, that expectations may prompt individuals to behave aggressively, even in the absence of an actual biological effect. However, in several prospective, randomized, double-blind, placebo-controlled studies,54,77,78 investigators administered supraphysiologic doses of AAS to normal volunteers and demonstrated hypomanic effects in some participants, suggesting that these effects cannot be entirely explained by psychosocial factors. Specifically, among 109 men in 4 studies who received doses of at least 500 mg/wk of AAS under blinded conditions, 5 (4.6%) developed hypomanic or manic syndromes on AAS, but none developed these syndromes on placebo.54 This figure is likely an underestimate of the true prevalence of hypomanic or manic episodes among AAS abusers, who frequently receive much larger doses.21 Abusers of AAS typically use several AAS simultaneously,1,4,8,9,21,41,45 thus possibly further increasing their psychiatric vulnerability. Finally, these studies generally excluded participants with preexisting psychopathology, whereas AAS abusers do not similarly screen themselves.
Other lines of evidence also suggest that AAS-associated mood syndromes cannot be entirely explained as the result of premorbid personality factors or expectations. For example, 3 reports collectively described 4 monozygotic twin pairs in which 1 twin abused AAS and the other did not.137–139 In each case, the AAS-abusing twin exhibited prominent AAS-associated symptoms, including suicide in 1 case139 and extreme violence in another.138 None of the non–AAS-abusing twins exhibited any significant psychopathology.137–139 The AAS also induce aggression in rodent models,50,140 again suggesting a biological component in these effects. However, the reasons for individual variability (in both humans and animals) remain unclear.
Evidence from naturalistic studies also suggests that episodes of major depression may arise in AAS abusers, especially during withdrawal.79 These depressive symptoms may be partially attributable to suppression of the hypothalamic-pituitary-testicular axis by AAS.7,11–14,22,51 As in hypomanic syndromes, depressive episodes appear to be highly idiosyncratic, with many individuals exhibiting virtually no depressive symptoms during withdrawal and occasional individuals becoming profoundly depressed and even suicidal.80 Again, the reasons for this variability are unknown.
For largely ethical reasons,11–13,23,52,55 no laboratory studies have been conducted on the withdrawal effects from supraphysiologic AAS doses. It is, of course, unethical to administer massive, prolonged AAS doses to normal volunteers and then deliberately precipitate withdrawal. Thus, evidence regarding these depressive effects necessarily stems almost entirely from naturalistic investigations and is subject to all related limitations, such as selection bias, recall bias, and the presence of confounding variables.84 Yet laboratory investigations of normal volunteers suggest that pharmacologically induced hypogonadism may indeed be associated with depressive reactions that occur idiosyncratically in some individuals.81
AAS Dependence
Increasingly acknowledged is that some AAS abusers develop a syndrome of AAS dependence.82,83 To date, 8 field studies conducted in the United States, Great Britain, and Australia have collectively noted AAS dependence in 197 (30.2%) of 653 AAS abusers.82 Even if one was to assume an estimate of much less than this 30% figure, it would follow that there are still hundreds of thousands of AAS-dependent Americans and millions more worldwide who have consumed AAS for prolonged periods, often despite adverse medical, psychiatric, and social consequences. Dependence on AAS may arise via any or all of 3 possible mechanisms: anabolic, androgenic, or hedonic.84 The anabolic mechanism refers to the muscle-building effects of AAS, which may be particularly appealing to men (and, in rare cases, women) with body-image disorders who wish to look leaner and more muscular.84 Some individuals experience a syndrome termed muscle dysmorphia, or reverse anorexia nervosa, a form of body dysmorphic disorder in which individuals become preoccupied with their perceived lack of muscularity.141 People with this form of body dysmorphic disorder may be particularly at risk for repeated AAS abuse because they constantly strive to be bigger.85,86 Paradoxically, however, some individuals develop worsening symptoms of muscle dysmorphia with AAS abuse, perhaps because they become increasingly preoccupied with their muscularity.84 Thus, they may continue to repeat AAS cycles, afraid of the possibility of losing even a little muscle size if they discontinue the drugs. Published descriptions of such individuals suggest this phenomenon is quite similar (albeit in reverse) to classical anorexia nervosa, in which the patient experiences severe anxiety about the prospect of gaining even a pound.142 Additionally, athletes may be further disposed to muscle dysmorphia and related body-image concerns due to pressures to be and appear athletically competent. This may encourage many to train with the dual intents of improving both performance and appearance. As such, some may decide to abuse AAS to achieve not only their athletic demands but also their psychological expectations.143
In susceptible individuals, AAS dependence may be further potentiated by the androgenic effects whereby these drugs affect the abuser's own endocrine function. Specifically, men who abuse AAS for prolonged periods may profoundly suppress their hypothalamic-pituitary-testicular functioning, to the point that protracted AAS-withdrawal hypogonadism develops every time they stop AAS.84,87 This hypogonadism, as mentioned earlier, may be associated with depression or with other symptoms, such as loss of sex drive and fatigue.144 Therefore, these individuals may soon be tempted to resume AAS to abort these dysphoric effects and may eventually develop a pattern of continuous AAS abuse.
It now appears that AAS dependence may evolve by a third pathway in a mechanism similar to that of classical drug addiction.84,88 Documentation of this hedonic pathway has occurred in laboratory animals. For instance, rodents will self-administer testosterone, even to the point of death142; some evidence suggests that this self-administration process may be at least partially mediated by opioidergic mechanisms.89 A similar process likely occurs in humans. It is notable that AAS-dependent humans often report other forms of classical substance dependence, indicating that certain individuals may have an innate susceptibility to substance-dependence syndromes in general, including dependence upon AAS.82 Clinical observations suggest that AAS dependence, as it evolves, may increasingly resemble classical substance dependence in its behavioral manifestations. In particular, AAS abuse and the surrounding lifestyle of the “gym culture” may become the central focus of an individual's life.145
Progression to Other Forms of Substance Dependence
Results from several studies suggest that AAS abusers may be prone to develop other forms of drug dependence, especially opioid dependence.83,90–93 For example, 40 (64%) of 62 male AAS abusers recruited in the field reported a lifetime history of some form of illicit drug dependence other than AAS.83 In another investigation, 21 (9%) of 227 sequentially admitted male heroin addicts appeared to have been first introduced to opioids via AAS abuse.91 Similarly, among 88 men with a primary diagnosis of opioid dependence, 22 (25%) reported prior AAS abuse, as compared with only 7 of 135 (5%) men with other forms of substance dependence (P < .001).90 Notably, recent observations indicate that AAS abusers may develop other forms of substance dependence before, during, or after their AAS abuse83,92; AAS abuse may not necessarily predispose an individual to other forms of substance dependence, but these various forms of drug abuse may arise from a common underlying susceptibility, as mentioned earlier.
Effects of AAS Abuse on the Cardiovascular System
A growing literature, including anecdotal reports, animal trials, clinical studies, and comprehensive reviews, describes possible adverse cardiovascular effects of AAS abuse.67,94–97 The mechanisms of AAS-induced cardiovascular toxicity remain uncertain, although one group98 proposed that AAS may be directly toxic to cardiac tissue, resulting in a cardiomyopathy characterized by decreased myocardial compliance.
Myocardial function studies in AAS abusers, using sensitive tissue Doppler imaging and strain imaging, documented marked cardiac deficits in AAS abusers.96,99,100 For instance, AAS abusers had lower early and late diastolic tissue velocities and reduced peak systolic strain versus nonusers.96 Other authors100 reported similar impairment of diastolic function in 6 AAS abusers, and tissue Doppler imaging showed impaired right ventricular function in AAS-using weight lifters.99 A recent US study compared 12 long-term AAS abusers and 7 non–AAS-using weight lifters matched for age and exercise history and, alarmingly, found cardiac deficits in the former group.101 In particular, AAS abusers showed strikingly lower left ventricular ejection fractions and strain measures, together with evidence of diastolic impairment, as illustrated by markedly lower early versus late diastolic transmitral blood-flow velocities (ie, a reduced ratio of early to late ventricular filling velocity).101 As such, cardiotoxicity from long-term AAS abuse may be more serious than previously recognized and may increase the risk for heart failure.101
The cardiovascular effects of AAS are not limited to the myocardium, as evidenced by a number of studies and summarized in 3 systematic reviews or meta-analyses.25,94,95 The evidence indicates that AAS abuse increases low-density lipoprotein cholesterol and decreases high-density lipoprotein cholesterol: a lipid profile recognized as a major risk factor for coronary heart disease.25,95,102 However, despite the well-documented changes in circulating lipids caused by AAS, direct evidence that AAS causes atherosclerosis remains limited, albeit suggestive.55,103 These effects are a concern because widespread AAS abuse is a relatively recent phenomenon.3,8,9,34–47,49,128 Thus, most long-term illicit AAS abusers are not yet 50 years old and may soon display increasing rates of cardiac problems as they age.67
Effects of AAS Abuse on the Hepatic System
The abuse of AAS may occasionally be associated with liver abnormalities, such as cholestatic hepatis, cholestatic jaundice, peliosis hepatis, and liver adenomas and carcinomas.7,11–14,22,24,51,55,104 These effects are rare and occur almost entirely from the abuse of oral C17α-alkylated AAS (ie, methyltestosterone, oxymetholone, fluoxymesterone, norethandrolone, methandienone).7,12,24,55,102,104 The frequency of AAS-induced hepatic abnormalities may be overestimated because many liver function tests involve enzymes present in muscle, which may be elevated from muscular trauma after high-intensity training.51 Consequently, to test for genuine liver abnormalities possibly attributable to AAS abuse, it is critical to assess γ-glutamyl transpeptidase because this enzyme is present in liver but not in muscle tissue.51 Although it may take an extended time, many AAS-induced liver conditions appear to resolve after AAS disuse.12,22,24,51
Effects of AAS Abuse on the Neuroendocrine System
Abuse of AAS suppresses the hypothalamic-pituitary-testicular axis in males.7,11–14,22,51 The resulting hypogonadism is thought to relate to AAS dosage, the specific agent abused, and the duration of abuse.11 Hypogonadism can lead to testicular atrophy,7,11–14,22,51,55 sexual dysfunction,11,12,51,55 and infertility.7,11–13,22,51,55 Many of these conditions appear to be reversible upon cessation, although complete resolution may take more than 1 year.7,11–13,22,51,55
Other possible male endocrine effects of AAS abuse include gynecomastia due to AAS conversion to estrogens, which typically requires surgical intervention because cessation does not reverse this change.11–14,22,51,55 Irreversible male-pattern baldness is another potential side effect as testosterone is converted to dihydrotestosterone.11–14,22,51,55 Effects on the prostate gland include hyperplasia, hypertrophy, and possibly cancer.11,12,55 As shown in a meta-analysis,17 therapeutic AAS use in middle-aged to older men led to an increased number of prostate problems.
Well-designed research involving female use of AAS is limited.51 Abuse of AAS in females apparently leads to increased masculinization, including voice deepening, hirsutism, male-pattern baldness, clitoral hypertrophy, and breast reduction.11–14,22,51 Related changes often seen in females include acne and changes in libido and menstruation.11,12,14,22,51 Some changes in females appear to be permanent.12,14,22 One review12 suggested that AAS abuse may lead to birth defects.
Limited evidence11,12,22 indicates that AAS abuse may influence thyroid function, with possible decreased production of triiodothyronine (T3), thyroxine (T4), and thyroid-binding globulin and increased production of thyrotropin and free T4. The clinical relevance of possible thyroid changes is presently unclear.12 Moreover, AAS abuse may decrease glucose tolerance and increase insulin resistance in humans.12,22,105
Effects of AAS Abuse on the Musculoskeletal System
Skeletally immature individuals who abuse AAS risk decreased adult height due to premature epiphyseal closure.11,12,14,22,51 Further evidence suggests a potential link between AAS abuse and assorted musculoskeletal conditions, including various tendinopathies and tendon ruptures.7,11–13,22,51 Possible causes include relative tendon versus muscle weakness and actual collagen fiber dysplasia.7,11–13,22,51 The use of AAS for sport injury recovery has limited support in the literature. Most wound-healing studies were of burn patients,7 but one group106 did report improved recovery time with therapeutic doses of AAS after induced muscle contusions in rats. Further study is needed.
Effects of AAS Abuse on the Other Biologic Systems and Areas
Additional health effects on other human biologic systems or regions may result from AAS abuse.
Immune System
Literature reviews of both animal and human studies suggest that immune system changes depend on AAS dose and type, but AAS generally act as immunosuppressants.107 Specifically, AAS appear to decrease antibody synthesis, limit T- and B-lymphocyte maturation, and diminish natural killer cell activity.11,22,107 Additionally, unhygienic needle or syringe use may lead to local abscesses or systemic infections, including human immunodeficiency and the hepatitis viruses.11,12,14,22
Integumentary System
Nontherapeutic abuse of AAS affects various aspects of human skin.11,12,14,22,108,109 Two reviews108,109 summarized the possible deleterious effects of high-dose AAS abuse related to the integumentary system. High doses of AAS promote the hypertrophy of the sebaceous glands and production of sebum and skin surface lipids and increase the concentration of Propionibacterium acnes. These changes increase the risk for conditions such as acne (conglobata, vulgaris, or fulminans), seborrhea, striae distensae, hypertrichosis, hirsutism, rosacea, folliculitis, keloids, and furunculosis.108,109 Furthermore, preexisting dermatologic conditions may be exacerbated by AAS abuse, including psoriasis, rosacea, angiolipomatosis, and acne vulgaris.108
Nephritic System
Although less studied and reported, renal side effects may occur with AAS abuse.11,22,51,55,110,111 Possible nephrologic conditions include kidney stones, cholestasis, tubular necrosis, hyperbilirubinemia, proteinuria, focal segmental glomerulosclerosis, renal cell cancer, and Wilms tumor.11,51,55,110,111 The pathophysiology of AAS-induced kidney conditions is unclear.110 Complicating factors in athletic and active individuals may include the frequent use of nonsteroidal anti-inflammatory agents, nutritional supplements, high-protein diets, and dehydration (purposeful or otherwise).110,111
Oral Region
Limited research suggests a possible association between AAS abuse and maladies related to the gingival tissues52 and masticatory system.112 A preliminary paper52 indicated that prolonged AAS abuse led to gingival tissue enlargement, including significantly thicker gingival tissue, gingival encroachment, and total gingival enlargement scores compared with AAS nonusers. These changes sometimes require surgical intervention.52 Another study112 indicated that AAS abuse may play a role in altering the masticatory structures, thus increasing the incidence of temporomandibular dysfunction.
AAS Abuse Prevention
Individuals typically abuse AAS to gain performance advantages and enhance their physique or body image (or both).1,2,4,5 The abuse of AAS often begins during adolescence,35,36,41–43,46,47 and thus prevention strategies should also begin at a young age. Prevention techniques generally include educational or screening paradigms (or both). Although relatively few scholarly publications specifically address AAS abuse prevention, general drug abuse prevention requires detailed understanding, including resistance skill development, learning peer norms, and being involved in a multisubstance abuse prevention program that does not focus solely on one substance of abuse. Using related theory in prevention efforts may be clinically helpful. For example, the transtheoretical model helped to explain and address smoking behaviors in specific populations and may also apply to AAS abuse.125,126 Understanding the reasons for AAS abuse or intent to abuse, as well as abuse progressions, may be an effective AAS prevention strategy in conjunction with other prevention initiatives.125,126
Drug Testing for AAS Abuse Prevention
Over the past half century, screening for general drug abuse evolved as part of prevention programs. Drug-tested groups are many and varied: for example, military personnel, civilian and governmental employees, and amateur and professional athletes. For some testing agencies, AAS are not drugs of interest, but the more common screening is for illicit abuse of recreational and other prescription drugs. Athlete AAS testing began more than 30 years ago.1,4,5 Testing for AAS typically involves only organized, competitive sport participants and thus is generally not targeted at the noncompetitive fitness or bodybuilding population.146 Screens for AAS initially focused on elite athletes but have since evolved to include collegiate and some high school student-athletes.114 Numerous international, national, and regional bodies oversee sport AAS testing.113 The World Anti-Doping Agency and related national organizations have strict, year-round, no-notice, accredited-laboratory drug testing, whereas other programs are less stringent and sometimes limited by collective bargaining agreements.4,113 Although these programs are varied, expensive, and time intensive, their effectiveness as preventive measures remains unclear.114 The many inherent testing variables make conducting related studies and drawing appropriate conclusions challenging.
The authors of one early study115 reported that more frequent drug testing resulted in a gradual decline in the number of positive drug tests for Norwegian athletes from 1977 to 1995. Only one group114 conducted a prospective randomized controlled study to investigate the deterrent efficacy of AAS testing for adolescent athletes. Student-athletes (N = 1396) from 11 high schools (5 drug testing, 6 controls) completed the 2-year project. Using a method similar to that used for out-of-competition Olympic testing, the investigators found that AAS abuse remained unaffected over a 2-year period. In addition, those high school student-athletes randomized to testing had increased risk factors for future drug abuse. No known prospective randomized trials exist to demonstrate the deterrent efficacy or inefficacy of AAS abuse testing in other populations such as collegiate, professional, or Olympic athletes. With only 1 well-designed study investigating AAS screening in a very specific population,114 the overall deterrent efficacy of such testing remains understudied and unclear. Additional research on the deterrent effect of AAS testing is greatly needed.
Education for AAS Abuse Prevention
When implemented as a stand-alone intervention, AAS testing focuses on identifying the abuser, but health care professionals should also be concerned about identifying the potential AAS abuser. This paradigm involves education of not only AAS abusers but also potential abusers, educators, and medical professionals.
Numerous authors investigated educational programs as a preventive measure for AAS abuse.114,116–120,147 One 2004 European group120 reported positive results after the implementation of a health promotion program to prevent AAS misuse among adolescents. The 2-year teaching session and group-discussion intervention program focused on appearance and self-confidence, rather than AAS abuse. Group discussions included both sexes. Results indicated that AAS abuse in adolescent boys, ages 16 through 17 years, might decrease after an educational program focused on appearance and self-confidence, although the authors recommended further study. Another project by this group120 emphasized the varied attitudes between AAS abusers and AAS nonusers and the need to understand these differences in designing an effective prevention program. For example, understanding is limited about the many reasons for AAS abuse, such as body-image enhancement, improved performance, and others. Gaining insight into the phenomenologic patterns of AAS abuse warrants further study. Similar to eating disorder prevention and screening protocols, simple body-image screening instruments may need to be included during the preparticipation physical examination process or during neuropsychological testing to help identify those more at risk for AAS abuse.
One US-based research group147,148 developed and tested a number of AAS prevention protocols for adolescents. An early study147 suggested that educational interventions stressing solely the negative effects of AAS were not only ineffective but increased adolescents' desire to abuse androgens. Programs focusing on knowledge of risks and benefits of AAS improved knowledge but did not change attitudes toward abuse; thus, they were deemed ineffective prevention approaches.148 Since then, the authors have developed team-centered, sex-specific educational programs designed to deter AAS abuse among adolescent boys.
Three distinct cohorts implemented Adolescents Training and Learning to Avoid Steroids (ATLAS), which successfully inhibited AAS abuse among adolescent boys involved in team sports.117,118 Results from the 1996 randomized, prospective study,117 involving more than 1500 male youth from 31 high schools, suggested the ATLAS intervention decreased factors that encourage AAS misuse and lowered the intent to abuse AAS while enhancing nutrition behaviors. A follow-up 2000 ATLAS study118 reconfirmed the earlier results with 31 high school football teams and 3207 student-athletes. Results suggested a significant reduction in the intent to abuse AAS as well as the actual AAS abuse within the ATLAS intervention group. Other abuse patterns also were reduced, including ingestion of sport supplements, alcohol, and illicit drugs and illicit drinking and driving behaviors.118
Athletes Targeting Healthy Exercise and Nutrition Alternatives (ATHENA) is a scripted, athletic team-centered, peer-taught, and coach-facilitated program similar to ATLAS that focuses on adolescent female risk factors. More than 925 students from 18 high schools in 2 states participated. Compared with the control group, the ATHENA group used fewer athletic-enhancing substances, including AAS, amphetamines, supplements, and diet pills. In addition, ATHENA participants improved their diets and displayed fewer risky behaviors.116 Long-term follow-up (1 to 3 years after high school graduation) indicated persistently lower rates of substance abuse.122 Both ATLAS and ATHENA have undergone mediation analysis to assess the reasons for the programs' effectiveness.123,124 A systematic review of educational programs commissioned by the World Anti-Doping Agency showed that ATLAS and ATHENA provided the only high-quality evidence available as to the best way to educate adolescents about doping and are the only programs that have been monitored regularly over an extended follow-up period.121 Sex-specific, sport-centered, and coach-facilitated educational programs that promote performance-enhancing alternatives (eg, sport nutrition and strength training) appear to be effective AAS abuse deterrents for adolescent student-athletes.116–120 Presently, other than adolescent educational AAS abuse-prevention programs, no additional studies involving other populations are found in the scientific literature.
AAS Abuse Identification and Intervention
Health care professionals and educators have a responsibility to assist individuals in making informed, positive health decisions.22 The Board of Certification details the following areas of professional athletic training practice related to AAS abuse: (1) injury/illness prevention and wellness protection, (2) treatment and rehabilitation, and (3) organizational and professional health and well-being.149 Recognizing AAS abuse or the intent to abuse (one aspect of prevention); knowing when and how to intervene (which guides referral options); and knowing how to implement health care administration for those in need all are responsibilities of athletic trainers.
AAS Abuse Identification
Health care professionals must understand the epidemiology of AAS abuse in strength and power athletes, as well as other, sometimes less obvious groups and individuals.3,8,9,34–49 A vigilant approach by athletic trainers is critical due to their extended time spent with these populations. The health care professional should be aware of related conversations, as well as changes in relationships,90,93 behaviors,54,67,68,82–85,128,145 and general health,11,13,22,51,55,127 all of which can provide valuable clues to AAS abuse. Although every situation varies, a number of behavioral changes and other signs and symptoms may indicate AAS abuse (Table 6).* If AAS abuse is suspected, consider comparing the patient's current medical records with his or her baseline.
Table 6.
Possible Indicators of Anabolic-Androgenic Steroid Abuse
| Behaviors | Secretive actions, excessive dietary supplement use, mood swings, aggressiveness, excessive exercise, periods of abnormal healthy patterns,a physique and/or diet obsession, high-risk behaviors, rapid performance increases |
| Dermatologic | Abnormal, excessive, or unexpected acne; jaundice, needle marks, abscesses |
| Cardiovascular | Changes toward hypertension, dyslipidemia |
| Neuroendocrine | Male-pattern baldness, gynecomastia, testicular atrophy, female virilization (clitoral hypertrophy, hirsutism, amenorrhea, breast reduction, voice changes) |
| Musculoskeletal | Extreme musculature, rapid growth, extreme leanness, unexpected or unexplainable tendinopathies, premature growth-plate closure |
During an anabolic-androgenic steroid cycle (time period of abuse), individuals may simultaneously focus on healthy behaviors such as a healthful diet, sufficient sleep, and regimented workouts.
AAS Abuse Intervention
To date, no validated management strategies are available for AAS abuse84; thus, much of the following stems from anecdotal evidence or experiences. Referral and counseling are paramount to avoiding possible consequences of AAS abuse, such as negative health effects and psychological dependence.149 However, not all health care professionals or educators understand AAS pharmacology, the mentality of athletes and AAS abusers, or common AAS abuse patterns.67,82,84 Few AAS abusers seek treatment,84 and they are historically skeptical of the medical-scientific establishment.5 As such, health care professionals should develop a referral network with other well-qualified colleagues, possibly including specialists such as endocrinologists and psychiatrists. Considering the various AAS abuse (or intent to abuse) stages may assist health care professionals in offering specific support, as described in the following examples.
-
•
Keep athletes' self-esteem high with alternatives to AAS, such as evidence-based nutrition and weight-training principles, along with collaboration from conditioning experts and sport nutritionists, among others.
-
•
Build trust with patients, clients, athletes, and coaches through consistent, year-round, evidence-based educational meetings and in-service sessions.
-
•
Avoid unfounded scare tactics and hype concerning the negative consequences of AAS.
-
•
Be alert for excessive discussion about and focus on supplements and nutrition practices or self-education about AAS (eg, types, stacks, and cycles).
-
•
Do not threaten suspected AAS abusers but instead cautiously state your suspicions (eg, “I have noticed a change in your behavior, supplement use, or interest in AAS…”) and offer evidence-based information and assistance. Be an accessible agent of positive behavioral changes.
-
•
Acknowledge the minimal, short-term (physical) consequences of AAS abuse but stress education from health, moral and ethical, and legal perspectives.
-
•
Comment on the negative aspects of AAS abuse, such as increased acne, stunted growth, or jaundice (ie, the issues younger adolescents and adults will likely be concerned about). Be sure not to compliment their physical appearance (eg, muscularity) or strength gains because this may reinforce or encourage AAS abuse.
-
•
Discuss personal sacrifices that accompany AAS abuse, such as money, time, social experiences, risk of disease and injury, and counterfeit or tainted products, among others.
-
•
Remain attentive because AAS abusers typically are secretive and good at not getting caught. A passed drug screen does not prove AAS nonuse.
Consider using available tools, such as validated body-image screening instruments: for example, the male version of the eating disorder inventory93 or the muscle dysmorphia version145 of the Body Dysmorphic Disorder Modification of the Yale Brown Obsessive-Compulsive Scale. Objective measures, such as the fat-free mass index, use basic anthropometric data to determine the upper physiologic limits of muscularity. Involve colleagues and other professionals as part of a holistic approach. For instance, integrate evidence-based strength and nutrition principles into all activities and programs. Cooperatively implementing these techniques and principles may allow us to better fulfill our comprehensive responsibility to athletes and the physically active. That is, health care professionals should constantly seek high-quality new resources and readily call on available professionals, while coordinating the process as with any other health care issue. Referral to an AAS-knowledgeable specialist for counseling and possible pharmacologic intervention may be beneficial.84
Health care professionals, including athletic trainers and others, manage many illnesses and injuries in their clinical practices.149 Each condition has unique characteristics and clinical features, just as each AAS abuser is different. Health care professionals may have a close relationship with those in their care. Such relationships provide excellent, critical opportunities to prevent AAS abuse through an active approach that includes being alert to abuse patterns, forthright in terms of approaching the issue, and effective in offering counseling and referral services. Health care professionals and educators serve as advocates and resources for all those with health-related issues, including AAS abuse.
CONCLUSIONS
Anabolic-androgenic steroids are powerful Schedule III pharmaceuticals related to human androgens, primarily testosterone. They act as both anabolic and androgenic agents. Although these drugs have therapeutic roles, athletes and nonathletes often abuse AAS to improve their physical performance, physique, and body image. Abusers of AAS most frequently use a cyclic, high-dose, multidrug regimen, the consequences of which are poorly understood. Rarely do they obtain their information or their drugs from valid and reliable sources. Anabolic-androgenic steroids carry a dose-related potential for increased lean body mass, and these agents generally improve strength- and power-related performances. Conversely, when abused, AAS may lead to negative health effects on the central nervous, cardiovascular, hepatic, reproductive, musculoskeletal, immune, integumentary, and nephritic systems and possibly others. Investigations into useful techniques for preventing AAS abuse are limited. Testing for AAS, by itself or in combination with other interventions, is not well studied, and its deterrent efficacy remains uncertain. Well-designed and properly implemented education appears to be useful for adolescent athletes, but other populations remain understudied. Health care professionals and educators must manage the AAS abuser and potential abuser based on the best available evidence, including studies detailing prevention and intervention strategies. Athletic trainers are health care professionals who are often in a unique position to assess and assist AAS abusers and those who may become AAS abusers.
ACKNOWLEDGMENTS
We gratefully acknowledge the efforts of Don Hooton; Cindy Thomas, MS, ATC; Teresa Seefeldt, PhD; and Andrew P. Winterstein, PhD, ATC in the review of this position statement.
DISCLAIMER
The NATA and NATA Foundation publish their position statements as a service to promote the awareness of certain issues to its members. The information contained in the position statement is neither exhaustive nor exclusive to all circumstances or individuals. Variables such as institutional human resource guidelines, state or federal statutes, rules, or regulations, as well as regional environmental conditions, may impact the relevance and implementation of these recommendations. The NATA and NATA Foundation advise members and others to carefully and independently consider each of the recommendations (including the applicability of same to any particular circumstance or individual). The position statement should not be relied upon as an independent basis for care but rather as a resource available to NATA members or others. Moreover, no opinion is expressed herein regarding the quality of care that adheres to or differs from the NATA and NATA Foundation position statements. The NATA and NATA Foundation reserve the right to rescind or modify position statements at any time.
Footnotes
References 11, 14, 22, 51, 54, 67, 68, 82–85, 90, 93, 127, 128, 133, 141, 145.
REFERENCES
- 1.Dotson JL, Brown RT. The history of the development of anabolic-androgenic steroids. Pediatr Clin North Am. 2007;54(4):761–769. doi: 10.1016/j.pcl.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Hoberman JM, Yesalis CE. The history of synthetic testosterone. Sci Am. 1995;272(2):76–81. doi: 10.1038/scientificamerican0295-76. [DOI] [PubMed] [Google Scholar]
- 3.McCabe SE, Brower KJ, West BT, Nelson TF, Wechsler H. Trends in non-medical use of anabolic steroids by U.S. college students: results from four national surveys. Drug Alcohol Depend. 2007;90((2–3)):243–251. doi: 10.1016/j.drugalcdep.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitch KD. Androgenic-anabolic steroids and the Olympic Games. Asian J Androl. 2008;10(3):384–390. doi: 10.1111/j.1745-7262.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- 5.Todd T. Anabolic steroids: the gremlins of sport. J Sport Hist. 1987;14(1):87–107. [PubMed] [Google Scholar]
- 6.Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA, Ostir GV. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc. 2006;54(11):1666–1673. doi: 10.1111/j.1532-5415.2006.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basaria S, Wahlstrom JT, Dobs AS. Clinical review: anabolic-androgenic steroid therapy in the treatment of chronic diseases. J Clin Endocrinol Metab. 2001;86(11):5108–5117. doi: 10.1210/jcem.86.11.7983. [DOI] [PubMed] [Google Scholar]
- 8.Kersey RD. Anabolic-androgenic steroid use among California community college student-athletes. J Athl Train. 1996;31(3):237–241. [PMC free article] [PubMed] [Google Scholar]
- 9.Perry PJ, Lund BC, Deninger MJ, Kutscher EC, Schneider J. Anabolic steroid use in weightlifters and bodybuilders: an Internet survey of drug utilization. Clin J Sport Med. 2005;15(5):326–330. doi: 10.1097/01.jsm.0000180872.22426.bb. [DOI] [PubMed] [Google Scholar]
- 10.Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69(3):548–556. [PubMed] [Google Scholar]
- 11.Buttner A, Thieme D. Side effects of anabolic androgenic steroids: pathological findings and structure-activity relationships. Handb Exp Pharmacol. 2010;195:459–484. doi: 10.1007/978-3-540-79088-4_19. [DOI] [PubMed] [Google Scholar]
- 12.Casavant MJ, Blake K, Griffith J, Yates A, Copley LM. Consequences of use of anabolic androgenic steroids. Pediatr Clin North Am. 2007;54(4):677–690. doi: 10.1016/j.pcl.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Thevis M, Schanzer W. Synthetic anabolic agents: steroids and nonsteroidal selective androgen receptor modulators. Handb Exp Pharmacol. 2010;195:99–126. doi: 10.1007/978-3-540-79088-4_5. [DOI] [PubMed] [Google Scholar]
- 14.Kicman A. Pharmacology of anabolic steroids. Br J Pharmacol. 2008;154(3):502–521. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivas-Shankar U, Wu FC. Drug insight: testosterone preparations. Nat Clin Pract Urol. 2006;3(12):653–665. doi: 10.1038/ncpuro0650. [DOI] [PubMed] [Google Scholar]
- 16.Freeman ER, Bloom DA, McGuire EJ. A brief history of testosterone. J Urol. 2001;165(2):371–373. doi: 10.1097/00005392-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60(11):1451–1457. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 18.Isidori AM, Giannetta E, Granfilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf) 2005;63(4):381–394. doi: 10.1111/j.1365-2265.2005.02350.x. [DOI] [PubMed] [Google Scholar]
- 19.Bolona ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20–28. doi: 10.4065/82.1.20. [DOI] [PubMed] [Google Scholar]
- 20.Tracz MJ, Sideras K, Bolona ER, et al. Clinical review: testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91(6):2011–2016. doi: 10.1210/jc.2006-0036. [DOI] [PubMed] [Google Scholar]
- 21.Pope HG, Katz D. Psychiatric effects of exogenous anabolic-androgenic steroids. In: Wolkowitz OM, Rothschild AJ, editors. Psychoneuroendocrinology: The Scientific Basis of Clinical Practice. Washington, DC: American Psychiatric Press; 2003. pp. 331–358. In. eds. [Google Scholar]
- 22.Maravelias C, Dona A, Stefanidou M, Spiliopoulou C. Adverse effects of anabolic steroids in athletes: a constant threat. Toxicol Lett. 2005;158(3):167–175. doi: 10.1016/j.toxlet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Kawada S, Okuno M, Ishii N. Testosterone causes decrease in the content of skeletal muscle myostatin. Int J Sport Health Sci. 2006;4:44–48. [Google Scholar]
- 24.Orr R. Fiatarone Singh M. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: review of efficacy and safety. Drugs. 2004;64(7):725–750. doi: 10.2165/00003495-200464070-00004. [DOI] [PubMed] [Google Scholar]
- 25.Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 2005;63(3):280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 26.Grimes DA, Gallo MF, Grigorieva V, Nanda K, Schulz KF. Steroid hormones for contraception in men: systematic review of randomized controlled trials. Contraception. 2005;71(2):89–94. doi: 10.1016/j.contraception.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Johns K, Beddall MJ, Corrin RC. Anabolic steroids for the treatment of weight loss in HIV-infected individuals. Cochrane Database Syst Rev. (4) 2005 doi: 10.1002/14651858.CD005483. ;CD005483. [DOI] [PubMed] [Google Scholar]
- 28.Kong A, Edmonds P. Testosterone therapy in HIV wasting syndrome: systematic review and meta-analysis. Lancet Infect Dis. 2002;2(11):692–699. doi: 10.1016/s1473-3099(02)00441-3. [DOI] [PubMed] [Google Scholar]
- 29.Moyle GJ, Schoelles K, Fahrbach K, et al. Efficacy of selected treatments of HIV wasting: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2004;37((suppl 5)):S262–S276. doi: 10.1097/01.qai.0000144381.09350.5b. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira IM, Brooks D, Lacasse Y, Goldstein RS. Nutritional intervention in COPD: a systematic overview. Chest. 2001;119(2):353–363. doi: 10.1378/chest.119.2.353. [DOI] [PubMed] [Google Scholar]
- 31.Rambaldi A, Iaquinto G, Gluud C. Anabolic-androgenic steroids for alcoholic liver disease: a Cochrane revew. Am J Gastroenterol. 2002;97(7):1674–1681. doi: 10.1111/j.1572-0241.2002.05826.x. [DOI] [PubMed] [Google Scholar]
- 32.Villaca DS, LerariO MC, Dal Corso S, Neder JA. New treatments for chronic obstructive pulmonary disease using ergogenic aids. J Bras Pneumol. 2005;32(1):66–74. doi: 10.1590/s1806-37132006000100013. [DOI] [PubMed] [Google Scholar]
- 33.Conway AJ, Handelsman DJ, Lording DW, Stuckey B, Zajac JD. Use, misuse and abuse of androgens: The Endocrine Society of Australia consensus guidelines for androgen prescribing. Med J Aust. 2000;172(5):220–224. doi: 10.5694/j.1326-5377.2000.tb123913.x. [DOI] [PubMed] [Google Scholar]
- 34.Wroble RR, Gray M, Rodrigo JA. Anabolic steroids and pre-adolescent athletes: prevalence, knowledge, and attitudes. Sport J. 2002;5(3):1–8. [Google Scholar]
- 35.Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance—United States: surveillance summaries, 2009. MMWR Morbid Mortal Wkly Rep. 2010;59 (SS–5). [Google Scholar]
- 36.Kokkevi A, Fotiou A, Chileva A, Nociar A, Miller P. Daily exercise and anabolic steroids use in adolescents: a cross-national European study. Subst Use Misuse. 2008;43(14):2053–2065. doi: 10.1080/10826080802279342. [DOI] [PubMed] [Google Scholar]
- 37.National Collegiate Athletic Association. National Study of Substance Use Trends Among NCAA College Student-Athletes. Indianapolis, IN: National Collegiate Athletic Association; 2012. [Google Scholar]
- 38.Tricker R, O'Neill MR, Cook D. The incidence of anabolic steroid use among competitive bodybuilders. J Drug Educ. 1989;19(4):313–325. doi: 10.2190/EGT5-4YWD-QX15-FLKK. [DOI] [PubMed] [Google Scholar]
- 39.Horn S, Gregory P, Guskiewicz KM. Self-reported anabolic-androgenic steroids use and musculoskeletal injuries: findings from the Center for the Study of Retired Athletes health survey of retired NFL players. Am J Phys Med Rehabil. 2009;88(3):192–200. doi: 10.1097/PHM.0b013e318198b622. [DOI] [PubMed] [Google Scholar]
- 40.Wagman DF, Curry LA, Cook DL. An investigation into anabolic androgenic steroid use by elite U.S. powerlifters. J Strength Cond Res. 1995;9(3):149–154. [Google Scholar]
- 41.Buckley WE, Yesalis CE, 3rd, Friedl KE, Anderson WA, Streit AL, Wright JE. Estimated prevalence of anabolic steroid use among male high school seniors. JAMA. 1988;260(23):3441–3445. [PubMed] [Google Scholar]
- 42.Stilger VG, Yesalis CE. Anabolic-androgenic steroid use among high school football players. J Community Health. 1999;24(2):131–145. doi: 10.1023/a:1018706424556. [DOI] [PubMed] [Google Scholar]
- 43.vandenBerg P, Neumark-Sztainer D, Cafri G, Wall M. Steroid use among adolescents: longitudinal findings from Project EAT. Pediatrics. 2007;119(3):476–486. doi: 10.1542/peds.2006-2529. [DOI] [PubMed] [Google Scholar]
- 44.Bolding G, Sherr L, Elford J. Use of anabolic steroids and associated health risks among gay men attending London gyms. Addiction. 2002;97(2):195–203. doi: 10.1046/j.1360-0443.2002.00031.x. [DOI] [PubMed] [Google Scholar]
- 45.Baker JS, Graham MR, Davies B. Steroid and prescription medicine abuse in the health and fitness community: a regional study. Eur J Intern Med. 2006;17(7):479–484. doi: 10.1016/j.ejim.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Dodge TL, Jaccard JJ. The effect of high school sports participation on the use of performance-enhancing stustances in young adulthood. J Adolesc Health. 2006;39(3):367–373. doi: 10.1016/j.jadohealth.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 47.The European School Survey Project on Alcohol and Other Drugs. The 2007 ESPAD Report: Substance Use Among Students in 35 European Countries. Stockholm, Sweden: The Swedish Council for Information on Alcohol and Other Drugs; 2009. [Google Scholar]
- 48.Kanayama G, Gruber AJ, Pope HG, Jr, Borowiecki JJ, Hudson JI. Over-the-counter drug use in gymnasiums: an unrecognized substance abuse problem? Psychother Psychosom. 2001;70(3):137–140. doi: 10.1159/000056238. [DOI] [PubMed] [Google Scholar]
- 49.Santos AM, da Rocha MS, da Silva M. Illicit use and abuse of anabolic-androgenic steroids among Brazilian bodybuilders. Subst Use Misuse. 2011;46(6):742–748. doi: 10.3109/10826084.2010.534123. [DOI] [PubMed] [Google Scholar]
- 50.Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci Biobehav Rev. 2003;27(5):413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 51.Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34(8):513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- 52.Ozcelik O, Haytac MC, Seydaoglu G. The effects of anabolic androgenic steroid abuse on gingival tissues. J Periodontol. 2006;77(7):1104–1109. doi: 10.1902/jop.2006.050389. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman JR, Faigenbaum AD, Ratamess NA, Ross R, Kang J, Tenenbaum G. Nutritional supplementation and anabolic steroid use in adolescents. Med Sci Sports Exerc. 2008;40(1):15–24. doi: 10.1249/mss.0b013e31815a5181. [DOI] [PubMed] [Google Scholar]
- 54.Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch Gen Psychiatry. 2000;57(2):133–140. doi: 10.1001/archpsyc.57.2.133. [DOI] [PubMed] [Google Scholar]
- 55.Parssinen M, Seppala T. Steroid use and long-term health risks in former athletes. Sports Med. 2002;32(2):83–94. doi: 10.2165/00007256-200232020-00001. [DOI] [PubMed] [Google Scholar]
- 56.Hoffman JR, Ratamess NA. Medical issues associated with anabolic steroid use: are they exaggerated? J Sports Sci Med. 2006;5(2):182–193. [PMC free article] [PubMed] [Google Scholar]
- 57.Lichtenbelt WDVM, Hartgens F, Vollaard NBJ, Ebbing S, Kuipers H. Bodybuilders' body composition: effect of nandrolone decanoate. Med Sci Sports Exerc. 2004;36(3):484–489. doi: 10.1249/01.mss.0000117157.06455.b0. [DOI] [PubMed] [Google Scholar]
- 58.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90(2):678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 59.Rogerson S, Weatherby RP, Deakin GB, et al. The effect of short-term use of testosterone enanthate on muscular strength and power in healthy young men. J Strength Cond Res. 2007;21(2):354–361. doi: 10.1519/R-18385.1. [DOI] [PubMed] [Google Scholar]
- 60.Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiological doses of testosterone on muscle size and strength in normal males. New Engl J Med. 1996;335(1):1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 61.Giorgi A, Weatherby RP, Murphy PW. Muscular strength, body composition and health responses to the use of testosterone enanthate: a double blind study. J Sci Med Sport. 1999;2(4):341–355. doi: 10.1016/s1440-2440(99)80007-3. [DOI] [PubMed] [Google Scholar]
- 62.Borst SE. Interventions for sarcopenia and muscle weakness in older people. Age Ageing. 2004;33(6):548–555. doi: 10.1093/ageing/afh201. [DOI] [PubMed] [Google Scholar]
- 63.O'Shea JP. The effects of an anabolic steroid on dynamic strength levels of weightlifters. Nutr Rep Int. 1971;4:363–370. [Google Scholar]
- 64.Johnson LC, Fisher G, Silvester JL, Hofheins CC. Anabolic steroid: effects on strength, body weight, oxygen uptake and spermatogenesis upon mature males. Med Sci Sports. 1972;4(1):43–45. [PubMed] [Google Scholar]
- 65.Hervey G, Knibbs A, Burkinshaw L, et al. Effects of methandienone on the performance and body composition of men undergoing athletic training. Clin Sci (Lond) 1981;60(4):457–461. doi: 10.1042/cs0600457. [DOI] [PubMed] [Google Scholar]
- 66.Storer TW, Magliano L, Woodhouse L, et al. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88(4):1478–1485. doi: 10.1210/jc.2002-021231. [DOI] [PubMed] [Google Scholar]
- 67.Kanayama G, Hudson JI, Pope HG., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98((1–2)):1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pope HG, Jr, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use: a controlled study of 160 athletes. Arch Gen Psychiatry. 1994;51(5):375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- 69.Gruber AJ, Pope HG., Jr Psychiatric and medical effects of anabolic-androgenic steroid use in women. Psychother Psychosom. 2000;69(1):19–26. doi: 10.1159/000012362. [DOI] [PubMed] [Google Scholar]
- 70.Choi PY, Pope HG., Jr Violence toward women and illicit androgenic-anabolic steroid use. Ann Clin Psychiatry. 1994;6(1):21–25. doi: 10.3109/10401239409148835. [DOI] [PubMed] [Google Scholar]
- 71.Perry PJ, Kutscher EC, Lund BC, Yates WR, Holman TL, Demers L. Measures of aggression and mood changes in male weightlifters with and without androgenic anabolic steroid use. J Forensic Sci. 2003;48(3):646–651. [PubMed] [Google Scholar]
- 72.Petersson A, Garle M, Granath F, Thiblin I. Morbidity and mortality in patients testing positively for the presence of anabolic androgenic steroids in connection with receiving medical care: a controlled retrospective cohort study. Drug Alcohol Depend. 2006;81(3):215–220. doi: 10.1016/j.drugalcdep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Petersson A, Garle M, Holmgren P, Druid H, Krantz P, Thiblin I. Toxicological findings and manner of death in autopsied users of anabolic androgenic steroids. Drug Alcohol Depend. 2006;81(3):241–249. doi: 10.1016/j.drugalcdep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Thiblin I, Kristiansson M, Rajs J. Anabolic androgenic steroids and behavioral patterns among violent offenders. J Forensic Sci. 1997;8(2):299–310. [Google Scholar]
- 75.Thiblin I, Parlklo T. Anbolic androgenic steroids and violence. Acta Psychiatrica Scand Suppl. 2002;106:125–128. doi: 10.1034/j.1600-0447.106.s412.27.x. [DOI] [PubMed] [Google Scholar]
- 76.Klotz F, Garle M, Granath F, Thiblin I. Criminality among individuals testing positive for the presence of anabolic-androgenic steroids. Arch Gen Psychiatry. 2006;63(11):1274–1279. doi: 10.1001/archpsyc.63.11.1274. [DOI] [PubMed] [Google Scholar]
- 77.Yates WR, Perry PJ, MacIndoe J, Holman T, Ellingrod V. Psychosexual effects of three doses of testosterone cycling in normal men. Biol Psychiatry. 1999;45(3):254–460. doi: 10.1016/s0006-3223(98)00028-6. [DOI] [PubMed] [Google Scholar]
- 78.Su TP, Pagliaro M, Schmidt PJ, Pickar D, Wolkewitz D, Rubinow DR. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;269(21):2760–2764. [PubMed] [Google Scholar]
- 79.Brower KJ. Anabolic steroid abuse and dependence. Curr Psychiatry Rep. 2002;4(5):377–387. doi: 10.1007/s11920-002-0086-6. [DOI] [PubMed] [Google Scholar]
- 80.Malone DA, Jr, Dimeff RJ, Lombardo JA, Sample BR. Psychiatric effects and psychoactive substance use in anabolic-androgenic steroid users. Clin J Sport Med. 1996;5(1):25–31. doi: 10.1097/00042752-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt PJ, Berlin KL, Danaceau MA, et al. The effects of pharmacologically induced hypogonadism on mood in healthy men. Arch Gen Psychiatry. 2004;61(10):997–1004. doi: 10.1001/archpsyc.61.10.997. [DOI] [PubMed] [Google Scholar]
- 82.Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG., Jr Anabolic-androgenic steroid dependence: an emerging disorder. Addiction. 2009;104(12):1966–1978. doi: 10.1111/j.1360-0443.2009.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kanayama G, Hudson JI, Pope HG., Jr Features of men with anabolic-androgenic steroid dependence: a comparison with nondependent AAS users and with AAS nonusers. Drug Alcohol Depend. 2009;102((1–3)):130–137. doi: 10.1016/j.drugalcdep.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG., Jr Treatment of anabolic-androgenic steroid dependence: emerging evidence and its implications. Drug Alcohol Depend. 2010;109((1–3)):6–13. doi: 10.1016/j.drugalcdep.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kanayama G, Barry S, Hudson JI, Pope HG., Jr Body image and attitudes toward male roles in anabolic-androgenic steroid users. Am J Psychiatry. 2006;163(4):697–703. doi: 10.1176/ajp.2006.163.4.697. [DOI] [PubMed] [Google Scholar]
- 86.Cole JC, Smith R, Halford JCG, Wagstaff GF. A preliminary investigation into the relationship between anabolic-androgenic steroid use and the symptoms of reverse anorexia in both current and ex-users. Psychopharmacology (Berl) 2003;166(4):424–429. doi: 10.1007/s00213-002-1352-3. [DOI] [PubMed] [Google Scholar]
- 87.Tan RS, Scally MC. Anabolic steroid-induced hypogonadism: towards a unified hypothesis of anabolic steroid action. Med Hypotheses. 2009;72(6):723–728. doi: 10.1016/j.mehy.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 88.Frye CA. Some rewarding effects of androgens may be mediated by actions of its 5α-reduced metabolite 3α-androstanediol. Pharmacol Biochem Behav. 2007;86(2):354–367. doi: 10.1016/j.pbb.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peters KD, Wood RI. Androgen dependence in hamsters: overdose, tolerance, and potential opioidergic mechanism. Neuroscience. 2004;130(4):971–981. doi: 10.1016/j.neuroscience.2004.09.063. [DOI] [PubMed] [Google Scholar]
- 90.Kanayama G, Pope HG, Cohane GH, Hudson JI. Risk factors for anabolic-androgenic steroid use among weightlifters: a case-control study. Drug Alcohol Depend. 2003;71(1):77–86. doi: 10.1016/s0376-8716(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 91.Arvary D, Pope HG., Jr Anabolic-androgenic steroids as a gateway to opioid dependence. New Engl J Med. 2000;342(20):1532. doi: 10.1056/NEJM200005183422018. [DOI] [PubMed] [Google Scholar]
- 92.Garevik N, Rane A. Dual use of anabolic-androgenic steroids and narcotics in Sweden. Drug Alcohol Depend. 2010;109((1–3)):144–146. doi: 10.1016/j.drugalcdep.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 93.Pope HG, Jr, Kanayama G, Hudson JI. Risk factors for illicit anabolic-androgenic steroid use in male weightlifters: a cross-sectional cohort study. Biol Psychiatry. 2012;71(3):254–261. doi: 10.1016/j.biopsych.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dhar R, Stout CW, Link MS, Homoud MK, Weinstock J, Estes NM., III Cardiovascular toxicities of performance-enhancing substances in sport. Mayo Clin Proc. 2005;80(10):1307–1315. doi: 10.4065/80.10.1307. [DOI] [PubMed] [Google Scholar]
- 95.Vanberg P, Atar D. Androgenic anabolic steroid abuse and the cardiovascular system. Handb Exp Pharmacol. 2010;195:411–457. doi: 10.1007/978-3-540-79088-4_18. [DOI] [PubMed] [Google Scholar]
- 96.D'Andrea A, Caso P, Salerno G, et al. Left ventricular early myocardial dysfunction after chronic misuse of anabolic androgenic steroids: a Doppler myocardial and strain imaging analysis. Br J Sports Med. 2007;41(3):149–155. doi: 10.1136/bjsm.2006.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hasson N, Salem M, Sayed M. Doping and effects of anabolic-androgenic steroids on the heart: histological, ultrastructural, and echocardiographic assessment in strength athletes. Hum Exp Toxicol. 2009;28(5):273–283. doi: 10.1177/0960327109104821. [DOI] [PubMed] [Google Scholar]
- 98.Melchert RB, Welder AA. Cardiovascular effects of androgenic-anabolic steroids. Med Sci Sports Exerc. 1995;27(9):1252–1262. [PubMed] [Google Scholar]
- 99.Kasikcioglu E, Oflaz H, Umman B, Burgra Z. Androgenic anabolic steroids also impair right ventricular function. Int J Cardiol. 2009;134(1):123–125. doi: 10.1016/j.ijcard.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 100.Nottin S, Nguyen LD, Terbah M, Obert P. Cardiovascular effects of androgenic-anabolic steroids in male bodybuilders determined by tissue Doppler imaging. Am J Cardiol. 2006;97(6):912–915. doi: 10.1016/j.amjcard.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 101.Baggish A, Weiner R, Kanayama G, et al. Long-term anabolic-androgenic steroid use is associated with left ventricular dysfunction. Circ Heart Fail. 2010;3(4):472–476. doi: 10.1161/CIRCHEARTFAILURE.109.931063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hartgens F, Rietjens G, Keizer HA, Kuipers HA, Wilffenbuttel BH. Effects of androgenic-anabolic steroids on apolipoproteins and lipoprotein (a) Br J Sports Med. 2004;38(3):253–259. doi: 10.1136/bjsm.2003.000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santora LJ, Marin J, Vangrow J, et al. Coronary calcification in body builders using anabolic steroids. Prev Cardiol. 2006;9(4):198–201. doi: 10.1111/j.1559-4564.2006.05210.x. [DOI] [PubMed] [Google Scholar]
- 104.Soe KL, Soe M, Gluud C. Liver pathology associated with the use of anabolic-androgenic steroids. Liver. 1992;12(2):73–79. doi: 10.1111/j.1600-0676.1992.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 105.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 106.Beiner JM, Jokl P, Cholewicki J, Panjabi MM. The effect of anabolic steroids and corticosteroids on healing of muscle contusion injury. Am J Sports Med. 1999;27(1):2–9. doi: 10.1177/03635465990270011101. [DOI] [PubMed] [Google Scholar]
- 107.Marshall-Gradisnik S, Green R, Brenu EW, Weatherby RP. Anabolic androgenic steroids effects on the immune system: a review. Cent Eur J Biol. 2009;4(1):19–33. [Google Scholar]
- 108.Walker J, Adams B. Cutaneous manifestations of anabolic-androgenic steroid use in athletes. Int J Dermatol. 2009;48(10):1044–1048. doi: 10.1111/j.1365-4632.2009.04139.x. [DOI] [PubMed] [Google Scholar]
- 109.Melnik B, Jansen T, Grabbe S. Abuse of anabolic-androgenic steroids and bodybuilding acne: an underestimated health problem. J Dtsch Dermatol Ges. 2007;5(2):110–117. doi: 10.1111/j.1610-0387.2007.06176.x. [DOI] [PubMed] [Google Scholar]
- 110.Daher EF, Silva GB, Jr, Queiroz AL, et al. Acute kidney injury due to anabolic steroid and vitamin supplement abuse: report of two cases and a literature review. Nephrology. 2009;41(3):717–723. doi: 10.1007/s11255-009-9571-8. [DOI] [PubMed] [Google Scholar]
- 111.Herlitz LC, Markowitz GS, Farris AB, et al. Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol. 2009;21(1):163–172. doi: 10.1681/ASN.2009040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barros TSP, Santos MBF, Shinozaki EB, Santos JFF, Marchini L. Effects of use of anabolic steroids on the masticatory system: a pilot study. J Oral Sci. 2008;51(1):19–24. doi: 10.2334/josnusd.50.19. [DOI] [PubMed] [Google Scholar]
- 113.Masteralexis LP. Drug testing provisions: an examination of disparities in rules and collective bargaining agreement provisions. New Engl Law Rev. 2006;40:775–788. [Google Scholar]
- 114.Goldberg L, Elliot DL, MacKinnon DP, et al. Outcomes of a prospective trial of student-athlete drug testing: the Student Athlete Testing Using Random Notification (SATURN) study. J Adolesc Health. 2007;41(5):421–429. doi: 10.1016/j.jadohealth.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 115.Bahr RT, Tjornhom M. Prevalence of doping in sports: doping control in Norway, 1977–1995. Clin J Sport Med. 1998;8(1):32–37. doi: 10.1097/00042752-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 116.Elliot DL, Goldberg L, Moe EL, Defrancesco CA, Durham MB, Hix-Small H. Preventing substance use and disordered eating: initial outcomes of the ATHENA (Athletes Targeting Healthy Exercise and Nutrition Alternatives) program. Arch Pediatr Adolesc Med. 2004;158(11):1043–1049. doi: 10.1001/archpedi.158.11.1043. [DOI] [PubMed] [Google Scholar]
- 117.Goldberg L, Elliot DL, Clarke GN, et al. Effects of a multidimensional anabolic steroid prevention intervention: the Adolescents Training and Learning to Avoid Steroids (ATLAS) program. JAMA. 1996;276(19):1555–1562. [PubMed] [Google Scholar]
- 118.Goldberg L, MacKinnon DP, Elliot DL, Moe EL, Clarke G, Cheong J. The Adolescents Training and Learning to Avoid Steroids program: preventing drug use and promoting healthy behaviors. Arch Pediatr Adolesc Med. 2000;154(4):332–338. doi: 10.1001/archpedi.154.4.332. [DOI] [PubMed] [Google Scholar]
- 119.Elliot DL, Moe EL, Goldberg L, DeFrancesco CA, Durham MB, Hix-Small H. Definition and outcome of a curriculum to prevent disordered eating and body-shaping drug use. J Sch Health. 2006;76(2):67–73. doi: 10.1111/j.1746-1561.2006.00070.x. [DOI] [PubMed] [Google Scholar]
- 120.Nilsson S, Allebeck P, Marklund B, Baigi A, Fridlund B. Evaluation of a health promotion programme to prevent the misuse of androgenic anabolic steroids among Swedish adolescents. Health Promot Int. 2004;19(1):61–67. doi: 10.1093/heapro/dah108. [DOI] [PubMed] [Google Scholar]
- 121.Backhouse S, McKenna J, Patterson L. Social Science Research Fund: Prevention through Education: A Review of Current International Social Science Literature. Leeds, UK: Leeds Metropolitan University, Carnegie Research Institute; 2009. [Google Scholar]
- 122.Elliot DL, Goldberg L, Moe EL, et al. Long-term outcomes of the ATHENA (Athletes Targeting Healthy Exercise & Nutrition Alternatives) program for female high school athletes. J Alcohol Drug Educ. 2008;52(2):73–92. [PMC free article] [PubMed] [Google Scholar]
- 123.Ranby KW, Aiken LS, MacKinnon DP, et al. A mediation analysis of the ATHENA intervention for female athletes: prevention of athletic-enhancing substance use and unehealthy weight loss behaviors. J Pediatr Psychol. 2009;34(10):1069–1083. doi: 10.1093/jpepsy/jsp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.MacKinnon DP, Goldberg L, Clarke GN, et al. Mediating mechanisms in a program to reduce intentions to use anabolic steroids and improve exercise self-efficacy and dietary behavior. Prev Sci. 2001;2(1):15–28. doi: 10.1023/a:1010082828000. [DOI] [PubMed] [Google Scholar]
- 125.Leone JE, Gray KA, Rossi JM. Using the transtheoretical model to explain androgenic-anabolic steroid use in adolescents and young adults, part one. Strength Cond J. 2008;30(6):47–54. [Google Scholar]
- 126.Leone JE, Gray KA, Rossi J, Colandreo RM. Using the transtheoretical model to explain androgenic-anabolic steroid use in adolescents and young adults, part two. Strength Cond J. 2009;31(1):13–22. [Google Scholar]
- 127.Graham MR, Davies B, Grace FM, Kicman A, Baker JS. Anabolic steroid use: patterns of use and detection of doping. Sports Med. 2008;38(6):505–525. doi: 10.2165/00007256-200838060-00005. [DOI] [PubMed] [Google Scholar]
- 128.Kanayama G, Cohane GH, Weiss RD, Pope HG., Jr Past anabolic-androgenic steroid use among men admitted for substance abuse treatment: an underrecognized problem? J Clin Psychiatry. 2003;64(2):156–160. doi: 10.4088/jcp.v64n0208. [DOI] [PubMed] [Google Scholar]
- 129.Kanayama G, Boynes M, Hudson JI, Field A, Pope HG., Jr Anabolic steroid abuse among teenage girls: an illusionary problem? Drug Alcohol Depend. 2007;88((2–3)):156–162. doi: 10.1016/j.drugalcdep.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fahey TD, Brown CH. The effects of an anabolic steroid on the strength, body composition, and endurance of college males when accompanied by a weight training program. Med Sci Sports. 1973;5(4):272–276. [PubMed] [Google Scholar]
- 131.Stromme S, Meen HD, Hakvaag A. Effects of an androgenic-anabolic steroid on strength development and plasma testosterone levels in normal males. Med Sci Sports Exerc. 1974;6(3):203–208. [PubMed] [Google Scholar]
- 132.Baume N, Schumacher YO, Sottas PE, et al. Effect of multiple oral doses of androgenic anabolic steroids on endurance performance and serum indices of physical stress in healthy male subjects. Eur J Appl Physiol. 2006;98(4):329–340. doi: 10.1007/s00421-006-0271-0. [DOI] [PubMed] [Google Scholar]
- 133.Parssinen M, Kujala U, Vartiainen E, Sarna S, Seppala T. Increased premature mortality of competitive powerlifters suspected to have used anabolic agents. Int J Sports Med. 2000;21(3):225–227. doi: 10.1055/s-2000-304. [DOI] [PubMed] [Google Scholar]
- 134.Thibliin I, Lindquist O, Rajs J. Cause and manner of death among users of anabolic androgenic steroids. J Forensic Sci. 2000;45(1):16–23. [PubMed] [Google Scholar]
- 135.Bahrke MS, Yesalis CE, III, Wright JE. Psychological and behavioural effects of endogenous testosterone and anabolic-androgenic steroids: an update. Am J Sports Med. 1996;22(6):367–390. doi: 10.2165/00007256-199622060-00005. [DOI] [PubMed] [Google Scholar]
- 136.Midgley SJ, Heather N, Davies JB. Levels of aggression among a group of anabolic-androgenic steroid users. Med Sci Law. 2001;41(4):309–314. doi: 10.1177/002580240104100407. [DOI] [PubMed] [Google Scholar]
- 137.Pagonis TA, Angelopoulos NV, Koukoulis GN, Hadjichristodoulou CS, Toli PN. Psychiatric and hostility factors related to use of anabolic steroids in monozygotic twins. Eur Psychiatry. 2006;21(8):563–569. doi: 10.1016/j.eurpsy.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 138.Pope HG, Jr, Katz DL. Homicide and near-homicide by anabolic steroid users. J Clin Psychiatry. 1990;51(1):28–31. [PubMed] [Google Scholar]
- 139.Thiblin I, Runeson B, Rajs J. Anabolic androgenic steroids and suicide. Ann Clin Psychiatry. 1999;11(4):223–231. doi: 10.1023/a:1022313529794. [DOI] [PubMed] [Google Scholar]
- 140.Melloni RH, Jr, Connor DF, Hang PTX, Harrison RJ, Ferris CF. Anabolic-androgenic steroid exposure during adolescence and aggressive behavior in golden hamsters. Physiol Behav. 1997;61(3):359–364. doi: 10.1016/s0031-9384(96)00373-3. [DOI] [PubMed] [Google Scholar]
- 141.Pope HG, Jr, Gruber AJ, Choi PY, Olivardia R, PHillips KA. Muscle dysmorphia: an underrecognized form of body dysmorphic disorder. Psychosomatics. 1997;38(6):548–557. doi: 10.1016/S0033-3182(97)71400-2. [DOI] [PubMed] [Google Scholar]
- 142.Wood RI. Anabolic steroids: a fatal attraction? J Neuroendocrinol. 2006;18(3):227–228. doi: 10.1111/j.1365-2826.2006.01407.x. [DOI] [PubMed] [Google Scholar]
- 143.Leone JE, Sedory EJ, Gray KA. Helping athletes: recognition and treatment of muscle dysmorphia and related body image disorders. J Athl Train. 2005;40(4):352–359. [PMC free article] [PubMed] [Google Scholar]
- 144.Brower KJ. Anabolic steroids abuse and dependence in clinical practice. Phys Sportsmed. 2009;37(4):130–140. doi: 10.3810/psm.2009.12.1751. [DOI] [PubMed] [Google Scholar]
- 145.Pope HG, Kean J, Nash A, et al. A diagnostic interview module for anabolic-androgenic steroid dependence: preliminary evidence of reliability and validity. Exp Clin Psychopharmacol. 2010;18(3):203–213. doi: 10.1037/a0019370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harmer PA. Anabolic-androgenic steroid use among young male and female athletes: is the game to blame? Br J Sports Med. 2010;44(1):26–31. doi: 10.1136/bjsm.2009.068924. [DOI] [PubMed] [Google Scholar]
- 147.Goldberg L, Bents R, Bosworth E, Trevisan L, Elliot DL. Anabolic steroid education and adolescents: do scare tactics work? Pediatrics. 1991;87(3):283–286. [PubMed] [Google Scholar]
- 148.Goldberg L, Bosworth EE, Bents RT, Trevisan L. Effect of an anabolic steroid education program on knowledge and attitudes of high school football players. J Adolesc Health Care. 1990;11(3):210–214. doi: 10.1016/0197-0070(90)90350-b. [DOI] [PubMed] [Google Scholar]
- 149.Board of Certification. Role Delineation Study: Practice Analysis. 6th ed. 2011 Omaha, NE: [Google Scholar]