Abstract
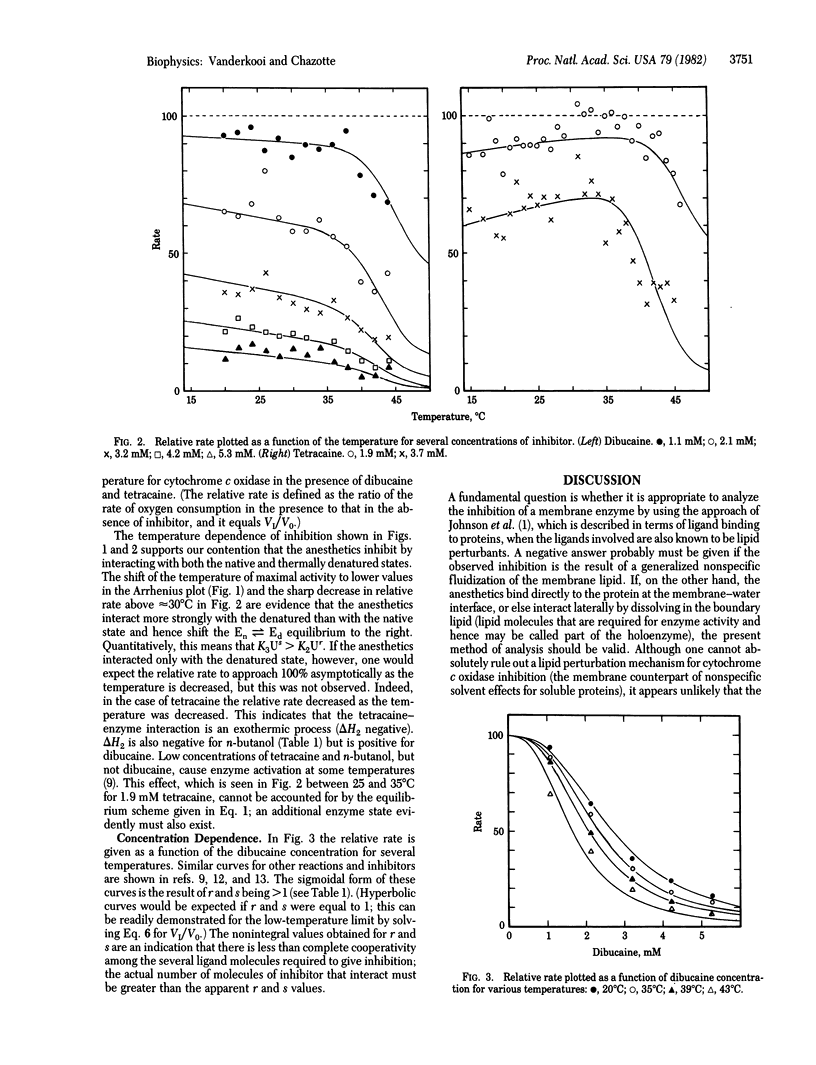
The thermodynamic parameters that characterize the inhibition of cytochrome c oxidase activity, in rat liver submitochondrial particles, by n-butanol, tetracaine, and dibucaine were obtained. Three equilibria were assumed in order to account for the data: for the interaction of inhibitor with the native state of the enzyme, for the interaction of inhibitor with the thermally (reversibly) denatured state, and for the change between the native and thermally denatured states. Inhibition results from interaction with both the native and denatured states but, because the interaction is stronger with the denatured than with the native state, the native/denatured equilibrium is shifted to the right by the anesthetics. The enthalpies of interaction are -2.3, -4.7, and 3.7 kcal/mol (1 cal = 4.18 J) for the native state and -10, -6, and -14 kcal/mol for the denatured state, for n-butanol, tetracaine, and dibucaine, respectively. These values are much smaller than the previous estimates obtained by using the assumption that anesthetics interact only with the thermally denatured state of enzymes (e.g., -81 kcal/mol for tetracaine inhibition of luciferase). Our results suggest that local anesthetics inhibit enzyme activity by causing a reversible perturbation of protein conformation. The magnitude of the perturbation is much smaller (in energetic terms) than that which accompanies thermal denaturation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chazotte B., Vanderkooi G. Multiple sites of inhibition of mitochondrial electron transport by local anesthetics. Biochim Biophys Acta. 1981 Jul;636(2):153–161. doi: 10.1016/0005-2728(81)90088-8. [DOI] [PubMed] [Google Scholar]
- Eyring H., Woodbury J. W., D'Arrigo J. S. A molecular mechanism of general anesthesia. Anesthesiology. 1973 May;38(5):415–424. doi: 10.1097/00000542-197305000-00001. [DOI] [PubMed] [Google Scholar]
- JOHNSON F. H., FLAGLER E. A. Hydrostatic pressure reversal of narcosis in tadpoles. Science. 1950 Jul 21;112(2899):91–92. doi: 10.1126/science.112.2899.91-a. [DOI] [PubMed] [Google Scholar]
- Lee M. P., Gear A. R. The effect of temperature on mitochondrial membrane-linked reactions. J Biol Chem. 1974 Dec 10;249(23):7541–7549. [PubMed] [Google Scholar]
- Lenaz G., Sechi A. M., Parenti-Castelli G., Landi L., Bertoli E. Activation energies of different mitochondrial enzymes: breaks in Arrhenius plots of membrane-bound enzymes occur at different temperatures. Biochem Biophys Res Commun. 1972 Oct 17;49(2):536–542. doi: 10.1016/0006-291x(72)90444-5. [DOI] [PubMed] [Google Scholar]
- Ueda I., Kamaya H., Eyring H. Molecular mechanism of inhibition of firefly luminescence by local anesthetics. Proc Natl Acad Sci U S A. 1976 Feb;73(2):481–485. doi: 10.1073/pnas.73.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda I., Kamaya H. Kinetic and thermodynamic aspects of the mechanism of general anesthesia in a model system of firefly luminescence in vitro. Anesthesiology. 1973 May;38(5):425–436. doi: 10.1097/00000542-197305000-00002. [DOI] [PubMed] [Google Scholar]
- Vanderkooi G., Chazotte B., Biethman R. Temperature dependence of anesthetic effects on succinate oxidase activity in uncoupled submitochondrial particles. FEBS Lett. 1978 Jun 1;90(1):21–23. doi: 10.1016/0014-5793(78)80289-0. [DOI] [PubMed] [Google Scholar]
- Vanderkooi G., Shaw J., Storms C., Vennerstrom R., Chignell D. On the mechanism of action of anesthetics. Direct inhibition of mitochondrial F1-ATPase by n-butanol and tetracaine. Biochim Biophys Acta. 1981 Mar 12;635(1):200–203. doi: 10.1016/0005-2728(81)90019-0. [DOI] [PubMed] [Google Scholar]


