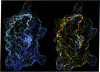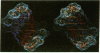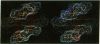Abstract
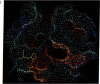
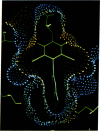
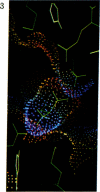
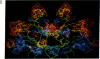
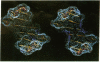
Color-coded computer graphics representations of the electrostatic potentials of trypsin, trypsin-inhibitor, prealbumin and its thyroxine complex, fragments of double-helical DNA, and a netropsin--DNA complex illustrate the electrostatic and topographic complementarity in macromolecule-ligand interactions. This approach is powerful in revealing intermolecular specificity and shows promise of having predictive value in drug design.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F., Ohlendorf D. H., Takeda Y., Matthews B. W. Structure of the cro repressor from bacteriophage lambda and its interaction with DNA. Nature. 1981 Apr 30;290(5809):754–758. doi: 10.1038/290754a0. [DOI] [PubMed] [Google Scholar]
- Andrea T. A., Cavalieri R. R., Goldfine I. D., Jorgensen E. C. Binding of thyroid hormones and analogues to the human plasma protein prealbumin. Biochemistry. 1980 Jan 8;19(1):55–63. doi: 10.1021/bi00542a009. [DOI] [PubMed] [Google Scholar]
- Berman H. M., Neidle S., Zimmer C., Thrum H. Netropsin, a DNA-binding oligopeptide structural and binding studies. Biochim Biophys Acta. 1979 Jan 26;561(1):124–131. doi: 10.1016/0005-2787(79)90496-9. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Oatley S. J. Protein-DNA and protein-hormone interactions in prealbumin: a model of the thyroid hormone nuclear receptor? Nature. 1977 Jul 14;268(5616):115–120. doi: 10.1038/268115a0. [DOI] [PubMed] [Google Scholar]
- Dean P. M. Drug-receptor recognition: electrostatic field lines at the receptor and dielectric effects. Br J Pharmacol. 1981 Sep;74(1):39–46. doi: 10.1111/j.1476-5381.1981.tb09953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Wakelin L. P. Electrostatic components of drug-receptor recognition. I. Structural and sequence analogues of DNA polynucleotides. Proc R Soc Lond B Biol Sci. 1980 Oct 15;209(1177):453–471. doi: 10.1098/rspb.1980.0107. [DOI] [PubMed] [Google Scholar]
- Dearing A., Weiner P., Kollman P. A. Molecular mechanical studies of proflavine and acridine orange intercalation. Nucleic Acids Res. 1981 Mar 25;9(6):1483–1497. doi: 10.1093/nar/9.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol. 1981 Jul 15;149(4):761–786. doi: 10.1016/0022-2836(81)90357-0. [DOI] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Side-chain torsional potentials: effect of dipeptide, protein, and solvent environment. Biochemistry. 1979 Apr 3;18(7):1256–1268. doi: 10.1021/bi00574a022. [DOI] [PubMed] [Google Scholar]
- Hayes D. M., Kollman P. A. Electrostatic potentials of proteins. 1. Carboxypeptidase A. J Am Chem Soc. 1976 May 26;98(11):3335–3345. doi: 10.1021/ja00427a048. [DOI] [PubMed] [Google Scholar]
- Hayes D. M., Kollman P. A. Electrostatic potentials of proteins. 2. Role of electrostatics in a possible catalytic mechanism for carboxypeptidase A. J Am Chem Soc. 1976 Nov 24;98(24):7811–7814. doi: 10.1021/ja00440a057. [DOI] [PubMed] [Google Scholar]
- Hayes D. M., Kollman P. A. Electrostatic potentials of proteins. 2. Role of electrostatics in a possible catalytic mechanism for carboxypeptidase A. J Am Chem Soc. 1976 Nov 24;98(24):7811–7814. doi: 10.1021/ja00440a057. [DOI] [PubMed] [Google Scholar]
- Helene C. Specific recognition of guanine bases in protein-nucleic acid complexes. FEBS Lett. 1977 Feb 15;74(1):10–13. doi: 10.1016/0014-5793(77)80740-0. [DOI] [PubMed] [Google Scholar]
- Hol W. G., van Duijnen P. T., Berendsen H. J. The alpha-helix dipole and the properties of proteins. Nature. 1978 Jun 8;273(5662):443–446. doi: 10.1038/273443a0. [DOI] [PubMed] [Google Scholar]
- Kollman P. A., Murray W. J., Nuss M. E., Jorgensen E. C., Rothenberg S. Molecular orbital studies of thyroid hormone analogs. J Am Chem Soc. 1973 Dec 26;95(26):8518–8525. doi: 10.1021/ja00807a004. [DOI] [PubMed] [Google Scholar]
- Kollman P. A., Weiner P. K., Dearing A. Studies of nucleotide conformations and interactions. The relative stabilities of double-helical B-DNA sequence isomers. Biopolymers. 1981 Dec;20(12):2583–2621. doi: 10.1002/bip.1981.360201208. [DOI] [PubMed] [Google Scholar]
- Langridge R., Ferrin T. E., Kuntz I. D., Connolly M. L. Real-time color graphics in studies of molecular interactions. Science. 1981 Feb 13;211(4483):661–666. doi: 10.1126/science.7455704. [DOI] [PubMed] [Google Scholar]
- Lavery R., Pullman B. The molecular electrostatic potential, steric accessibility and hydration of Dickerson's B-DNA dodecamer d(CpGpCpGpApApTpTpCpGpCpG). Nucleic Acids Res. 1981 Aug 11;9(15):3765–3777. doi: 10.1093/nar/9.15.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod K. N., Baxter J. D. DNA binding of thyroid hormone receptors. Biochem Biophys Res Commun. 1975 Feb 3;62(3):577–583. doi: 10.1016/0006-291x(75)90437-4. [DOI] [PubMed] [Google Scholar]
- Nuss M. E., Kollman P. A. Electrostatic potentials of deoxydinucleoside monophosphates. 1. Deoxydinucleoside monophosphates and actinomycin chromophore interactions. J Med Chem. 1979 Dec;22(12):1517–1524. doi: 10.1021/jm00198a016. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Electrostatic effects in proteins. Science. 1978 Sep 29;201(4362):1187–1191. doi: 10.1126/science.694508. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Jeffrey A., Johnson A. D., Maurer R., Meyer B. J., Pabo C. O., Roberts T. M., Sauer R. T. How the lambda repressor and cro work. Cell. 1980 Jan;19(1):1–11. doi: 10.1016/0092-8674(80)90383-9. [DOI] [PubMed] [Google Scholar]
- Rask L., Anundi H., Peterson P. A. The primary structure of the human retinol-binding protein. FEBS Lett. 1979 Aug 1;104(1):55–58. doi: 10.1016/0014-5793(79)81084-4. [DOI] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Seeman N. C., Rosenberg J. M., Rich A. Sequence-specific recognition of double helical nucleic acids by proteins. Proc Natl Acad Sci U S A. 1976 Mar;73(3):804–808. doi: 10.1073/pnas.73.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Nucleotide sequence specificity of restriction endonucleases. Science. 1979 Aug 3;205(4405):455–462. doi: 10.1126/science.377492. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Lazdunski M. Trypsin-pancreatic trypsin inhibitor association. Dynamics of the interaction and role of disulfide bridges. Biochemistry. 1972 Aug 1;11(16):2967–2977. doi: 10.1021/bi00766a007. [DOI] [PubMed] [Google Scholar]
- Wartell R. M., Larson J. E., Wells R. D. Netropsin. A specific probe for A-T regions of duplex deoxyribonucleic acid. J Biol Chem. 1974 Nov 10;249(21):6719–6731. [PubMed] [Google Scholar]
- Weinstein H., Maayani S., Srebrenik S., Cohen S., Sokolovsky M. A theoretical and experimental study of the semirigid cholinergic agonist 3-acetoxyquinuclidine. Mol Pharmacol. 1975 Sep;11(5):671–689. [PubMed] [Google Scholar]
- Zimmer C., Puschendorf B., Grunicke H., Chandra P., Venner H. Influence of netropsin and distamycin A on the secondary structure and template activity of DNA. Eur J Biochem. 1971 Jul 29;21(2):269–278. doi: 10.1111/j.1432-1033.1971.tb01466.x. [DOI] [PubMed] [Google Scholar]
- van Jaarsveld P. P., Edelhoch H., Goodman D. S., Robbins J. The interaction of human plasma retinol-binding protein and prealbumin. J Biol Chem. 1973 Jul 10;248(13):4698–4705. [PubMed] [Google Scholar]