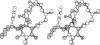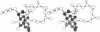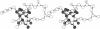Abstract
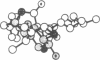
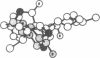
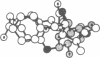
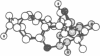
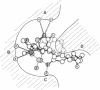
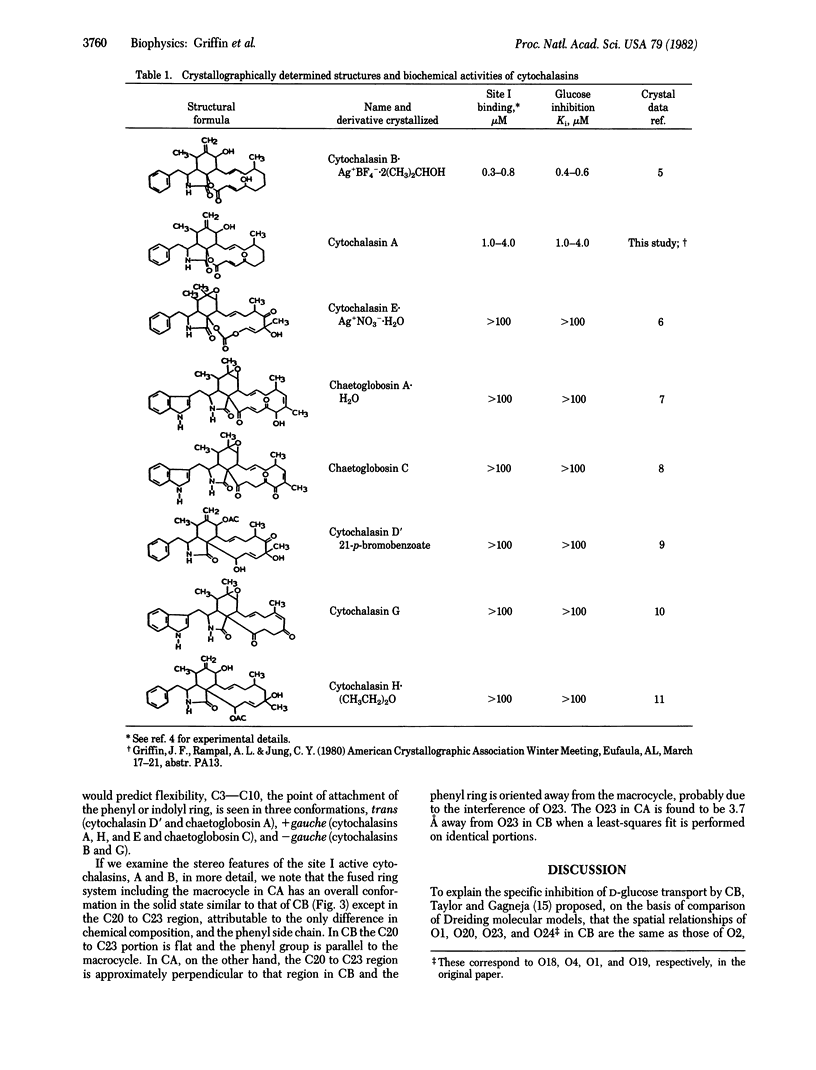
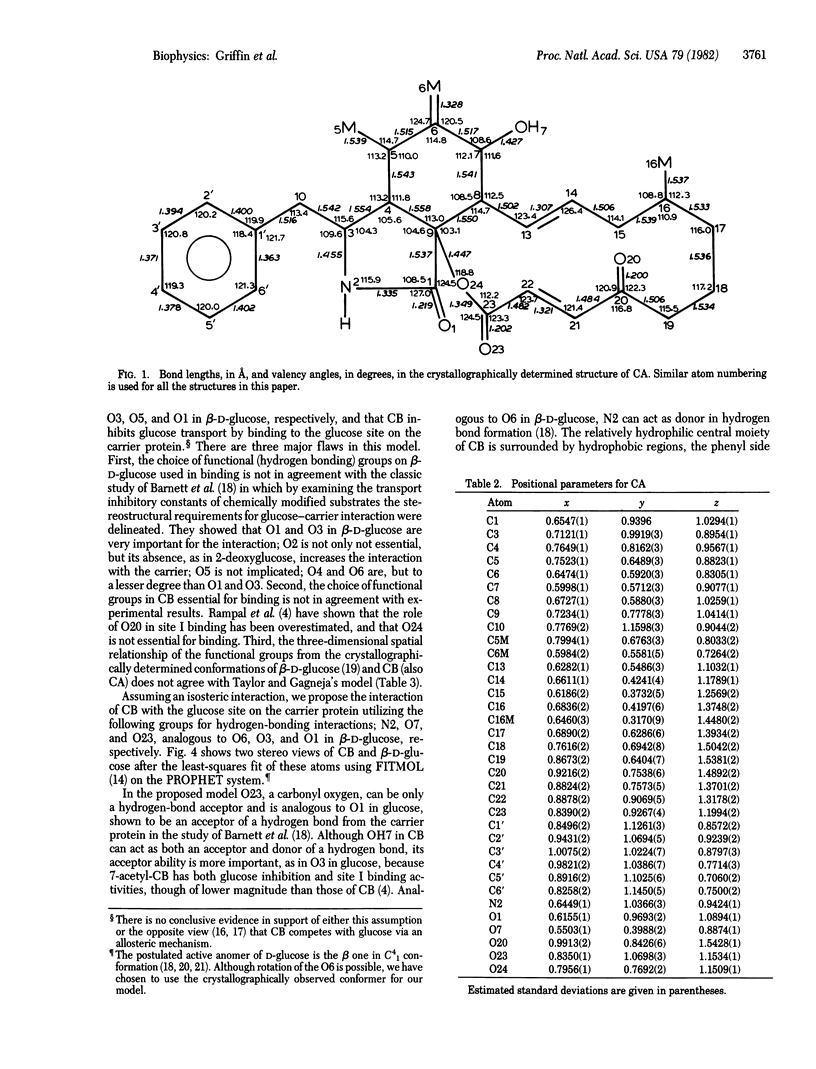
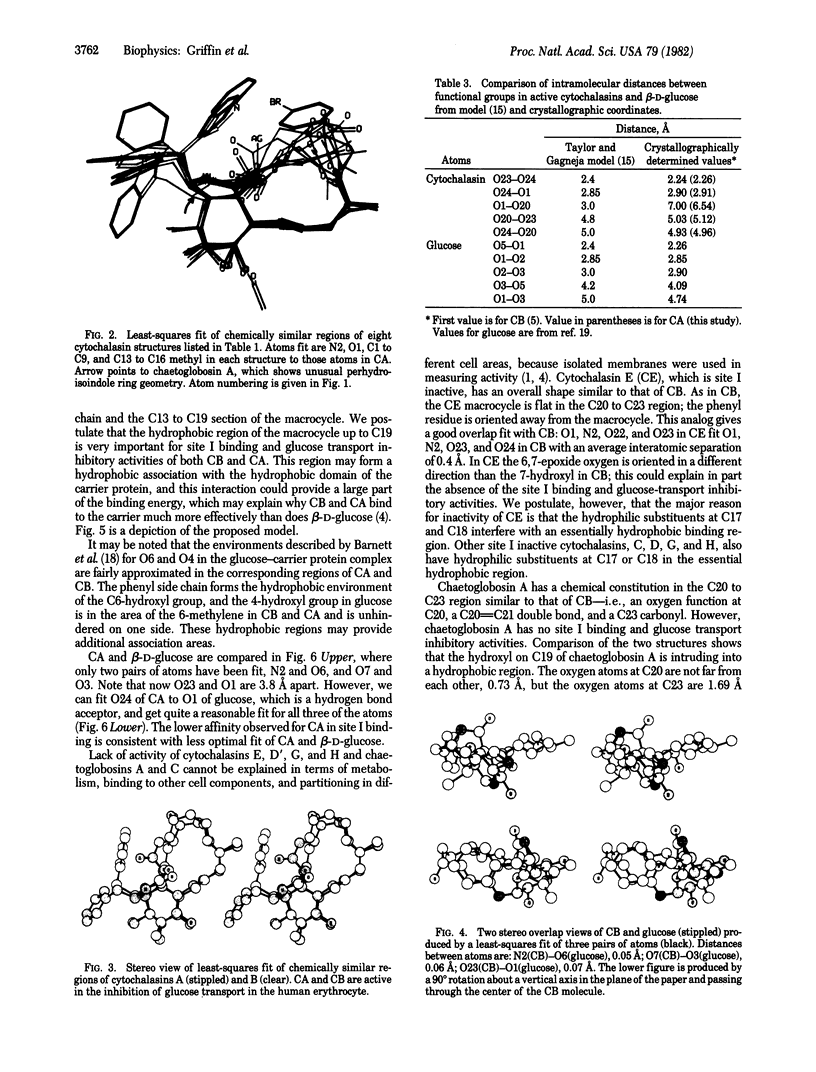
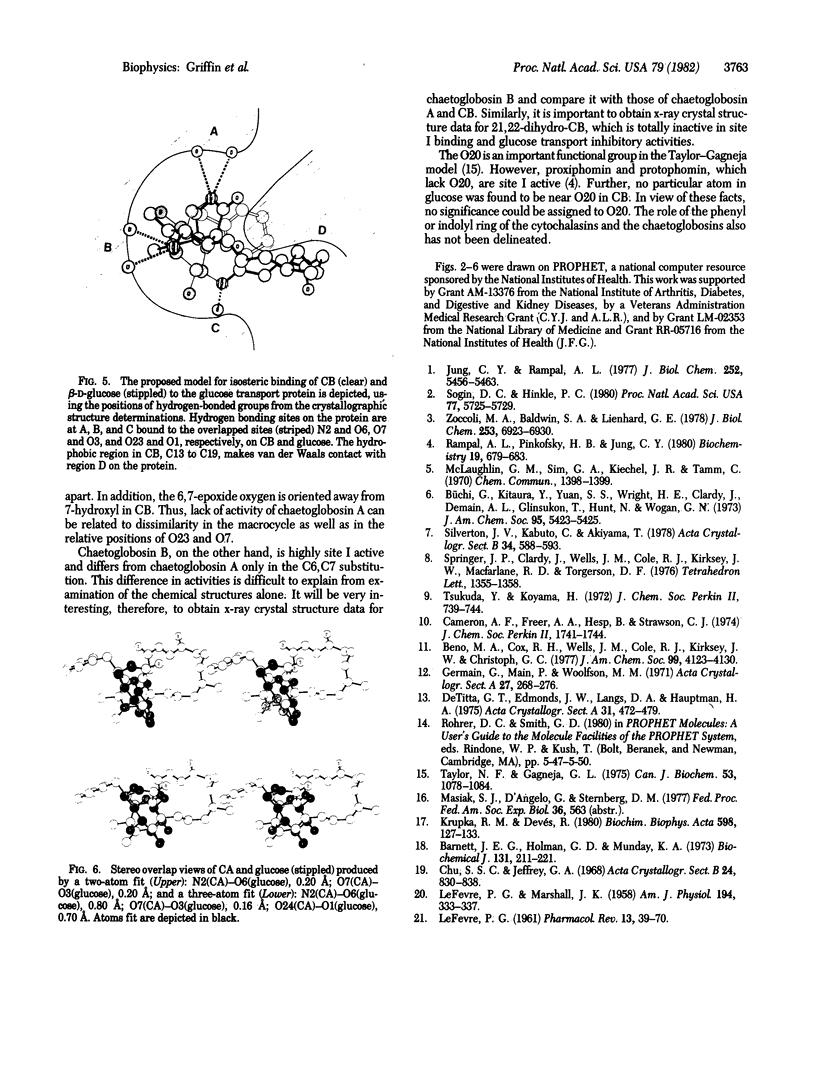
On the basis of details of the three-dimensional structures of beta-D-glucose and of cytochalasins, either previously published or reported here (cytochalasin A), we propose a model to explain the observed difference in activity of cytochalasins in the inhibition of glucose transport. In our model cytochalasin B binds to the glucose carrier through hydrogen bonds at N2 (donates), O7 (accepts), and O23 (accepts) analogous to O6, O3, and O1, respectively, on beta-D-glucose. The hydrophobic region from C13 to C19 is also essential in binding and appears to act as an anchor in a hydrophobic domain of the glucose carrier. The presence of hydrophilic groups in this essential hydrophobic region accounts, at least in part, for the inactivity of the other cytochalasins in the series.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. E., Holman G. D., Munday K. A. Structural requirements for binding to the sugar-transport system of the human erythrocyte. Biochem J. 1973 Feb;131(2):211–221. doi: 10.1042/bj1310211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beno M. A., Cox R. H., Wells J. M., Cole R. J., Kirksey J. W., Christoph G. G. Structure of a new [11]cytochalasin, cytochalasin H or kodo-cytochalasin-1. J Am Chem Soc. 1977 Jun 8;99(12):4123–4130. doi: 10.1021/ja00454a035. [DOI] [PubMed] [Google Scholar]
- Büchi G., Kitaura Y., Yuan S. S., Wright H. E., Clardy J., Demain A. L., Ginsukon T., Hunt N., Wogan G. N. Letter: structure of cytochalasin E, a toxic metabolite of Aspergillus clavatus. J Am Chem Soc. 1973 Aug 8;95(16):5423–5425. doi: 10.1021/ja00797a060. [DOI] [PubMed] [Google Scholar]
- Jung C. Y., Rampal A. L. Cytochalasin B binding sites and glucose transport carrier in human erythrocyte ghosts. J Biol Chem. 1977 Aug 10;252(15):5456–5463. [PubMed] [Google Scholar]
- Krupka R. M., Devés R. Evidence for allosteric inhibition sites in the glucose carrier of erythrocytes. Biochim Biophys Acta. 1980 May 8;598(1):127–133. doi: 10.1016/0005-2736(80)90270-9. [DOI] [PubMed] [Google Scholar]
- LEFEVRE P. G., MARSHALL J. K. Conformational specificity in a biological sugar transport system. Am J Physiol. 1958 Aug;194(2):333–337. doi: 10.1152/ajplegacy.1958.194.2.333. [DOI] [PubMed] [Google Scholar]
- LEFEVRE P. G. Sugar transport in the red blood cell: structure-activity relationships in substrates and antagonists. Pharmacol Rev. 1961 Mar;13:39–70. [PubMed] [Google Scholar]
- Rampal A. L., Pinkofsky H. B., Jung C. Y. Structure of cytochalasins and cytochalasin B binding sites in human erythrocyte membranes. Biochemistry. 1980 Feb 19;19(4):679–683. doi: 10.1021/bi00545a011. [DOI] [PubMed] [Google Scholar]
- Sogin D. C., Hinkle P. C. Immunological identification of the human erythrocyte glucose transporter. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5725–5729. doi: 10.1073/pnas.77.10.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. F., Gagneja G. L. A model for the mode of action of cytochalasin B inhibition of D-glucose transport in the human erythrocyte. Can J Biochem. 1975 Oct;53(10):1078–1084. doi: 10.1139/o75-148. [DOI] [PubMed] [Google Scholar]
- Zoccoli M. A., Baldwin S. A., Lienhard G. E. The monosaccharide transport system of the human erythrocyte. Solubilization and characterization on the basis of cytochalasin B binding. J Biol Chem. 1978 Oct 10;253(19):6923–6930. [PubMed] [Google Scholar]