Abstract
Immune system factors including complement pathway activation are increasingly linked to the etiology and pathophysiology of schizophrenia. Complement protein, C1q, binds to and helps to clear immune complexes composed of immunoglobulins coupled to antigens. The antigenic stimuli for C1q activation in schizophrenia are not known. Food sensitivities characterized by elevated IgG antibodies to bovine milk caseins and wheat glutens have been reported in individuals with schizophrenia. Here, we examined the extent to which these food products might comprise the antigen component of complement C1q immune complexes in individuals with recent onset schizophrenia (n=38), non-recent onset schizophrenia (n=61) and non-psychiatric controls (n=63). C1q seropositivity was significantly associated with both schizophrenia groups (recent onset, odds ratio (OR)=8.02, p≤0.008; non-recent onset, OR=3.15, p≤0.03) compared to controls (logistic regression models corrected for age, sex, race and smoking status). Casein- and/or gluten-IgG binding to C1q was significantly elevated in the non-recent onset group compared to controls (OR=4.36, p≤0.01). Significant amounts of C1q-casein/gluten-related immune complexes and C1q correlations with a marker for gastrointestinal inflammation in non-recent onset schizophrenia suggests a heightened rate of food antigens in the systemic circulation, perhaps via a disease-associated altered intestinal permeability. In individuals who are in the early stages of disease onset, C1q activation may reflect the formation of immune complexes with non-casein- or non-gluten-related antigens, the presence of C1q autoantibodies, and/or a dissociated state of immune complex components. In conclusion, complement activation may be a useful biomarker to diagnose schizophrenia early during the course of the disease. Future prospective studies should evaluate the impacts of casein- and gluten-free diets on C1q activation in schizophrenia.
Keywords: Neuroinflammation, schizoaffective disorder, bipolar disorder, psychosis, food sensitivity, gliadin, diet
Introduction
Immune system factors are becoming increasingly linked to the etiology and pathophysiology of complex neurodevelopmental brain diseases such as schizophrenia and autism (Abazyan et al., 2010; Brown, 2011; Brown and Patterson, 2011; Dohan, 1981; Onore et al., 2011; Reichelt and Landmark, 1995; Rossignol and Frye, 2011; Shi et al., 2009; Stefansson et al., 2009; Yolken and Torrey, 2008). We have reported strong associations between schizophrenia and a variety of infectious disease and food-derived antigens (Dickerson et al., 2010a; Dickerson et al., 2010b; Leweke et al., 2004; Niebuhr et al., 2008; Severance et al., 2012; Severance et al., 2010a; Severance et al., 2011a; Yolken and Torrey, 2008). If antigenic exposures play a part in the pathogenesis of schizophrenia, we might expect that immune components involved in early stages of antigen clearance are also affected.
The classic complement pathway immune mediator, C1q, is of particular relevance in this regard and to studies of brain disorders, because it functions both in antigen elimination and in synapse development (Boulanger, 2009; Stevens et al., 2007; Walport, 2001a; Walport, 2001b). C1q is best known for its role in binding to and facilitating the clearing of antigen-antibody immune complexes from systemic circulation (Walport, 2001a; Walport, 2001b). In recent years, however, C1q has been identified as a cellular component involved in synaptic pruning during perinatal central nervous system development (Boulanger, 2009; Stevens et al., 2007). Mouse models indicate a high level of early postnatal C1q expression in the brain, as well as a possible neural C1q reactivation in adults in response to cellular signals of neurodegeneration (Boulanger, 2009; Stevens et al., 2007).
Complement pathway activation may occur at increased rates in schizophrenia, and elevated levels of circulating immune complexes containing C1q have been found in individuals with schizophrenia compared to controls (Arakelyan et al., 2011; Boyajyan et al., 2008; Mailian et al., 2005; Mayilyan et al., 2008; Vetlugina et al., 1984). Complement factor C3 proteins were also found to be a part of these complexes, but the antigenic protein initiating the immune response was not identified (Arakelyan et al., 2011). Food sensitivities to bovine milk caseins and wheat glutens, as evident by antibody-based measures, have been reported in a number of studies of schizophrenia over the years (Cascella et al., 2011; Dickerson et al., 2010b; Dohan, 1981; Dohan et al., 1972; Karlsson et al., 2012; Niebuhr et al., 2011; Reichelt and Landmark, 1995; Samaroo et al., 2010; Severance et al., 2010a), an indication that these foods might be possible antigenic candidates for C1q binding. Indeed, we have previously reported that immune complexes containing casein are present in blood samples from people with psychiatric disorders (Severance et al., 2011b).
Here, we sought to examine if schizophrenia-associated C1q activation might co-occur with the formation of immune complexes that contain casein and gluten. We aimed to (1) quantify total IgG antibody binding to C1q, (2) define the subset of C1q immunoreactivity that can be attributed to the formation of immune complexes with food antigens, and (3) determine if either process occurs at altered rates in schizophrenia compared to controls. If food antigen exposure can be linked to a versatile, synaptically-active, basic immune protein such as C1q, future studies will be implemented to explore if caseins and glutens might impact neurodevelopment through a C1q-mediated pathway.
Materials and methods
Study participants
Sixty-one individuals with non-recent onset schizophrenia, 38 individuals with a recent onset of schizophrenia and 63 individuals who had no history of psychiatric disorders were recruited from the Sheppard Pratt Health System, Baltimore, MD, U.S.A. Identification and characterization of individuals into diagnostic groups was performed according to criteria defined by DSM-IV for schizophrenia, schizophreniform disorder and schizoaffective disorder, as previously described (Dickerson et al., 2010b; Severance et al., 2010a; Severance et al., 2011a). In these previous studies, the recent onset schizophrenia group was part of a larger designation of recent onset psychosis, but in the present paper, only those with these DSM-IV schizophrenia-related diagnoses were included (Dickerson et al., 2010b; Severance et al., 2010a; Severance et al., 2011a). The two schizophrenia groups (recent onset and non-recent onset) were distinguished from each other by the inclusion criteria that the recent onset group have an onset of psychotic symptoms for the first time within the past 24 months. Individuals without a history of psychiatric disorder were recruited from posted announcements and were screened to rule out current or past psychiatric disorders with the Structured Clinical Interview for DSM-IV Axis I Disorders (First, 1998). Basic demographic data and mean durations of illness of the study populations are shown in Table 1. Diagnostic groups differed significantly in age, sex, and smoking status as determined by t-tests and chi-square tests. These variables were included in the multivariate analyses described below.
Table 1.
Demographic information of the study population
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
| n | Age Mean years +SEMa | Duration of illness Mean years + SEM | Males n (%) | Females n (%) | African American n (%) | Caucasian/ Other n (%) | Smoker n (%) | |
| Controls | 63 | 31.76+1.40 | - | 15 (23.8) | 48 (76.2) | 24 (38.1) | 39 (61.9) | 17 (27) |
| Recent onset schizophrenia | 38 | 22.51+1.04b | 0.74+0.11 | 30 (78.9) | 8c (21.1) | 14 (36.8) | 24 (63.2) | 15 (39.5) |
| Non-recent onset schizophrenia | 61 | 42.25+1.44d | 20.84+1.50 | 31 (50.8) | 30e (49.2) | 31 (50.8) | 30 (49.2) | 39f (63.9) |
SEM refers to standard error of the mean
t=4.68, p≤0.00001
chi2=29.17, p≤0.0001
t=−5.21, p≤0.00001
chi2=9.69, p≤0.002
chi2=17.09, p≤0.0001
Blood samples were obtained by venipuncture, and serum separated and assessed for antibodies in the assays described below.
The studies were approved by the Institutional Review Boards (IRB) of the Sheppard Pratt Health System and the Johns Hopkins Medical Institution according to established guidelines. All participants provided written informed consent after study procedures were explained. The work described was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Laboratory procedures
Measurement of IgG antibody to C1q
Specific IgG antibodies were measured by ELISAs as previously described with some modification (Severance et al., 2012; Severance et al., 2010a). Human complement factor C1q was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). In brief, microplate wells were incubated with 100ng C1q protein in 50μl carbonate buffer overnight at 4°C, and microplates were blocked for 1 h at 37°C with 1% (wt/vol) human serum albumin (HSA; Sigma-Aldrich, St. Louis, MO, U.S.A.) diluted in Phosphate Buffered Saline (PBS). Microplates were then incubated with serum samples diluted 1:200 in PBS-Tween 20 (PBST) for 2h at 37°C. Microplates were washed and incubated with peroxidase-conjugated goat-anti-human IgG secondary antibodies for 30min at 37°C (Southern Biotech, Birmingham, AL, U.S.A.). 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate) hydrogen peroxide solution (KPL Protein Research Products, Gaithersburg, MD, U.S.A.) was added for color development, and absorbance was measured at 405 nm, with a reference wavelength of 490 nm, in an automated microplate reader (Molecular Devices, Menlo Park, CA, U.S.A.).
Detection of immune complexes
For immune complex detection immunoassays, 100ng of C1q protein in 50ul carbonate buffer was bound to microplates overnight, blocked with 1% HSA and incubated with serum samples as described above. Rabbit polyclonal antibodies directed at casein or gliadin (a major protein component of gluten) were diluted 1:5000 in PBST and were incubated on the microplates at 37°C for 1 hour. These polyclonal antibodies specific to different subunits of the casein protein molecule were produced as previously described (Senocq et al., 2001), and the rabbit anti-gliadin antibody was purchased from Sigma-Aldrich. Microplates were washed, incubated at 37°C for 30min with goat-anti rabbit peroxidase-conjugated IgG secondary antibodies, washed again and then developed as described above.
Statistical analyses
Overview
For each microplate, absorbance levels were mean-normalized to the value of that generated by control individuals in order to correct for plate-to-plate variation, as previously described (Severance et al., 2010a). Multivariate regression models were used to evaluate associations of quantitative antibody levels and seropositivity with diagnosis and to generate disease-specific odds ratios. All multivariate models incorporated the covariates of age, sex, race and smoking status. A p-value of 0.05 or below was considered statistically significant. Statistical analyses were performed with STATA version 12 (STATA Corp LP, College Station, Texas, U.S.A.).
Quantitative C1q antibody levels
In multiple linear regression models, we examined associations of C1q IgG antibody levels as the dependent variable with the independent variables, diagnosis, age, sex, race, smoking status. We also performed multiple linear regressions to detect correlations of C1q IgG antibody levels with previously generated antibody level data for a variety of antigens including casein, gliadin, anti-Saccharomyces cerevisiae (ASCA, a marker of gastrointestinal inflammation), Epstein Barr virus, influenza A virus, influenza B virus, measles and Toxoplasma gondii (Severance et al., 2012). To further quantify relative differences in quantitative antibody levels between diagnostic groups and between sex, race and smoking status subgroups, ANOVAs followed by post-hoc Bonferonni and t-tests were used. We also estimated the effect sizes for each schizophrenia group compared to controls using Cohens d definition and corresponding interpretation: small, d=0.2, medium d=0.5, large, d=0.8 (Cohen, 1988).
C1q antibody and C1q-immune complex seropositivity
C1q and C1q-food antigen seropositivity limits were established based on a cut-off value equivalent to 90% of the control group antibody levels, as previously described (Severance et al., 2010a). An individual was designated seropositive for an immune complex if she or he was then positive to any one of the casein or gluten polyclonal antibodies tested using the above C1q-food antigen seropositivity definition. Multinomial logistic regression models were used to generate odds ratios for disease association based on these seropositivity designations. In these logistic regressions, diagnostic group served as the dependent variable with C1q seropositivity status as the independent variable (along with age, sex, race and smoking status as covariates).
Results
Quantitative C1q IgG levels
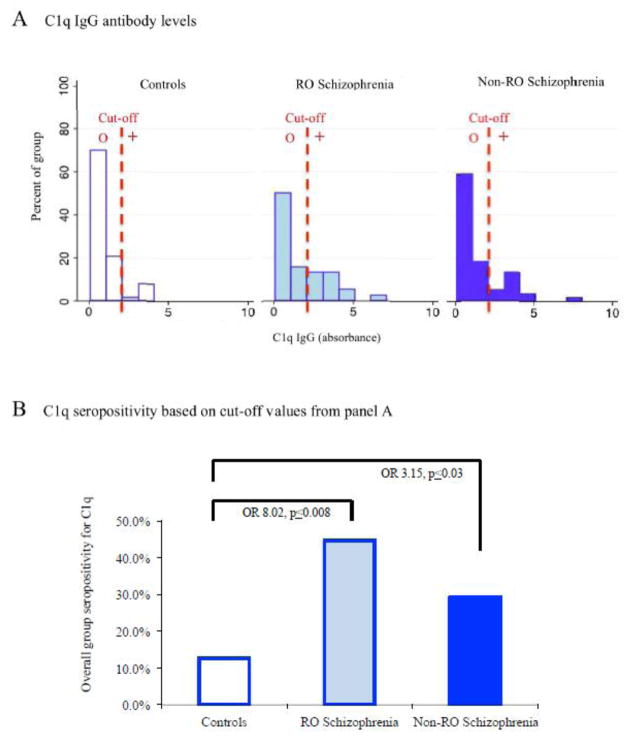
The immunoassay used to measure all C1q-related IgG is diagrammed in Figure 1 panel A. As shown, this assay detects C1q recognition of IgG-bound antigens in immune complexes as well as C1q autoantibodies. Quantitative levels of total C1q IgG are shown in histogram form in Figure 2 panel A. Multiple linear regressions containing the covariates age, sex, race and smoking status yielded significant associations of C1q antibody levels with diagnosis but not with any of the other covariates (coefficient=0.17, CI=0.06–0.27, p≤0.002). C1q antibody levels were highest in individuals with recent-onset schizophrenia and moderately elevated in non-recent onset schizophrenia compared to controls (mean + SEM: recent onset 1.81±0.26, non-recent onset 1.41±0.19, controls 1.00±0.12; ANOVA, F=4.50, p≤0.01; Bonferroni post hoc recent onset vs controls p≤0.01; one-tailed t-test non-recent onset vs controls (t=−1.84, p≤0.03). Effect size calculations yielded large effect sizes for both the recent onset group (Cohen’s d =4.06, r=0.90) and the non-recent onset group (Cohen’s d =2.58, r=0.80).
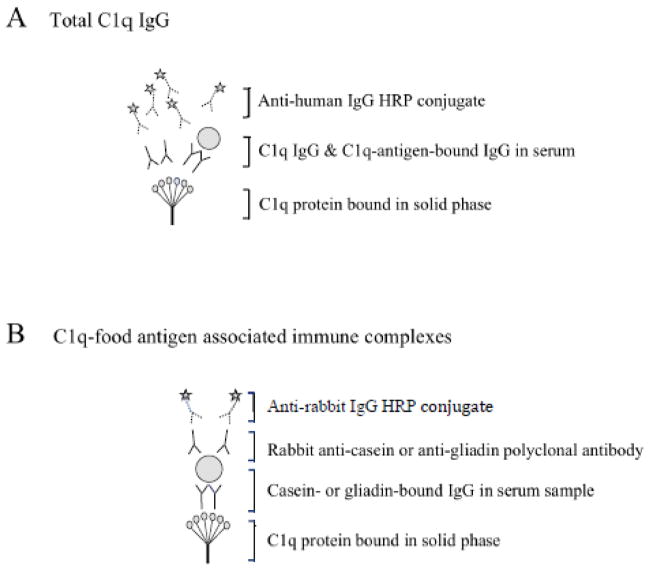
Figure 1.
Immunoassays to measure total C1q IgG levels and C1q-food antigen associated immune complexes.
Panel A: The immunoassay employed to measure total C1q IgG will detect C1q-associated immune complexes and autoantibodies generated against the C1q protein. HRP refers to the horseradish peroxidase conjugate of the secondary antibody. Panel B: The immunoassay developed here will detect casein- and gliadin-bound IgG contained in C1q-associated immune complexes.
Figure 2.
Disease association of C1q antibody levels and C1q seropositivity.
Panel A: C1q IgG antibody levels are displayed in histogram form. RO refers to recent onset. Cut-off refers to the absorbance value corresponding to 90% in the control population. “o” refers to values considered to be seronegative and “+” refers to values that are considered to be seropositive. Panel B: The percentage of individuals who were seropositive for total C1q immunoreactivity is diagrammed here. The p-value listed refers to the level of statistical significance following multinomial logistic regressions corrected for age, sex, race and smoking status. Diagnosis is the dependent variable and C1q seropositivity is the independent variable in these regressions. OR refers to odds ratio.
C1q seropositivity
C1q IgG seropositivity was calculated based on 90% C1q IgG levels found in control individuals. C1q seropositivity according to diagnosis is shown in Figure 2 panel B. Case-control differences in seropositivity generated significant age-, sex-, race- and smoking status-corrected odds ratios of 8.02 for recent onset schizophrenia (CI=1.71–37.57, p≤0.008) and 3.15 for non-recent onset schizophrenia (CI=1.09–9.13, p≤0.03). There were no significant associations of C1q IgG seropositivity with age, sex, race or smoking status.
C1q immune complexes containing food antigens
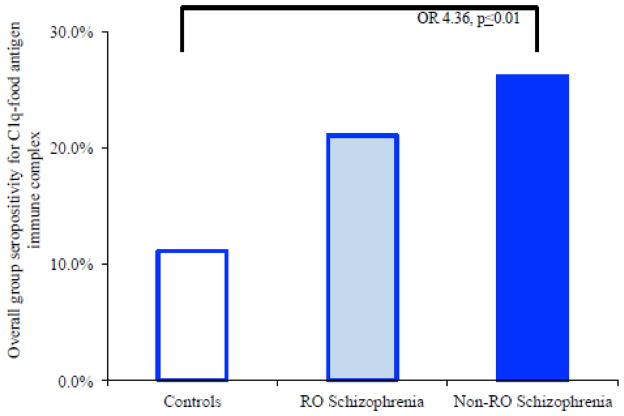
We employed the assay diagrammed In Figure 1 panel B to determine if the food antigens, milk casein or wheat gluten, might contribute to the formation of C1q-associated immune complexes. In multinomial logistic regressions, age-, sex-, race- and smoking status-corrected odds ratios revealed statistically significant associations of immune complex seropositivity with the non-recent onset group compared to controls (OR=4.36, CI=1.38–13.73, p≤0.01; Figure 3). Odds ratios generated separately for casein-containing immune complexes and for gluten-containing immune complexes were also statistically significant for the non-recent onset group compared to controls; these data are shown in Table 2. Immune complex seropositivity was not significantly associated with the recent onset group in these multivariate regression models.
Figure 3.
Prevalence of C1q-associated casein- and gliadin-IgG immune complexes per diagnosis and odds ratio association for disease.
The percentage of individuals who were seropositive for immune complexes containing these food antigens is diagrammed here. RO refers to recent onset. OR refers to odds ratio. The p-value listed refers to the level of statistical significance following multinomial logistic regressions corrected for age, sex, race and smoking status.
Table 2.
Disease-association of C1q immune complexes that contain a food antigen component in non-recent onset schizophrenia
| Food antigen component of C1q immune complex | Odds ratio (OR) | Confidence Interval (CI) | p-value |
|---|---|---|---|
| Casein | 3.30 | 1.00–11.00 | 0.05 |
| Gluten | 4.00 | 1.18–13.57 | 0.03 |
| Either casein or gluten | 4.36 | 1.38–13.73 | 0.01 |
Bolded values were statistically significant.
In the non-recent onset group, multinomial logistic regressions showed a significant association of C1q-food antigen seropositivity with age (OR=1.15, CI=1.06–1.26, p≤0.001), but not sex, race or smoking status. We further investigated this association and found that individuals in the non-recent onset group who were seropositive for immune complexes were younger (mean years±SEM 33.20±2.68 vs 45.21±1.48; t=3.99, two-tailed p≤0.0002) and had a shorter duration of illness (mean years±SEM 14.00±2.73 vs 23.08±1.66, t=2.75, two-tailed p≤0.008) than those who were seronegative. Similarly in the recent onset group, individuals who were seropositive for C1q-food antigen immune complexes were also significantly younger than those who were seronegative (19.17±1.46 vs 23.40±1.21, t=1.7, one-tailed p≤0.05). Conversely, in the control group, seropositive individuals were significantly older than those who were seronegative (38.42±5.42 vs 30.93±1.41, t=−1.7, one-tailed p≤0.05).
Association of C1q IgG antibodies with other antigens
C1q IgG antibody levels were significantly associated with casein IgG, gliadin IgG, and ASCA (a marker of GI inflammation) in multiple linear regressions including age, sex, race and smoking status covariates. These data are listed in Table 3. C1q IgG levels were positively correlated with casein and gluten IgG in both controls and the non-recent onset schizophrenia groups. C1q IgG was further significantly correlated with gliadin IgG in the recent onset group. The ASCA measure of GI inflammation was only correlated with C1q in the non-recent onset schizophrenia group. None of the other antigens tested (influenza A virus, influenza B virus, measles, or T. gondii) were significantly associated with C1q IgG, although the Epstein-Barr virus showed a significant negative correlation with C1q IgG.
Table 3.
Correlation of C1q IgG antibody levels with IgG from other antigens
| Antigen | Controls | Recent onset schizophrenia | Non-recent onset schizophrenia | |
|---|---|---|---|---|
| Casein | R2 | 0.31 | 0.26 | 0.37 |
| Coefficient CIa | 0.16–0.46 | 0.12–0.70 | 0.25–0.67 | |
| p-value | 0.0007 | 0.08 | 0.0001 | |
| Gluten | R2 | 0.41 | 0.42 | 0.44 |
| Coefficient CI | 0.30–0.64 | 0.24–0.64 | 0.44–0.98 | |
| p-value | 0.00001 | 0.003 | 0.00001 | |
| ASCA (GI inflammation) | R2 | 0.11 | 0.15 | 0.27 |
| Coefficient CI | −0.06–0.26 | −0.02–0.28 | 0.09–0.41 | |
| p-value | 0.22 | 0.37 | 0.003 | |
| Epstein-Barr virus | R2 | 0.15 | 0.08 | 0.22b |
| Coefficient CI | 0.002–0.87 | −1.33–0.59 | −1.59–−0.08 | |
| p-value | 0.09 | 0.72 | 0.02 | |
| Influenzavirus A | R2 | 0.09 | 0.07 | 0.16c |
| Coefficient CI | −0.58–0.58 | −2.77–1.77 | −1.47–0.56 | |
| p-value | 0.36 | 0.79 | 0.09 | |
| Influenzavirus B | R2 | 0.09 | 0.12 | 0.15d |
| Coefficient CI | −0.69–0.47 | −3.63–0.60 | −1.36–1.07 | |
| p-value | 0.34 | 0.50 | 0.12 | |
| Measles | R2 | 0.11 | 0.06e | 0.14f |
| Coefficient CI | −0.19–0.59 | −0.46–0.26 | −0.43–0.16 | |
| p-value | 0.26 | 0.85 | 0.14 | |
| Toxoplasma gondii | R2 | 0.10 | 0.07 | 0.15 |
| Coefficient CI | −0.26–0.12 | −0.24–0.43 | −0.33–0.36 | |
| p-value | 0.30 | 0.77 | 0.11 |
CI refers to confidence interval
Data were available for n=59
Data were available for n=60
Data were available for n=60
Data were available for n=37
Data were available for n=59
Bolded values were statistically significant.
Discussion
This study uncovered two major findings. First, we found significantly elevated levels of complement C1q activation and large effect sizes of C1q antibody levels in individuals with schizophrenia compared to controls. This C1q activation was particularly notable in the recent onset group where the odds ratio of C1q seropositivity for disease association was 8.02 and the effect size was 0.90. The corresponding odds ratio for disease association in the non-recent onset group was also significant at 3.15, with an effect size of 0.80. Secondly, we established that milk casein and wheat gluten comprise the antigen component of C1q-based immune complexes in a prominent portion of individuals with non-recent onset schizophrenia. In this chronically affected group, the presence of an immune complex containing C1q and a casein or gluten food antigen yielded a significant odds ratio for disease association of 4.36.
The recent onset group had a particularly high level of C1q activation, but this heightened C1q was not detected in a bound state to food antigen IgG, a finding that has several possible explanations. The measured C1q immunoreactivity could reflect C1q binding to immunoglobulins coupled to other types of bound antigens, such as antigens from microbial agents. C1q immunoreactivity, however, was not strongly correlated with IgG from a series of infectious disease antigens, but was correlated to IgG from food antigens. Therefore, even though the recent onset group did not show significant numbers of intact food antigen-related immune complexes, the relevant constituents, C1q and food antigen IgG, were nevertheless present and correlated. It may be that immune complexes were not yet formed in this early stage disease group, or perhaps other conditions led to the dissociation of the components.
Early clinical studies of food antigen-related immune complexes underscore the temporal dynamics of immune complex component interactions (Cunningham-Rundles et al., 1978; Cunningham-Rundles et al., 1979; Cunningham-Rundles and Good, 1979; Paganelli et al., 1983). Following milk ingestion, peak levels of unbound circulating bovine casein corresponded to a period when immune complex concentrations were at a minimum, an indication that antigen excess might be involved with complex dissociation (Cunningham-Rundles et al., 1979; Cunningham-Rundles and Good, 1979). One of the first studies published to examine the role of immune complex formation in schizophrenia reported that levels of immune complexes in patients tended to correlate with disease duration (Vetlugina et al., 1984). This result is consistent with our discovery of food antigen-based immune complexes predominately in the longer-term schizophrenia group as compared to those with a recent onset of disease. However, within each of the schizophrenia groups, we found that immune complexes were more often present in individuals who were younger compared to older seronegative counterparts. These data suggest that food sensitivities may more likely affect younger subsets of patients, even for those who have been afflicted with the disease for longer periods of time (i.e. recent onset vs non-recent onset).
Another explanation for the heightened C1q immunoreactivity in the recent onset group could be the presence of C1q autoantibodies produced early during disease pathogenesis. The assay used to determine total C1q activation would also detect antibodies generated against the C1q protein. Autoimmune diseases are known risk factors for the development of schizophrenia (Benros et al., 2011), and a number of studies have documented the increased presence of antibodies to a variety of cellular proteins such as the α7 nicotinic receptor, smooth muscle actin, cardiolipin, serotonin, and transglutaminase-6 in individuals with schizophrenia (Cascella et al., 2012; Chandley et al., 2009; Chang et al., 2011; Laske et al., 2008; Schott et al., 2003). In other reports, anti-gluten antibodies formed in children with autism were strongly associated with anti-peptidase antibodies, perhaps suggesting an autoimmune response directed at a bound gluten-peptidase complex (Vojdani et al., 2004a; Vojdani et al., 2004b; Vojdani et al., 2003). Anti-gluten and anti-casein peptide antibodies were also found to associate with the immune molecule anti-CD26 and anti-CD69 autoantibodies, thus suggesting another source of food antigen-associated autoantibody target (Vojdani et al., 2003).
We also found that in the non-recent onset schizophrenia group, C1q was associated with a marker of GI inflammation, ASCA, which is used to aid the diagnosis of Crohns Disease. We recently reported elevated levels of this type of inflammation in individuals with schizophrenia and found it to be correlated with antibodies to food antigens (Severance et al., 2012). GI permeability brought on by inflammation represents a logical means by which food antigens might be introduced into systemic circulation. Cunningham-Rundles and colleagues put forth years ago that the formation of milk casein-related complexes is likely a response to a greater circulating peptide load following the excessive absorption of milk proteins across the gastrointestinal tract (Cunningham-Rundles et al., 1978; Cunningham-Rundles et al., 1979; Cunningham-Rundles and Carr, 1986). As stated above, the general lack of correlation of C1q with the infectious disease antigen IgG and a strong correlation with food antigen antibodies further reinforces a GI origin of the observed C1q activation.
A number of issues limit the extent to which our results can be fully interpreted. The current study is cross-sectional, and therefore, any changing levels of immune complex components over time are not captured in this type of study. Age, sex and smoking status were all identified as possible confounding variables, and thus were included in the multivariate regression models utilized here. As discussed earlier, immune complexes were more often found in younger individuals in both schizophrenia groups; for sex and smoking status, we found no significant associations of these variables with the present results. In previous studies of mood disorders, we were able to disentangle a particular association of food antigen antibodies with clinical features of mania and psychosis (Dickerson et al., 2012; Severance et al., 2010b). In the current study, however, evaluation of the clinical impact of our findings in schizophrenia is limited. Large-scale, well-controlled, prospective clinical studies are required to identify specific symptoms that might be affected by casein and gluten intake in individuals with schizophrenia and to evaluate how dietary modifications might affect symptom severity.
The interaction of genes and environmental factors is increasingly implicated in complex neurodevelopmental disorders such as schizophrenia. In the context of the present study, we can speculate that an altered function of complement and other immune-related genes might allow the decreased clearance of circulating antigens, which might in turn reflect an imbalance in immune complex components. Genetic studies of the complement pathway in schizophrenia reveal that variants containing single-nucleotide polymorphisms in the C1QB gene, in complement control-related genes and in the complement surface receptor gene CD46 all segregate with the disorder (Havik et al., 2011; Zakharyan et al., 2011). Genes of the major histocompatibility complex (MHC) are also of interest to our C1q findings, because MHC class I proteins may similarly act to modify synaptic plasticity during neurodevelopment (Boulanger, 2009; Shatz, 2009). Genome-wide association studies in schizophrenia identified the chromosomal area housing the MHC genes as a potentially important candidate region (Purcell et al., 2009; Shi et al., 2009; Stefansson et al., 2009). These associations suggest the possibility that genetic variants in MHC alleles might control how antigens are recognized and which antibodies are actually generated.
We can further hypothesize that the presence of these anti-C1q or anti-food antigen antibodies during pregnancy or in breast milk could potentially expose the developing brain to unanticipated C1q activation. In a large birth registry-based study in Sweden, elevated levels of maternal anti-gliadin IgG were recently found to increase the risk of nonaffective psychosis in offspring (Karlsson et al., 2012). Maternally-derived food antigen IgG, therefore, could in theory contribute to fetal or postnatal activation of C1q. If this process occurs during a critical period of C1q-mediated synaptic pruning, then it is reasonable to predict that there will be adverse effects on synaptic organization in the brain. Upon re-exposure as adults, food antigens separately or in conjunction with C1q reactivation could contribute to and/or aggravate psychiatric symptoms and behaviors.
Conclusions
In conclusion, a significant portion of C1q activation in non-recent onset schizophrenia can be attributed to the formation of immune complexes with the food antigens, milk caseins and wheat glutens. In individuals who are in the earlier stages of their disease, C1q activation is most prominent and may reflect the generation of C1q autoantibodies, formation of immune complexes with non-food-related antigens, or the dissociated state of immune complex components. Our data provide a starting point to examine if perinatal exposure to food antigens in susceptible individuals represents a plausible means by which C1q synaptic pruning defects could be generated and ultimately result in symptoms and behaviors characteristic of schizophrenia.
Highlights.
We identified significant C1q activation in schizophrenia compared to controls.
Milk caseins and wheat glutens were prominent antigens of C1q immune complexes.
Especially elevated C1q activity in recent onset schizophrenia may aid early stage diagnosis.
Disease-associated C1q activation has implications in synapse pruning processes.
Acknowledgments
Role of funding source
This work was supported by a NIMH P50 Silvio O. Conte Center at Johns Hopkins (grant# MH-94268); by the Brain and Behavior Research Foundation (formerly NARSAD) where Dr. Severance is a Scott-Gentle Foundation Young Investigator; and by the Stanley Medical Research Institute. These funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations
- CI
confidence interval
- GI
gastrointestinal
- HRP
horseradish peroxidase
- HSA
human serum albumin
- IgG
Immunoglobulin G
- OR
odds ratio
- PBS
phosphate buffered saline
- PBST
phosphate buffered saline plus Tween20
- RO
recent onset
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, et al. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry. 2010;68:1172–81. doi: 10.1016/j.biopsych.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakelyan A, Zakharyan R, Khoyetsyan A, Poghosyan D, Aroutiounian R, Mrazek F, et al. Functional characterization of the complement receptor type 1 and its circulating ligands in patients with schizophrenia. BMC Clin Pathol. 2011;11:10. doi: 10.1186/1472-6890-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: A 30-year population-based register study. Am J Psychiatry. 2011;168:1303–10. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Boyajyan A, Khoyetsyan A, Tsakanova G, Sim RB. Cryoglobulins as indicators of upregulated immune response in schizophrenia. Clin Biochem. 2008;41:355–60. doi: 10.1016/j.clinbiochem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011;93:23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Patterson PH. Maternal infection and schizophrenia: Implications for prevention. Schizophr Bull. 2011;37:284–90. doi: 10.1093/schbul/sbq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella NG, Kryszak D, Bhatti B, Gregory P, Kelly DL, Mc Evoy JP, et al. Prevalence of celiac disease and gluten sensitivity in the united states clinical antipsychotic trials of intervention effectiveness study population. Schizophr Bull. 2011;37:94–100. doi: 10.1093/schbul/sbp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella NG, Santora D, Gregory P, Kelly DL, Fasano A, Eaton WW. Increased prevalence of transglutaminase 6 antibodies in sera from schizophrenia patients. Schizophr Bull. 2012 doi: 10.1093/schbul/sbs064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandley MJ, Miller MN, Kwasigroch CN, Wilson TD, Miller BE. Increased antibodies for the alpha7 subunit of the nicotinic receptor in schizophrenia. Schizophr Res. 2009;109:98–101. doi: 10.1016/j.schres.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Chang SH, Chiang SY, Chiu CC, Tsai CC, Tsai HH, Huang CY, et al. Expression of anti-cardiolipin antibodies and inflammatory associated factors in patients with schizophrenia. Psychiatry Res. 2011;187:341–6. doi: 10.1016/j.psychres.2010.04.049. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Cunningham-Rundles C, Brandeis WE, Good RA, Day NK. Milk precipitins, circulating immune complexes, and iga deficiency. Proc Natl Acad Sci U S A. 1978;75:3387–9. doi: 10.1073/pnas.75.7.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham-Rundles C, Brandeis WE, Good RA, Day NK. Bovine antigens and the formation of circulating immune complexes in selective immunoglobulin a deficiency. J Clin Invest. 1979;64:272–9. doi: 10.1172/JCI109448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham-Rundles C, Carr RI. Dietary bovine antigens and immune complex formation after intravenous immunoglobulin in common varied immunodeficiency. J Clin Immunol. 1986;6:381–8. doi: 10.1007/BF00915377. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C, Good RA. Immune complexes after milk ingestion. Lancet. 1979;2:361–2. doi: 10.1016/s0140-6736(79)90375-1. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Copp C, Khushalani S, Yolken R. Antibodies to measles in individuals with recent onset psychosis. Schizophr Res. 2010a;119:89–94. doi: 10.1016/j.schres.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, et al. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. 2010b;68:100–4. doi: 10.1016/j.biopsych.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Markers of gluten sensitivity in acute mania: A longitudinal study. Psychiatry Res. 2012;196:68–71. doi: 10.1016/j.psychres.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Dohan FC. Schizophrenia, celiac disease, gluten antibodies, and the importance of beta. Biol Psychiatry. 1981;16:1115–7. [PubMed] [Google Scholar]
- Dohan FC, Martin L, Grasberger JC, Boehme D, Cottrell JC. Antibodies to wheat gliadin in blood of psychiatric patients: Possible role of emotional factors. Biol Psychiatry. 1972;5:127–37. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders - non-patient edition (SCID I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 1998. [Google Scholar]
- Havik B, Le Hellard S, Rietschel M, Lybaek H, Djurovic S, Mattheisen M, et al. The complement control-related genes CSMD1 and CSMD2 associate to schizophrenia. Biol Psychiatry. 2011;70:35–42. doi: 10.1016/j.biopsych.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Karlsson H, Blomstrom A, Wicks S, Yang S, Yolken RH, Dalman C. Maternal antibodies to dietary antigens and risk for nonaffective psychosis in offspring. Am J Psychiatry. 2012 doi: 10.1176/appi.ajp.2012.11081197. [DOI] [PubMed] [Google Scholar]
- Laske C, Zank M, Klein R, Stransky E, Batra A, Buchkremer G, et al. Autoantibody reactivity in serum of patients with major depression, schizophrenia and healthy controls. Psychiatry Res. 2008;158:83–6. doi: 10.1016/j.psychres.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Gerth CW, Koethe D, Klosterkotter J, Ruslanova I, Krivogorsky B, et al. Antibodies to infectious agents in individuals with recent onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254:4–8. doi: 10.1007/s00406-004-0481-6. [DOI] [PubMed] [Google Scholar]
- Mailian KR, Boiadzhian AS, Sogoian AF, Sim RB, Manukian LA. [Concentration and protein composition of circulating immune complexes in the blood of patients with schizophrenia and subjects with positive familial history of disease] Zh Nevrol Psikhiatr Im S S Korsakova. 2005;105:55–60. [PubMed] [Google Scholar]
- Mayilyan KR, Weinberger DR, Sim RB. The complement system in schizophrenia. Drug News Perspect. 2008;21:200–10. doi: 10.1358/dnp.2008.21.4.1213349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebuhr DW, Li Y, Cowan DN, Weber NS, Fisher JA, Ford GM, et al. Association between bovine casein antibody and new onset schizophrenia among us military personnel. Schizophr Res. 2011;128:51–5. doi: 10.1016/j.schres.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Niebuhr DW, Millikan AM, Yolken R, Li Y, Weber NS. Results from a hypothesis generating case-control study: Herpes family viruses and schizophrenia among military personnel. Schizophr Bull. 2008;34:1182–8. doi: 10.1093/schbul/sbm139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganelli R, Atherton DJ, Levinsky RJ. Differences between normal and milk allergic subjects in their immune responses after milk ingestion. Arch Dis Child. 1983;58:201–6. doi: 10.1136/adc.58.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt KL, Landmark J. Specific IgA antibody increases in schizophrenia. Biol Psychiatry. 1995;37:410–413. doi: 10.1016/0006-3223(94)00176-4. [DOI] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: Immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaroo D, Dickerson F, Kasarda DD, Green PH, Briani C, Yolken RH, et al. Novel immune response to gluten in individuals with schizophrenia. Schizophr Res. 2010;118:248–55. doi: 10.1016/j.schres.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott K, Schaefer JE, Richartz E, Batra A, Eusterschulte B, Klein R, et al. Autoantibodies to serotonin in serum of patients with psychiatric disorders. Psychiatry Res. 2003;121:51–7. doi: 10.1016/s0165-1781(03)00137-9. [DOI] [PubMed] [Google Scholar]
- Senocq D, Dupont D, Rolet-Repecaud O, Faurie F, Levieux D. Antipeptide antibodies recognizing plasmin sensitive sites in bovine beta-casein sequence. J Agric Food Chem. 2001;49:1571–7. doi: 10.1021/jf001352s. [DOI] [PubMed] [Google Scholar]
- Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. 2012 doi: 10.1016/j.schres.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Dickerson FB, Halling M, Krivogorsky B, Haile L, Yang S, et al. Subunit and whole molecule specificity of the anti-bovine casein immune response in recent onset psychosis and schizophrenia. Schizophr Res. 2010a;118:240–7. doi: 10.1016/j.schres.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Severance EG, Dickerson FB, Viscidi RP, Bossis I, Stallings CR, Origoni AE, et al. Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr Bull. 2011a;37:101–7. doi: 10.1093/schbul/sbp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Dupont D, Dickerson FB, Stallings CR, Origoni AE, Krivogorsky B, et al. Immune activation by casein dietary antigens in bipolar disorder. Bipolar Disord. 2010b;12:834–42. doi: 10.1111/j.1399-5618.2010.00879.x. [DOI] [PubMed] [Google Scholar]
- Severance EG, Lin J, Sampson HA, Gimenez G, Dickerson FB, Halling M, et al. Dietary antigens, epitope recognition, and immune complex formation in recent onset psychosis and long-term schizophrenia. Schizophr Res. 2011b;126:43–50. doi: 10.1016/j.schres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. Mhc class i: An unexpected role in neuronal plasticity. Neuron. 2009;64:40–5. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, et al. Common variants on chromosome 6p22. 1 are associated with schizophrenia. Nature. 2009;460:753–7. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–7. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates cns synapse elimination. Cell. 2007;131:1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Vetlugina TP, Logvinovich GV, Maslennikova SN, Vasil’eva OA. [Circulating immune complexes in the serum of mental patients and healthy subjects] Zh Nevropatol Psikhiatr Im S S Korsakova. 1984;84:422–6. [PubMed] [Google Scholar]
- Vojdani A, Bazargan M, Vojdani E, Samadi J, Nourian AA, Eghbalieh N, et al. Heat shock protein and gliadin peptide promote development of peptidase antibodies in children with autism and patients with autoimmune disease. Clin Diagn Lab Immunol. 2004a;11:515–24. doi: 10.1128/CDLI.11.3.515-524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojdani A, O’Bryan T, Green JA, McCandless J, Woeller KN, Vojdani E, et al. Immune response to dietary proteins, gliadin and cerebellar peptides in children with autism. Nutr Neurosci. 2004b;7:151–61. doi: 10.1080/10284150400004155. [DOI] [PubMed] [Google Scholar]
- Vojdani A, Pangborn JB, Vojdani E, Cooper EL. Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major instigators of autoimmunity in autism. Int J Immunopathol Pharmacol. 2003;16:189–99. doi: 10.1177/039463200301600302. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001a;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement. Second of two parts. N Engl J Med. 2001b;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol Psychiatry. 2008;13:470–9. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
- Zakharyan R, Khoyetsyan A, Arakelyan A, Boyajyan A, Gevorgyan A, Stahelova A, et al. Association of C1QB gene polymorphism with schizophrenia in armenian population. BMC Med Genet. 2011;12:126. doi: 10.1186/1471-2350-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]