Abstract
Background
Whole-heart coronary MR angiography (MRA) is a promising method for non-invasive, radiation-free detection and exclusion of obstructive coronary artery disease (CAD); however, the required imaging time and robustness of the technique are not yet satisfactory. We evaluated the value of whole-heart coronary MRA at 3.0T using a 32-channal cardiac coil, which reduces image acquisition times and hence allows to increase the clinical throughput.
Methods and Results
A total of 110 consecutive patients with suspected CAD referred for clinically indicated conventional coronary angiography were included in this prospective study. 32-channel receiver coils were used for 3.0T coronary MRA data acquisition. An ECG-triggered, navigator-gated, inversion-recovery prepared, segmented gradient-echo sequence was used for image acquisition with an acceleration factor of three in the phase-encoding direction using GRAPPA reconstruction. Acquisition of coronary MRA was successfully completed in 101 of 110 (92%) patients with average imaging time of 7.0 ± 1.8 min. The sensitivity, specificity, positive and negative predictive value of coronary MRA on a patient-based analysis were 95.9% (47/49, 95% CI: 86.0% to 99.4%), 86.5% (45/52, 95% CI, 74.2% to 94.4%), 87.0% (47/54, 95% CI, 75.1% to 94.6%) and 95.7% (45/47, 95% CI, 85.4% to 99.4%), respectively.
Conclusions
Whole-heart coronary MRA at 3.0 T using a 32-channal cardiac coil allows high overall accuracy for detecting significant CAD with reduced imaging time. It has potential to be a robust and alternative technique for ruling out significant CAD.
Clinical Trial Registration
URL: http://www.chictr.org. Unique identifier: ChiCTR-DDT-07000121.
Keywords: magnetic resonance angiography, coronary arteries, 3.0 T
The current gold standard for the assessment of coronary artery disease (CAD) remains invasive X-ray coronary angiography, which exposes patients to ionizing radiation and involves certain risk of complications. Since the implementation of multi-slice CT (MSCT), non-invasive coronary imaging using 64-slice CT has proven to be highly accurate as a diagnostic tool for the detection of coronary artery stenoses in the clinical routine1, 2. However, radiation exposure to patients and its possible risk of cancer induction have remained issues of great concern3. Over the past 15 years, the continuous improvement in MRI technology allows non-invasive, radiation-free, comprehensive evaluation of CAD4-6. Initial experiences have shown that the diagnostic accuracy of contrast-enhanced whole-heart coronary MRA at 3.0T in detecting coronary artery stenosis approaches that of 64-slice CT7. Nevertheless, coronary MR angiography (MRA) procedure remains lengthy and has limited the general applicability of this test8, 9. Further reductions in coronary MRA acquisition time have been made possible recently with the novel multi-channel cardiac coils and high parallel imaging factors10. The use of parallel imaging at higher magnetic fields has been shown to be extremely promising for minimizing the many challenges for high-resolution, high-speed coronary MRA.
We have therefore conducted a prospective study to evaluate the diagnostic performance of 3.0 T whole-heart coronary MRA using a 32-channal cardiac coil compared with quantitative X-ray coronary angiography in patients with suspected CAD.
Methods
Study Population
From January 2009 to July 2010, a total of 130 consecutive patients scheduled for conventional coronary angiography were prospectively recruited in this study. Exclusion criteria included general contraindications to MR examination (claustrophobia, pacemaker), unstable angina, atrial fibrillation, patients with coronary stents or bypass grafts, and renal insufficiency (estimated glomerular filtration rate assessed by creatinine clearance < 60 ml/min/1.73 m2). 20 patients were excluded for these reasons and 110 patients (54 men, age 58 ± 11) underwent coronary MRA before conventional coronary angiography (Figure 1). The study protocol was approved by the institutional review board of our hospital. Written informed consent was obtained from each patient prior to the study.
Figure 1. Flow Diagram of Patient Recruitment.
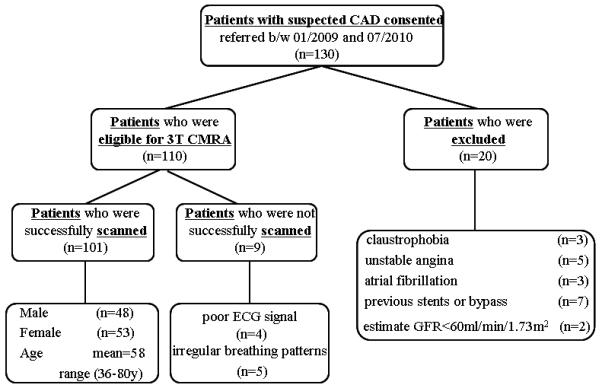
CAD = Coronary Artery Disease. 32-CC Coronary MRA = 32 channel cardiac coils coronary MRA.
Patient Preparation
A beta-blocker (metoprolol tartrate, 25 to 50 mg) was given orally to patients with heart rate >75 beats/min before coronary MRA. All images were collected under free breathing with the patient in supine position. Patients were trained to perform shallow breathing during coronary MRA data acquisition. Abdominal belt was wrapped non-tightly in patients with irregular breath pattern to suppress the vertical motion of the diaphragm.
Acquisition of 3.0T Whole-Heart Coronary MRA with 32-Channel Cardiac Coils
Contrast-enhanced whole-heart coronary MRA was performed on a 3.0T whole-body scanner (MAGNETOM Trio; Siemens AG Healthcare, Germany) with a 32-channel cardiac coil (Invivo, Gainesville, Florida, USA). The procedures were as follows: Two dimensional (2D) scout images were first obtained in three orthogonal orientations to identify the position of the heart and diaphragm. Four-chamber view cine images were then acquired with a fast low-angle shot (FLASH) sequence during free breathing. The global cardiac motion was visually assessed from the cine images and patient-specific data acquisition windows were determined either in the diastolic or systolic phase.
Free-breathing, contrast-enhanced coronary MRA was obtained using a ECG-triggered, navigator-gated, inversion-recovery prepared, segmented 3D FLASH sequence (TR /TE = 3.3/1.5 ms; TI = 200 ms; flip angle = 20°; readout bandwidth = 700 Hz/pixel; voxel size = 1.1 × 1.1 × 1.3 mm3 interpolated to 0.55 × 0.55 × 0.65 mm3) with slow infusion of 0.15 mmol/kg body weight of Gadobenate dimeglumine (MultiHance; Bracco Imaging SpA, Milan, Italy) at a rate of 0.3 ml/sec. Sixty seconds after the initiation of contrast administration, data acquisition was started. Navigator echo signal was acquired from a 2D beam perpendicular to the right hemi-diaphragm. The width of navigator acceptance window was ± 2.5 mm. The shift of 3D imaging volume was correlated to the shift of navigator tracking point using a prospective real-time adaptive motion correction with a constant (0.6) correction factor in the superior-inferior direction11. 3D whole- heart coronary MRA scan was accelerated by using a combination of partial Fourier in the slab-encoding direction with a factor of 6/8 and generalized autocalibrating partially parallel acquisitions (GRAPPA) acceleration factor of 3 in the phase encoding direction.
Conventional Coronary Angiography Studies
X-ray coronary angiography was performed in all patients and evaluated by quantitative coronary angiography (QuantCor QCA, Siemens Healthcare) by two cardiologists who was blinded to the coronary MRA results. Standard 15-segment American Heart Association classification system was used. Stenoses were quantitatively evaluated for segments with a reference diameter of 1.5 mm or more. Significant coronary artery disease was defined as a luminal diameter reduction of ≥50% in coronary arteries.
Contrast-Enhanced Whole-Heart Coronary MRA Image Analysis
Coronary MRA images were transferred to an external workstation (MMWP, Siemens AG Healthcare, Erlangen, Germany). Two experienced readers who were blinded to the patient information independently assessed coronary MRA using axial source images, curved multiplanar reformations (CPR), and thin-slab sliding maximum intensity projections (MIP) images. Coronary MRA image quality was graded on a 4-point scale (1, non-assessable with severe image artifacts, poor vessel contrast; 2, assessable with moderate image artifacts, fair vessel contrast; 3, assessable with minor artifacts, good vessel contrast; 4, assessable with no apparent artifacts, excellent vessel contrast). The scores generated by two readers were averaged. Significant narrowing of the coronary arteries (≥50% reduction in diameter) was visually assessed by two observers. Each observer independently recorded the presence or absence of significant stenosis to determine the interobserver agreement of binary judgment. All coronary arteries were included for the evaluation regardless the image quality of coronary MRA to avoid overestimation of the diagnostic accuracy. A consensus reading was performed for the segments in which there was disagreement between the two observers. Vessel-based data sets were constructed from the final segment data to generate the patient-based data sets.
Statistical Analysis
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy with 95% confidence intervals were calculated on a per-segment, per-vessel and per-patient basis using invasive x-ray coronary angiography as reference standard. The intention-to-read approach was used and non-assessable segments were considered to have a stenosis. A vessel was considered diseased if it presented at least 1 segment with a coronary stenosis ≥50%, and a patient was considered as having CAD if he or she had at least 1 vessel with a ≥50% stenosis. The level of agreement between the two readers with respect to the image quality grading was assessed by weighted Kappa statistics. The interobserver agreement for the binary judgments for the presence or absence of stenosis was evaluated using Kappa statistics. Number of cases without successfully coronary MRA was not taken into account in the calculation of diagnostic performance. All statistical analysis was performed using statistical software (SAS version 9.1, SAS Institute Inc., Cary, North Carolina, USA). Quantitative variables were expressed as mean value ± standard deviation, and categorical variables as percentages.
Results
Coronary MRA was successfully completed in 101 of 110 (92%) patients without complications. Nine patient studies were aborted due to poor ECG signal (n = 4), or extremely low respiratory gating efficiency (< 20%, n = 5). The characteristics of the study population are summarized in Table 1. The prevalence of having at least one significant coronary stenosis in patients with successful coronary MRA was 49% Mean heart rate during coronary MRA was 66 ± 8 beats/min. Coronary MRA was acquired during diastole in 67% (68/101) (acquisition window 124 ± 28ms) and during systole in 33%(33/101)(acquisition window 84 ± 6ms). The average navigator acceptance rate was 36%.
Table 1.
Characteristics of the study population
| Characteristics | Patients Who Underwent Coronary MRA (n =110) |
Patients With Successful Coronary MRA (n =101) |
|---|---|---|
| Age, yrs | 58±11 | 58±11 |
| Range | 39-84 | 36-80 |
| Sex, male/female | 54 / 46 | 48 / 53 |
| Body mass index (kg/m2) | 24±3 | 24±3 |
| Hypertension, n (%) | 45 (41%) | 43(47%) |
| Hypercholesterolemia, n (%) | 48(44%) | 45 (45%) |
| Diabetes mellitus | 28(23%) | 26(26%) |
| Current or prior cigarette smoking, n (%) | 45 (41%) | 42(42%) |
| Chest pain | 68(62%) | 65 (64%) |
| Prior myocardial infarction | 18 (16%) | 19 (19%) |
| Stenosis on x-ray coronary angiography | 54 (50%) | 49 (49%) |
| One vessel | 25 | 21 |
| Two vessel | 19 | 18 |
| Three vessel | 10 | 10 |
| Beta-blocker administered before scan | 27 (25%) | 23 (23%) |
| Heart rate on coronary MRA, beats/min | 66±8 | 66±8 |
| Range | 51-88 | 51-88 |
Image Quality of the Whole-Heart Coronary MRA
In 101 patients, a total of 403 vessels and 1181 coronary artery segments were evaluated. 1104 of 1181 (93.5%) segments with a reference luminal diameter ≥1.5 mm on QCA were evaluated as assessable (score 2 to 4). The reasons for 77 non-assessable segments were poor contrast-to-noise ratio (n=33), motion artifacts (n=29), and small diameter (n=15). The image score was 3.2 ± 0.8. Weighted Kappa value for interobserver agreement for image quality grading was 0.86. The whole-heart coronary MRA image quality of 101 patients is summarized in Table 2.
Table 2.
Image quality of 101 patients with successful CMRA
| No. of Segments ≥1.5 mm On QCA |
No. of Assessable Segments on CMRA |
Causes of Nonassessibility | Image Quality of CMRA |
|||
|---|---|---|---|---|---|---|
|
Poor
opacification |
Motion
artifacts |
Small
caliber |
||||
| LM | 101 | 101(100%) | 0 | 0 | 0 | 3.4 ± 0.5 |
| LAD | ||||||
| Proximal | 101 | 97(96%) | 3 | 1 | 0 | 3.3 ± 0.7 |
| Mid | 98 | 93(95%) | 3 | 2 | 0 | 3.3 ± 0.8 |
| Distal | 84 | 74(88%) | 3 | 5 | 2 | 3.1 ± 1.0 |
| Diagonal branches | 116 | 100(86%) | 7 | 4 | 5 | 3.1 ± 1.0 |
| LCX | ||||||
| Proximal | 100 | 97(97%) | 1 | 2 | 0 | 3.3 ± 0.7 |
| Distal | 79 | 74(94%) | 2 | 2 | 1 | 3.2 ± 0.8 |
| Marginal branches | 111 | 99(89%) | 4 | 5 | 3 | 2.9 ± 1.0 |
| RCA | ||||||
| Proximal | 101 | 101 (100%) | 0 | 0 | 0 | 3.4 ± 0.5 |
| Mid | 101 | 101 (100%) | 0 | 0 | 0 | 3.4 ± 0.5 |
| Distal | 94 | 91(97%) | 2 | 1 | 0 | 3.3 ± 0.7 |
| PDA/PL | 95 | 76(80%) | 8 | 7 | 4 | 2.8 ± 1.1 |
| Total | 1181 | 1104(94%) | 33 | 29 | 15 | 3.2 ± 0.8 |
Data are expressed as means ± SD. QCA = quantitative coronary angiography; LAD = left anterior descending coronary artery; LCX = left circumflex coronary artery; LM = left main coronary artery; RCA = right coronary artery; pro = proximal; mid = middle; dis = distal.
Diagnostic Performance of Coronary MRA compared with QCA
3.0T contrast-enhanced whole-heart coronary MRA correctly identified significant CAD in 47 out of 49 patients and correctly ruled out CAD in 45 out of 52 patients (Figure 2, 3). In all patients with left main or 3-vessel disease (100%, 11 of 11), coronary MRA detected at least one significant coronary stenosis, which means that on a per-patient basis all of these patients were correctly identified. Kappa value for the binary judgment was 0.89 (95% CI:0.80-0.98) on per-patient based analysis.
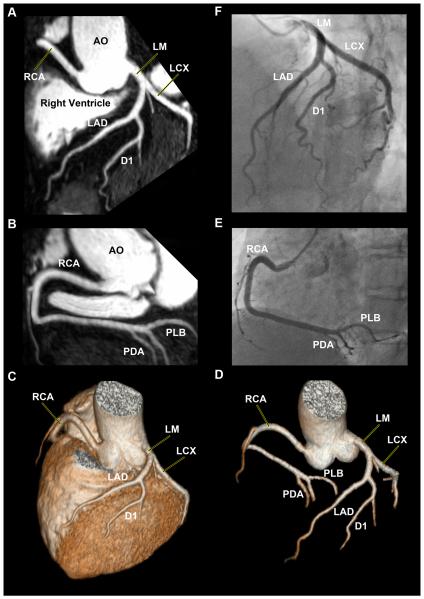
Figure 2. 32-Channel Cardiac Coils Based Whole-Heart Coronary MR Angiography Exclusion of Significant Coronary Artery Disease (Imaging time: 4 min 14 sec).
Left anterior oblique image reformatted with curved multiplanar reformation depicts LM, LAD, LCX and the first diagonal branch (A), right anterior oblique image reformatted with curved multiplanar reformation shows RCA, posterior descending artery (PDA), and posterior lateral branch (PLB) (B). Volume-rendered image provodes an overview of coronary anatomy (C). Better visualization of the entire coronary artery tree after removing the background of myocardium, long segments of all major coronary arteries are well depicted (D). The conventional coronary angiography confirms the absence of significant coronary artery disease (E, F). AO = aorta, LM = left main coronary artery, OM = obtuse marginal branch, D1 = first diagonal branch, D2 = second diagonal branch, LCX = left circumflex coronary artery, RCA = right coronary artery.
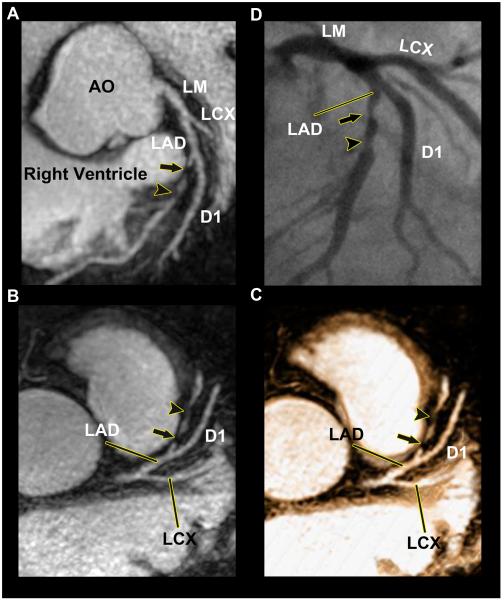
Figure 3. 32-Channal Cardiac Coils Based Whole-Heart CMRA Image of the LAD With Significant Coronary Artery Disease (Imaging time: 3 min 34 sec).
Sliding thin-slab maximum intensity projection images (A , B) and the thin-slab volume-rendered image (C) disclose a significant coronary stenosis (arrow) and distally an intermediate coronary stenosis (arrowhead) in the mid LAD, which were both corroborated by conventional coronary angiography (B). Abbreviations as in Figures 2.
In a total of 1104 assessable coronary segments, QCA detected a total of 107 lesions (≥50%). Coronary MRA correctly identified 91 of these lesions. In 986 segments, stenosis was ruled out correctly by coronary MRA. A detailed overview of the diagnostic performance of 3.0T coronary MRA compared with QCA is summarized in Table 3.
Table 3.
Accuracy of WH-CMRA Using 32 Channel Cardiac Coils for Detection of Coronary Stenosis
| Patient based n=101 |
Vessel based n=403 |
Segment based n=1181 |
|
|---|---|---|---|
| Sensitivity, % (95% CI) | 95.9(86.0–99.4) | 88.7(80.3-94.5) | 85.1(76.9-91.2) |
| Specificity, % (95% CI) | 86.5(74.2-94.4) | 91.1(87.9-93.4) | 91.8(90.0-93.4) |
| Positive predictive value, % (95% CI) | 87.0(75.1-94.6) | 68.7(59.4-77.0) | 50.8(43.3-58.4) |
| Negative predictive value, % (95% CI) | 95.7(85.4-99.4) | 97.4(95.2-98.7) | 98.4(97.4-99.1) |
Note.—Data are percentages, with raw data in parentheses and 95% confidence
Abbreviations: CI, confidence interval.
Discussion
This prospective, single-center study demonstrated high diagnostic performance of 3.0T contrast enhanced whole-heart coronary MRA using 32-channal cardiac coils in intermediate symptomatic patients for the detection of obstructive CAD. The patient-based sensitivity and specificity of whole-heart coronary MRA with 32-channel coils in the detection of significant stenoses were 95.9% and 86.5%, respectively. Thus, our data establish coronary MRA at 3.0T using a 32-channal cardiac coil as a robust technique to perform noninvasive and radiation-free coronary angiography.
To the best of our knowledge, this is the first study in which 32-channel cardiac coils, 3.0T MRI system, and highly accelerated parallel imaging were combined to perform whole-heart coronary MRA. A very recent study has investigated the use of 32-channel coils to perform whole-heart coronary MRA at 1.5T with high parallel imaging factors12. In their study, substantially shortened imaging time and high study success rate were observed. Nevertheless, it is known that the straightforward application of parallel imaging at field strengths of 1.5T dramatically reduces acquisition time, but at the cost of SNR. The baseline SNR gain at 3.0T can be used to tradeoff spatial and temporal resolution of coronary MRA with parallel imaging13, 14.
In our study, whole-heart coronary MRA was obtained with a substantially reduced acquisition time (7.0 ±1.8 min) compared to previous studies without using 32-channel coils15. Reduced acquisition time can be translated into an improvement of in-plane and through-plane spatial resolution, which resulted in an improved delineation of distal segments of the coronary arteries. In most of the previous coronary MRA studies, evaluation was limited to branches having a diameter >2 mm15, 16. We evaluated all segments being >1.5 mm in diameter, while only 6.5% of the coronary segments were non-diagnostic, significantly lower than findings of our previous study (12%)7. In addition, the time savings improves the clinical throughput of coronary MRA and potentially decrease patient discomfort resulting from long measurements. The resulting measurable improvements of coronary MRA using 32-channal cardiac coils in image quality are likely to translate into more stable, more diverse, and more widely accepted clinical applications.
MSCT has emerged as a rapid and noninvasive tool for the detection of CAD and numerous methods for dose reduction have been developed recently17, 18. However, the penetration of these techniques into widespread clinical practice has not yet established, and current guidelines recommend a heart rate of than 60 beats per minute both for optimal image quality and for reduction of radiation exposure19. MRI is the most promising cardiac imaging test due to its unique advantage of not requiring radiation exposure, allowing concurrent assessment of myocardial structure, function, myocardial edema, fibrosis, and coronary arteries in a single setting. In our study only patients with heart rate higher than 75 beats per minute received an oral beta-blocker before the scan. Slow heart rates relatively prolong cardiac phases with little cardiac motion, so a data acquisition window of patients with high heart rates can be safely set to allow artifact-free imaging. The high sensitivity (95.9%) of coronary MRA for CAD shown in our study is comparable to the sensitivity of 64-slice CT studies performed in multicenter trials1, 20. The specificity (86.5%) is on par with magnetic resonance myocardial perfusion imaging, whereas the diagnostic sensitivity and NPV are higher21. The NPV was 98.4%, 97.4%, and 95.7% on per-segment, per-vessel and per-patient basis, respectively, indicating that this technique can reliably rule out significant stenoses, consistent with findings from previous studies7, 15, 16.
The low PPV (50.8%) on segment basis is explained for the most part by the non-assessable segments on coronary MRA. We did not exclude these segments from the analysis but tended to grade these lesions as having a significant obstruction. Our coronary stenosis grading policy is based on the premise that patients with either positive coronary MRA results or non-assessable segments will undergo QCA in an intention-to-read approach. Because of this, coronary MRA is not ready to challenge invasive coronary angiography as a true alternative.
Nevertheless, developments in parallel imaging22, 23 and multi-channel phased-array coils may further reduce the imaging time24, 25, this will have a considerable impact on improving spatial resolution and image quality due to inconsistent cardiac and respiratory motion. Thus, if the diagnostic performance of coronary MRA can be further improved, this test may become the most important imaging tool for noninvasively and comprehensively assessing patients with suspected CAD. Whole-heart coronary MRA at 3.0T has great potential to become a valuable complement to other non-invasive imaging modalities if current limitations, such as navigator failure rate and low spatial resolution, can be overcome.
There are several limitations that need to be acknowledged in this study. The capability to perform cardiac function, perfusion, and viability, as well as coronary imaging in the same setting for a comprehensive exam of CAD is a major strength of cardiac MR imaging. The need for contrast agent in both coronary MRA and cardiac perfusion scans will either lead to increased total contrast dose, or decreased dose from optimal value for coronary MRA and/or perfusion scans. The performance of coronary MRA with further reduced contrast dose is not yet established. Second, coronary MRA could not be acquired from about 8% of the patients due to unstable breathing patterns or poor ECG signal. Not including all subjects may result in overestimation of the diagnostic accuracy of coronary MRA. Future studies are needed to define the method’s precise role in the diagnostic algorithm for the evaluation of patients with suspected CAD in multi-center trials. Third, despite the use of partial Fourier acquisition and parallel imaging, it is desirable to further improve the imaging speed with advanced acceleration and reconstruction techniques without compromising image quality. Finally, to acquire consistent coronary MRA images requires highly attentive and experienced operators on the setting of timing and imaging parameters. Broader utilization and acceptance of coronary MRA could be improved by simplifying or automating the protocol settings.
In conclusion, among patients who were scheduled to obtain conventional X-ray coronary angiography, we found that coronary MRA at 3.0 T using 32-channal cardiac coils demonstrates high accuracy for detection of significant coronary artery stenosis. The high NPV (95.8%) establishes coronary MRA as an effective noninvasive method to rule out significant coronary artery stenosis without exposure to ionizing radiation. The speed advantage and extra diagnostic value afforded by 32-channel cardiac coils at 3.0 T may be expected to drive future technological developments of more robust and reliable coronary MRA.
Coronary imaging with magnetic resonance has historically been challenging, but recent technical advantages may overcome prior limitations to reliable detection of coronary artery stenosis. This study evaluated whole-heart navigator-gated, ECG-triggered and free-breathing coronary magnetic resonance angiography (MRA) acquired after intravenous infusion of gadolinium-based contrast successfully performed in 101 patients also undergoing traditional invasive x-ray angiography. MRA acquisition time averaged 7.0±1.8 min. For a cutoff of 50% luminal stenosis by quantitative coronary angiography, 3D-MRA performed well with positive and negative predictive values averaging 87.0 and 95.7%, respectively. With contemporary techniques, coronary MRA can be performed efficiently, and warrants greater consideration as a suitable noninvasive method to exclude obstructive coronary artery disease.
Acknowledgments
Sources of Funding
National Basic Research Program 973 (Grant No. 2010CB732600) from Ministry of Science and Technology, China; National Natural Science Foundation of China grant number 30900355; National Institute of Health grants numbers NIBIB EB002623 and NHLBI HL38698.
Abbreviations
- CT
computed tomography
- MRI
magnetic resonance imaging
- SNR
signal-to-noise ratio
- FLASH
fast low-angle shot
- GRAPPA
Generalized auto calibrating partially parallel acquisitions
- LM
left main coronary artery
- LAD
left anterior descending coronary artery
- LCX
left circumflex coronary artery
- RCA
right coronary artery
- QCA
quantitative coronary angiography
- SSFP
steady-state free precession
- RF
radio-frequency
- TR
repetition time
- TE
echo time
Footnotes
Disclosures
Dr Kuncheng reported receiving honoraria for lectures from Siemens Medical Systems and Bracco.
Journal Subject Codes: Diagnostic Testing [30] CT and MRI [29] Coronary imaging
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row ct. N Engl J Med. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 2.Mollet NR, Cademartiri F, van Mieghem CA, Runza G, McFadden EP, Baks T, Serruys PW, Krestin GP, de Feyter PJ. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–2323. doi: 10.1161/CIRCULATIONAHA.105.533471. [DOI] [PubMed] [Google Scholar]
- 3.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. Jama. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 4.Constantine G, Shan K, Flamm SD, Sivananthan MU. Role of mri in clinical cardiology. Lancet. 2004;363:2162–2171. doi: 10.1016/S0140-6736(04)16509-4. [DOI] [PubMed] [Google Scholar]
- 5.Kim WY, Danias PG, Stuber M, Flamm SD, Plein S, Nagel E, Langerak SE, Weber OM, Pedersen EM, Schmidt M, Botnar RM, Manning WJ. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345:1863–1869. doi: 10.1056/NEJMoa010866. [DOI] [PubMed] [Google Scholar]
- 6.Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, Pennell DJ. Abnormal subendocardial perfusion in cardiac syndrome x detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–1953. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 7.Yang Q, Li K, Liu X, Bi X, Liu Z, An J, Zhang A, Jerecic R, Li D. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3.0-t: A comparative study with x-ray angiography in a single center. Journal of the American College of Cardiology. 2009;54:69–76. doi: 10.1016/j.jacc.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamdan A, Asbach P, Wellnhofer E, Klein C, Gebker R, Kelle S, Kilian H, Huppertz A, Fleck E. A prospective study for comparison of mr and ct imaging for detection of coronary artery stenosis. JACC. Cardiovascular imaging. 2011;4:50–61. doi: 10.1016/j.jcmg.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Pouleur AC, le Polain de Waroux JB, Kefer J, Pasquet A, Vanoverschelde JL, Gerber BL. Direct comparison of whole-heart navigator-gated magnetic resonance coronary angiography and 40- and 64-slice multidetector row computed tomography to detect the coronary artery stenosis in patients scheduled for conventional coronary angiography. Circulation. Cardiovascular imaging. 2008;1:114–121. doi: 10.1161/CIRCIMAGING.107.756304. [DOI] [PubMed] [Google Scholar]
- 10.Niendorf T, Hardy CJ, Giaquinto RO, Gross P, Cline HE, Zhu Y, Kenwood G, Cohen S, Grant AK, Joshi S, Rofsky NM, Sodickson DK. Toward single breath-hold whole-heart coverage coronary mra using highly accelerated parallel imaging with a 32-channel mr system. Magn Reson Med. 2006;56:167–176. doi: 10.1002/mrm.20923. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Ehman RL. Retrospective adaptive motion correction for navigator-gated 3d coronary mr angiography. Journal of magnetic resonance imaging: JMRI. 2000;11:208–214. doi: 10.1002/(sici)1522-2586(200002)11:2<208::aid-jmri20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Nagata M, Kato S, Kitagawa K, Ishida N, Nakajima H, Nakamori S, Ishida M, Miyahara M, Ito M, Sakuma H. Diagnostic accuracy of 1.5-t unenhanced whole-heart coronary mr angiography performed with 32-channel cardiac coils: Initial single-center experience. Radiology. 2011;259:384–392. doi: 10.1148/radiol.11101323. [DOI] [PubMed] [Google Scholar]
- 13.Stuber M, Botnar RM, Fischer SE, Lamerichs R, Smink J, Harvey P, Manning WJ. Preliminary report on in vivo coronary mra at 3 tesla in humans. Magn Reson Med. 2002;48:425–429. doi: 10.1002/mrm.10240. [DOI] [PubMed] [Google Scholar]
- 14.Bi X, Carr JC, Li D. Whole-heart coronary magnetic resonance angiography at 3 tesla in 5 minutes with slow infusion of gd-bopta, a high-relaxivity clinical contrast agent. Magn Reson Med. 2007;58:1–7. doi: 10.1002/mrm.21224. [DOI] [PubMed] [Google Scholar]
- 15.Sakuma H, Ichikawa Y, Chino S, Hirano T, Makino K, Takeda K. Detection of coronary artery stenosis with whole-heart coronary magnetic resonance angiography. Journal of the American College of Cardiology. 2006;48:1946–1950. doi: 10.1016/j.jacc.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 16.Kato S, Kitagawa K, Ishida N, Ishida M, Nagata M, Ichikawa Y, Katahira K, Matsumoto Y, Seo K, Ochiai R, Kobayashi Y, Sakuma H. Assessment of coronary artery disease using magnetic resonance coronary angiography: A national multicenter trial. Journal of the American College of Cardiology. 2010;56:983–991. doi: 10.1016/j.jacc.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 17.Achenbach S, Marwan M, Ropers D, Schepis T, Pflederer T, Anders K, Kuettner A, Daniel WG, Uder M, Lell MM. Coronary computed tomography angiography with a consistent dose below 1 msv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. European heart journal. 2010;31:340–346. doi: 10.1093/eurheartj/ehp470. [DOI] [PubMed] [Google Scholar]
- 18.Scheffel H, Alkadhi H, Leschka S, Plass A, Desbiolles L, Guber I, Krauss T, Gruenenfelder J, Genoni M, Luescher TF, Marincek B, Stolzmann P. Low-dose ct coronary angiography in the step-and-shoot mode: Diagnostic performance. Heart. 2008;94:1132–1137. doi: 10.1136/hrt.2008.149971. [DOI] [PubMed] [Google Scholar]
- 19.Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, Weigold WG. Scct guidelines for performance of coronary computed tomographic angiography: A report of the society of cardiovascular computed tomography guidelines committee. Journal of cardiovascular computed tomography. 2009;3:190–204. doi: 10.1016/j.jcct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, Nieman K, van Werkhoven JM, Pundziute G, Weustink AC, de Vos AM, Pugliese F, Rensing B, Jukema JW, Bax JJ, Prokop M, Doevendans PA, Hunink MG, Krestin GP, de Feyter PJ. Diagnostic accuracy of 64-slice computed tomography coronary angiography: A prospective, multicenter, multivendor study. Journal of the American College of Cardiology. 2008;52:2135–2144. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 21.Al-Saadi N, Nagel E, Gross M, Bornstedt A, Schnackenburg B, Klein C, Klimek W, Oswald H, Fleck E. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation. 2000;101:1379–1383. doi: 10.1161/01.cir.101.12.1379. [DOI] [PubMed] [Google Scholar]
- 22.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (grappa) Magn Reson Med. 2002;47:1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 23.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. Sense: Sensitivity encoding for fast mri. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- 24.Park J, Zhang Q, Jellus V, Simonetti O, Li D. Artifact and noise suppression in grappa imaging using improved k-space coil calibration and variable density sampling. Magn Reson Med. 2005;53:186–193. doi: 10.1002/mrm.20328. [DOI] [PubMed] [Google Scholar]
- 25.Park J, Larson AC, Zhang Q, Simonetti O, Li D. 4d radial coronary artery imaging within a single breath-hold: Cine angiography with phase-sensitive fat suppression (caps) Magn Reson Med. 2005;54:833–840. doi: 10.1002/mrm.20627. [DOI] [PubMed] [Google Scholar]