Abstract
In this issue, three papers report the distribution of the RNA polymerase III (Pol III) machinery, including transcription factor IIIB, transcription factor IIIC and Pol III itself, across the human genome. These studies reveal cell type–specific expression of Pol III genes, functional interplay between the Pol II and Pol III transcriptional machineries and the potential involvement of Pol III genes in chromosome organization.
Eukaryotic transcription is mediated by three distinct RNA polymerases and regulated by several sequence-specific transcription factors. Pol I and Pol III are involved solely in the transcription of non–protein coding RNAs, whereas Pol II transcribes protein-coding genes as well as noncoding RNAs (ncRNAs)1. To better define the Pol III transcriptome and to understand the relationship with the Pol II transcriptome, multiple research groups have now mapped the distribution of Pol III transcription factors in human cells2–5. These data have led to the intriguing observation that there is a high degree of sharing of factors between Pol II and III, and cross-regulation between these systems may be possible.
Although the bulk of studies on transcription have focused on the regulation of Pol II transcription, the vast majority of RNA in a cell is generated by the other polymerases. Pol I transcribes ribosomal RNA, and Pol III mediates transcription of the 5S rRNA, tRNA, 7SK, 7SL and U6 genes as well as SINE elements (MIR and Alu elements)6 in human cells. Transcription factors IIIA (TFIIIA), TFIIIB and TFIIIC are used in various combinations for transcription of these genes. Based on the factors used during transcription, there are three types of Pol III–transcribed genes. Type 1 genes (5S rRNA) use TFIIIA, TFIIIB1 (composed of TATA box–binding protein (TBP), Bdp1 and Brf1) and TFIIIC for transcription, with TFIIIA binding specific sequences within the gene. Type 2 genes (tRNA) recruit TFIIIB1 and TFIIIC, where TFIIIC binds within the gene to two elements called the A Box and B Box and TFIIIB1 binds AT-rich sequences upstream of the gene. Type 3 genes (U6) have external promoters (proximal sequence element (PSE) and distal sequence element (DSE)); these genes recruit the factors OCT1 and TFIIIB2 (composed of TBP, Bdp1 and Brf2) and seem not to require TFIIIC7.
RNA Pol III distribution
Mapping Pol III subunits in different human cell types and lines led to the observation that only a fraction of the predicted Pol III genes (and some pseudogenes) are bound by the polymerase, and only 75% of the bound genes are transcribed2,4,5. For instance, roughly 50% of tRNA genes are occupied by Pol III in HeLa cells. This is in contrast to the situation in yeast, where almost all of the Pol III genes are occupied and transcriptionally active8–10.
Another notable observation is that specific Pol III genes are active in specific cell types, revealing active regulation of these genes. Whereas the majority of Pol III genes are active in all cell types analyzed, 10–26% of tRNA genes are active in one cell type versus another. This cannot be explained simply by differences in Pol III promoter architecture and likely reflects active regulation mediated by factors bound to flanking sequences2,4. These data are consistent with earlier observations that Pol III–transcribed genes are subject to regulation during stress, development, differentiation and the cell cycle11–13.
Relationships between Pol III and Pol II transcription
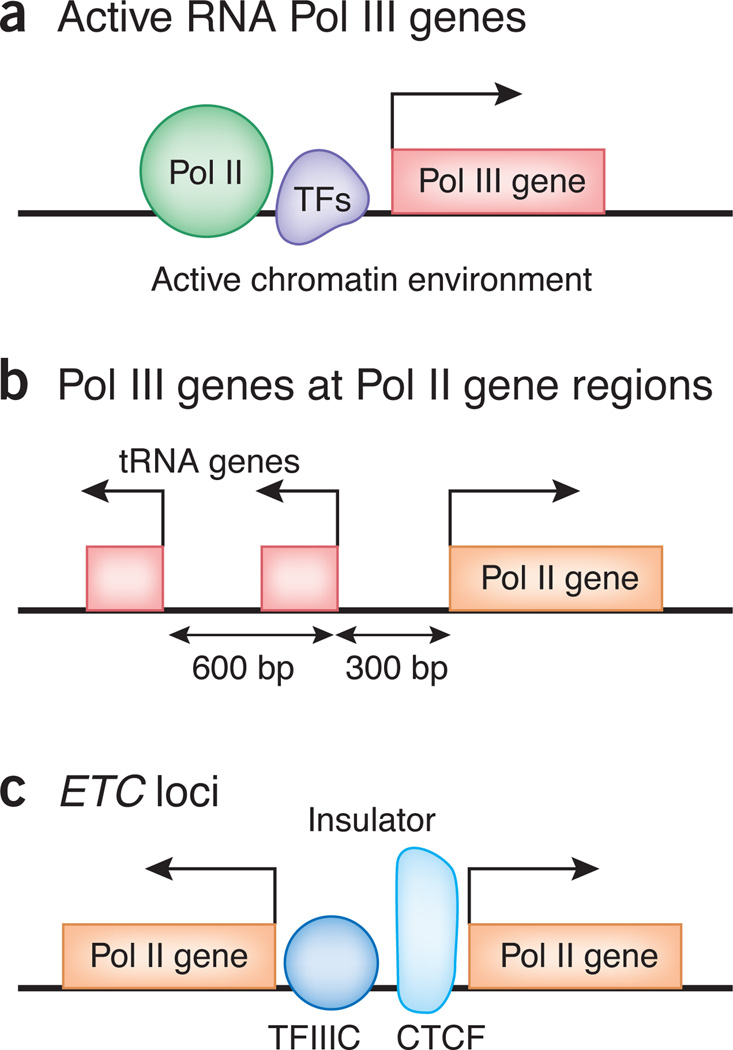
Pol III binds upstream of many Pol II transcribed genes2–5, and TFIIIC is highly overrepresented between closely spaced, divergently transcribed Pol II genes. Unexpectedly, Pol II is found upstream of many transcriptionally active Pol III genes even in Drosophila melanogaster S2 cells2–5 (Fig. 1a). At some of the loci, Pol II activity is necessary for transcription by Pol III, consistent with previous observations that Pol II facilitates Pol III transcription at the U6 gene14.
Figure 1.
Schematic representation of three typical loci exemplifying cross-regulation of Pol III and Pol II transcription. (a) Pol II and transcription factors (TFs) bind active Pol III gene regions. Chromatin marks generally present at active Pol II genes are observed at active Pol III genes. (b) Active tRNA genes, frequently two tandem tRNA genes, are present at Pol II gene promoters. (c) ETC loci, bound by TFIIIC without recruiting Pol III, are often positioned near CTCF binding sites, suggesting the potential role of ETC loci in chromatin insulation.
Another related observation is that 20% of active tRNA genes reside within 1 kilobase of a transcription start site (TSS) of a Pol II–transcribed gene (Fig. 1b). There are usually two tRNA genes, 600 base pairs (bp) apart, divergently oriented from the nearby Pol II gene2,4. The significance of the 600-bp spacing between the two tRNA genes, and the role, if any, of these genes in Pol II gene regulation awaits further studies.
Histone modifications and Pol III transcription
Most active chromatin marks present at Pol II genes are also observed at active Pol III genes, though the precise distribution of the modifications with respect to the Pol III genes is different. Histone acetylation (acetylation of histone H3 Lys9 (H3K9ac), H3K18ac, H4K16ac), methylation (mono-, di- or trimethylation of histone H3 Lys4 (H3K4me1/2/3), H3K27me1, H4K20me1), H2A.Z and H3.3 and CpG islands are all present in the vicinity of active Pol III genes, whereas repressive marks H3K27me3 and H3K9me3 are associated with inactive Pol III genes3,4. This is to be expected for the genes that reside immediately upstream of active Pol II promoters, but the genes that are not near Pol II TSSs also have histone modifications that are typically observed at Pol II–transcribed sites, and it is likely that the chromatin modifications are being targeted to these loci by transcription factors (see below) that bind in their vicinity. These data suggest that Pol III gene transcription occurs if the genes reside within an active chromatin domain, but the genes themselves do not create an active environment.
It is also possible that the Pol III machinery is recruited to poised chromatin domains and then creates additional alterations to the chromatin, enabling Pol III transcription. This would be consistent with the observation that Pol III factors either possess or recruit histone-modifying activities15. The active chromatin structure created by the Pol III machinery and its associated factors, in turn, might stabilize the binding of Pol II factors in the vicinity, generating a self-reinforcing mechanism that leads to stable transcription of both Pol II and Pol III genes.
Binding of transcription factors at Pol III genes
The distribution of various Pol II– and Pol III– specific transcription factors is also very informative. Similar to the yeast system, there is high co-occupancy between Pol III and TFIIIB factors, but this concordance is less pronounced for the TFIIIC subunits3–5. Brf1 and Brf2 are almost entirely mutually exclusive in their distribution, and Brf2-bound sites do not appear to recruit or require TFIIIC for their binding, suggesting that genes using Brf2 (such as U6) may be using other factors for transcription.
Almost two-thirds of tRNA genes have ETS1 or STAT1 bound in their immediate vicinity4, and genes that are not near Pol II promoters but contain ETS1 also bind the acetyltransferase CREB-binding protein. This suggests that ETS1 might help recruit these enzymes, aiding in Pol III transcription. Differences in the ETS1 sequences at various loci suggest that differential regulation by this factor might have a role in tissue-specific regulation of Pol III genes. In addition to ETS1 and STAT1, ‘genome-wide’ mapping of Myc, Fos and Jun indicates that over 75% of Pol III–transcribed genes have these factors bound within 100 bp of the TSS2 (Fig. 1a). It has previously been shown that, in addition to its function in Pol II transcription activation, Myc can stimulate Pol III transcription through its association with TFIIIB (see ref. 16 and references therein). Furthermore, Pol III transcription is upregulated in cancer cells due to increased binding of Myc to TFIIIB, and overexpression of a single tRNA gene has been shown to contribute to cell transformation17, indicating that misregulation of Pol III transcription can have significant consequences. Understanding the functional interplay between Pol II and Pol III factors will aid in a more thorough understanding of oncogenesis.
ETC sites
The vast majority of TFIIIC-bound sites are not found at Pol III–transcribed genes but are located at ETC sites3–5. A substantial number of ETC loci also have binding motifs for the ETS transcription factor, but the role of ETS and its relationship with TFIIIC at these sites remains unknown5. In budding yeast, ETC loci function as heterochromatin barriers blocking the spread of heterochromatin into surrounding euchromatin18,19 whereas ETC loci in fission yeast, referred to as COCs, also function as boundary elements at the matingtype locus20,21. In addition, tRNA genes function as chromatin boundaries in yeast22,23. In mammalian cells, the mouse B2 and human Alu short interspersed elements, which are transcribed by Pol III, have been implicated in insulator activities24. Whether the newly discovered ETC sites in human cells function as insulators is not known, but the strongest TFIIIC-bound loci were within 200 bp of CCCTC-binding factor (CTCF) sites, a protein required for mammalian insulation4,5. Many ETC loci are present between divergently transcribed Pol II genes (Fig. 1c), where they might function as chromatin insulators.
Cross-talk regulation
It had been assumed that the regulation of transcription by the three polymerases is autonomous, with little cross-regulation. However, the current results, along with other data, have begun to question this dogma. Pol III–transcribed RNAs have already been shown to directly regulate Pol II transcription by binding Pol II transcription factors (7SK RNA binding P-TEFb) or the enzyme itself (B2 RNA binding Pol II)25. The current data now raise questions regarding how Pol II promoter and enhancer architecture might be used to regulate the transcription of Pol III genes and also raise questions about gene information flows. It raises further questions about how the chromatin architecture set up at Pol II promoters by Pol II factors affects Pol III transcription and is used by the Pol III machinery to initiate transcription. In yeast, TFIIIC-bound loci regulate Pol II genes by regulating chromatin domains. Do TFIIICbound loci also regulate Pol II genes in human cells, and if so, how?
The role of TBP in recruiting both Pol II and Pol III needs to be further evaluated as well. It was believed that TBP in TFIID recruited Pol II and TBP in TFIIIB recruited Pol III. The current data suggest a more complex scenario. What do the flanking elements and bound factors alter or affect in the recruitment choice by TBP, and how do they exert this effect? Most importantly, what is the role of recruiting both polymerases to specific loci? The recent data even raise the issue of whether Pol II factors like Myc and STAT1 should be recharacterized as ‘general’ transcription activators. Taken together, these new studies revealing the human Pol III transcriptome have just discovered functional interplay between Pol III and Pol II transcription. Future studies addressing the mechanisms of this cross-regulation should lead to new insights into ‘general’ transcriptional regulation.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Ken-ichi Noma, The Wistar Institute, Philadelphia, Pennsylvania, USA. noma@wistar.org.
Rohinton T Kamakaka, Department of Molecular Cell & Developmental Biology, University of California, Santa Cruz, California, USA. rohinton@biology.ucsc.edu.
References
- 1.Paule MR, White RJ. Nucleic Acids Res. 2000;28:1283–1298. doi: 10.1093/nar/28.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raha D, et al. Proc. Natl. Acad. Sci. USA. 2010;107:3639–3644. doi: 10.1073/pnas.0911315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barski A, et al. Nat. Struct. Mol. Biol. 2010;17:629–634. doi: 10.1038/nsmb.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oler AJ, et al. Nat. Struct. Mol. Biol. 2010;17:620–628. doi: 10.1038/nsmb.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moqtaderi Z, et al. Nat. Struct. Mol. Biol. 2010;17:635–640. doi: 10.1038/nsmb.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieci G, Fiorino G, Castelnuovo M, Teichmann M, Pagano A. Trends Genet. 2007;23:614–622. doi: 10.1016/j.tig.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Geiduschek EP, Kassavetis GA. J. Mol. Biol. 2001;310:1–26. doi: 10.1006/jmbi.2001.4732. [DOI] [PubMed] [Google Scholar]
- 8.Moqtaderi Z, Struhl K. Mol. Cell. Biol. 2004;24:4118–4127. doi: 10.1128/MCB.24.10.4118-4127.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts DN, Stewart AJ, Huff JT, Cairns BR. Proc. Natl. Acad. Sci. USA. 2003;100:14695–14700. doi: 10.1073/pnas.2435566100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harismendy O, et al. EMBO J. 2003;22:4738–4747. doi: 10.1093/emboj/cdg466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stutz F, Gouilloud E, Clarkson SG. Genes Dev. 1989;3:1190–1198. doi: 10.1101/gad.3.8.1190. [DOI] [PubMed] [Google Scholar]
- 12.Fairley JA, Scott PH, White RJ. EMBO J. 2003;22:5841–5850. doi: 10.1093/emboj/cdg544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittmar KA, Goodenbour JM, Pan T. PLoS Genet. 2006;2:e221. doi: 10.1371/journal.pgen.0020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Listerman I, Bledau AS, Grishina I, Neugebauer KM. PLoS Genet. 2007;3:e212. doi: 10.1371/journal.pgen.0030212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mertens C, Roeder RG. Mol. Cell. Biol. 2008;28:5764–5776. doi: 10.1128/MCB.01262-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Roman N, et al. Biochem. Soc. Symp. 2006;73:141–154. doi: 10.1042/bss0730141. [DOI] [PubMed] [Google Scholar]
- 17.Marshall L, Kenneth NS, White RJ. Cell. 2008;133:78–89. doi: 10.1016/j.cell.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 18.Simms TA, et al. Eukaryot. Cell. 2008;7:2078–2086. doi: 10.1128/EC.00128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valenzuela L, Dhillon N, Kamakaka RT. Genetics. 2009;183:131–148. doi: 10.1534/genetics.109.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noma K, Cam HP, Maraia RJ, Grewal SI. Cell. 2006;125:859–872. doi: 10.1016/j.cell.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki O, Tanaka A, Tanizawa H, Grewal SI, Noma K. Mol. Biol. Cell. 2010;21:254–265. doi: 10.1091/mbc.E09-09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott KC, Merrett SL, Willard HF. Curr. Biol. 2006;16:119–129. doi: 10.1016/j.cub.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 23.Donze D, Adams CR, Rine J, Kamakaka RT. Genes Dev. 1999;13:698–708. doi: 10.1101/gad.13.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunyak VV, et al. Science. 2007;317:248–251. doi: 10.1126/science.1140871. [DOI] [PubMed] [Google Scholar]
- 25.Goodrich JA, Kugel JF. Nat. Rev. Mol. Cell Biol. 2006;7:612–616. doi: 10.1038/nrm1946. [DOI] [PubMed] [Google Scholar]