Abstract
Background:
A number of drugs have been reported as risk factors for acute lung injury (ALI) and ARDS. However, evidence is largely limited to case reports, and there is a paucity of data on the incidence and outcome of drug-associated ALI (DALI).
Methods:
Using a population-based retrospective cohort study design, critically ill patients with a diagnosis of ALI were studied. These patients were classified as having DALI or non-DALI, based on whether they were exposed to prespecified drugs prior to development of ALI. Outcomes were compared between the two groups and frequencies and incidences reported.
Results:
Among 514 patients with ALI, 49 (9.5%) had DALI with an estimated population-based incidence of 6.6 (95% CI, 4.8-8.5) per 100,000 person-years. Of the 49 patients with DALI, 36 received chemotherapeutic/antiinflammatory agents, and 14 received amiodarone. Twelve patients had no additional risk factors for ALI (probable DALI), whereas 37 had alternative risk factors (possible DALI). Patients with and without DALI had similar baseline characteristics. However, the APACHE (Acute Physiology and Chronic Health Evaluation) III scores (median, 83 vs 70, P = .03), ICU mortality (35% vs 20%, P = .03), and hospital mortality (63% vs 32%, P < .001) were significantly higher in the DALI group compared with those of the non-DALI group. Hospital mortality remained significantly higher after adjusting for APACHE III score on admission and the presence of malignancy in logistic regression analysis (OR, 2.8; 95% CI, 1.3-6.4; P = .009).
Conclusions:
Drugs are important risk factors for ALI, and recognizing them as such may have important implications for early identification of patients at risk, discontinuation of the offending agent, and prognosis.
Acute lung injury (ALI) and ARDS are severe clinical sequelae of both pulmonary and nonpulmonary conditions, including sepsis, shock, aspiration, pneumonia, major trauma, pancreatitis, and massive transfusion (since all patients with ARDS fulfill the criteria for ALI, we will refer to both conditions as ALI unless otherwise specified). ALI is a common clinical manifestation of drug-associated lung diseases. However, its overall incidence remains largely unknown.1
Extensive lists of cytotoxic and noncytotoxic agents have been reported as causes of ALI.1‐9 However, the role of drugs as ALI risk factors is unclear because of the absence of universal diagnostic criteria. Although criteria to guide the diagnosis of adverse drug reactions have been defined, diagnosis remains a challenge.10 The clinical, laboratory, and radiographic features of drug-associated ALI (DALI) are generally indistinguishable from other causes, and specific markers are absent.1,11 Hence, diagnosis rests on the exclusion of other risk factors for ALI.6
Pathophysiologic mechanisms for DALI and ARDS remain unknown for most agents. Idiosyncratic reactions rather than adverse reactions, capillary leak syndrome, or anaphylaxis are postulated explanations.1 Some drugs cause direct damage to the pulmonary tissue by producing reactive oxygen species, whereas others cause indirect damage by releasing inflammatory cytotoxic mediators.12 The latent period from exposure to adverse effect is highly variable for most drugs, ranging from minutes to several years; hence, temporal eligibility is difficult to define. For these reasons, most cases of DALI are considered probable or possible rather than definitive.10 The overall prognosis of DALI appears to be good; however, fatalities have been reported.7,13 Corticosteroids have been used in several instances with good response, although their therapeutic role is uncertain and randomized controlled studies concerning their efficacy are currently lacking.1
There is a paucity of studies in this field and most literature is in the form of case reports and review articles. Incidence and mortality have been defined only for the common precipitating causes of ALI/ARDS and not for drugs.14 The objective of this study is to fill these knowledge gaps using a population-based study design.
Materials and Methods
The Mayo Clinic Institutional Review Board approved the study protocol and waived the need for informed consent (IRB No. 08-007804). This is a retrospective, population-based cohort study of adult Olmsted County, Minnesota, residents. We included all patients ≥ 18 years of age who had been admitted to the ICU (medical, surgical, or mixed) at a tertiary care center over an 8-year period (January 2001 to December 2008). The characteristics of the ICUs and Olmsted County population have been described previously.15 Patients who did not provide research authorization were excluded from the study (about 5%). A previously validated electronic surveillance system, the “ALI sniffer,” was used to screen patients for ALI. This system queries the electronic medical records for matching of Pao2/Fio2 < 300 on arterial blood gas results and radiologist interpretation of chest radiographs for the words “edema” or “bilateral infiltrates” within a 24-h period. It has a sensitivity of 96% (95% CI, 94%-98%), making it an excellent screening tool.16 “Sniffer-positive” patients were confirmed or excluded for ALI by manual review by a team of critical care experts, including clinical/research fellows and staff. The interobserver variability among the reviewers for the year 2006 had a κ value of 0.86.
Drugs potentially related to the development of noncardiogenic pulmonary edema (NCPE) and ALI were identified from an extensive review of the literature and two online platforms (www.drugs.com and www.pneumotox.com) (e-Table 1 (379.6KB, pdf) ). However, we restricted ourselves to chemotherapeutic/antiinflammatory agents, amiodarone, and nitrofurantoin to limit to agents specifically known to cause ALI and not just NCPE (Table 1).1
Table 1.
—Drugs Reported to Cause DALI and Their Frequency of Application Within the Cohort
| Drug | No.a | Drug | No.a |
| Amiodarone | 14 | Leuprorelin | 0 |
| Arsenic trioxide | 0 | Medroxyprogesterone | 0 |
| Basiliximab | 0 | Melphalan | 2 |
| Bleomycin | 3 | Methotrexate | 11 |
| Busulfan | 0 | Mitomycin C | 1 |
| Carmustine (BCNU) | 1 | Nitrofurantoin | 0 |
| Cyclophosphamide | 10 | Muromunab-CD3 | 0 |
| Cyclosporine | 4 | Oxaliplatin | 1 |
| Cytarabine | 5 | Paclitaxel | 3 |
| Docetaxel | 0 | Pentostatin | 0 |
| Doxorubicin | 8 | Recombinant GM-CSF | 0 |
| Fludarabine | 1 | Rituximab | 4 |
| Gefitinib | 0 | Teniposide (VM-26) | 0 |
| Gemcitabine | 2 | Tretinoin/retinoic acid | 0 |
| Gold salts | 0 | Tumor necrosis factor | 0 |
| Immunglobulin | 0 | Vinblastine | 3 |
| Infliximab | 1 | Vincristine | 5 |
| IL-2 | 0 | Vindesine | 0 |
| Irinotecan | 3 | Vinorelbine | 0 |
DALI = drug-associated acute lung injury; GM-CSF = granulocyte monocyte colony-stimulating factor.
Frequency of application.
Records of patients with ALI were reviewed manually to identify patients exposed to one or more of the prespecified drugs. Because of the lack of an established consensus on the length of exposure period, a cutoff of 1 year prior to ALI development was chosen. Exposure time was calculated in each patient from the latest dose of any listed agent received to the development of ALI and the median and interquartile ranges (IQRs) were calculated to estimate the distribution of time courses.
ALI was defined using the standard American-European Consensus Conference criteria.17 All patients in our cohort were true cases of ALI as per this definition. Because of the absence of a consensus definition for DALI, we used the following definition: “development of ALI within 1 year after exposure to any of the pre-specified drugs.” DALI cases were further divided into the following:
•Probable DALI: patients with no established risk factors for ALI except for the specific drug exposure within 1 year
•Possible DALI: patients with at least one risk factor for ALI and history of specific drug exposure within 1 year
•Conditional DALI: patients who, independent of their risk factor status, received drugs that had not been previously reported to cause ALI but that may have been culprits of DALI due to their similarity to known causative agents
•Non-DALI: patients who were not exposed to drugs reported or assumed to cause ALI
The primary outcomes measured were ICU and hospital mortalities. Secondary outcomes included ICU and hospital lengths of stay. Baseline characteristics included demographics such as age, sex, and race, APACHE (Acute Physiology and Chronic Health Evaluation) III scores on admission, Charlson scores, and Sequential Organ Failure Assessment (SOFA) scores on days 1, 2 and 3. Traditionally identified risk factors for ALI, such as sepsis, septic shock, pneumonia, pancreatitis, trauma, massive blood transfusion, and gastric aspiration, were reported. Other baseline data on malignancies, including solid tumor, metastatic cancer, leukemia, lymphoma, and multiple myeloma, and history of bone marrow transplant were also reported. The collection of baseline and outcome data was done blinded to the DALI status.
Statistical Analysis
Primary analysis compared probable and possible DALI with non-DALI. Additional sensitivity analyses were performed to compare different definitions of DALI (probable, possible, and conditional) with non-DALI.
Age- and sex-adjusted incidence rates for DALI were calculated using the incident cases as the numerator. The denominator was the age- and sex-specific person-years derived from Olmsted County decennial census figures from the year 2000 and based on an expected 1.9% population growth per year. Continuous data were summarized as median (IQR), and categorical data were summarized as frequencies (percentage) for each group. The Wilcoxon rank sum test was used for comparisons between continuous variables, whereas the Fisher exact test was used for comparisons between categorical data. Multivariate logistic regression analysis was used to examine the association between the development of DALI and hospital mortality adjusted by APACHE III score on admission and the presence of malignancies. JMP statistical software (version 8.0; SAS Institute, Inc) was used for all data analyses. A P value < .05 was considered statistically significant.
Results
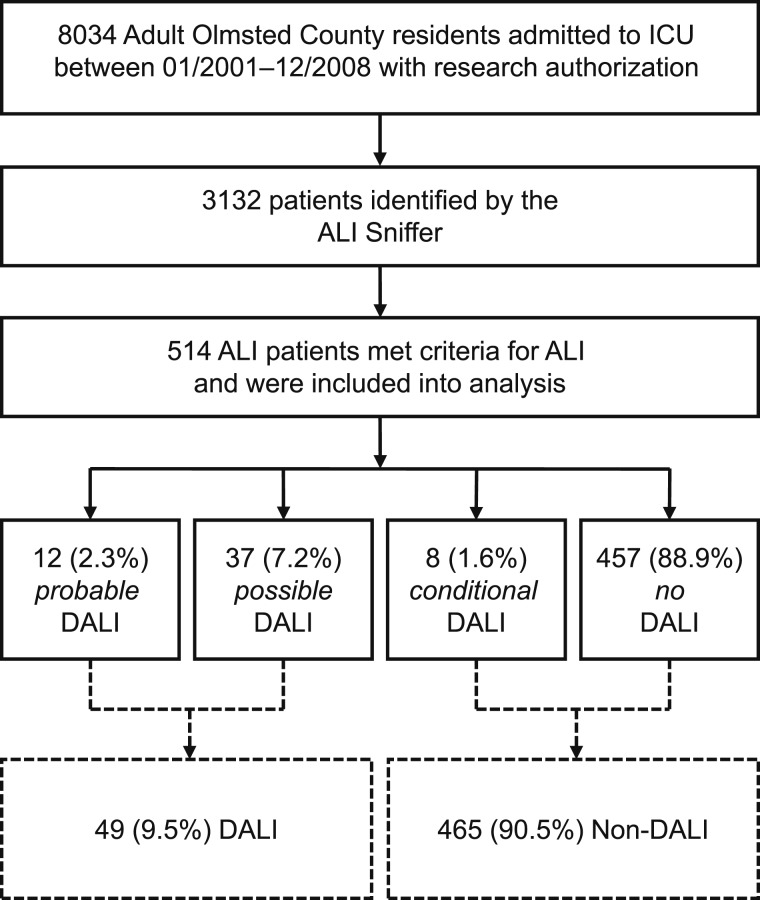
Of 8,034 Olmsted County residents who required ICU care between 2001 and 2008, 3,132 were identified with the ALI sniffer, and 514 met the criteria for ALI. Among these were 12 (2.3%) probable DALI, 37 (7.2%) possible DALI, eight (1.6%) conditional DALI, and 457 (88.9%) no DALI (Fig 1). Thus, the frequency of DALI (possible and probable) in this population-based ALI cohort was 9.5%, with an estimated population-based incidence of 6.6 per 100,000 person-years (95% CI, 4.8-8.5).
Figure 1.
Study flowchart for the main analysis. The probable- and possible-DALI groups were combined (DALI) and compared with the conditional- and non-DALI groups (non-DALI). ALI = acute lung injury; DALI = drug-associated acute lung injury.
The median time from the last dose of the last culprit drug to the development of ALI was 2 days (IQR, 1-58 days). Twenty-seven patients (55%) progressed within 72 h, and 14 patients (28%) progressed in > 4 weeks.
Most antineoplastic and antiinflammatory agents identified in our cohort were drugs reported in the past as causes of ALI (Table 1). We also found other drugs from these two classes that had not been reported previously that might be potential risk factors (included as conditional DALI) (Table 2). Many drugs, especially the chemotherapeutic agents, were given as combination therapy. Of the 49 patients with DALI, 36 (73.5%) had received chemotherapeutic/antiinflammatory agents and 14 (28.6%) amiodarone (e-Table 2 (379.6KB, pdf) ).
Table 2.
—Drugs Not Previously Reported to Cause DALI and Their Frequency of Application Within the Cohort
| Drug | No.a | Drug | No.a |
| Alvocidib (Flavopiridol) | 1 | Idarubicin | 3 |
| Azathioprine | 1 | Ifosphamide | 1 |
| Carboplatin | 4 | Leucovorin | 3 |
| Cisplatin | 6 | Mycophenolate | 1 |
| Dacarbazine | 1 | Sunitinib | 1 |
| Etoposide | 4 | Temozolamide | 2 |
| 5-Flurouracil | 7 |
See Table 1 for expansion of abbreviation.
Frequency of application.
The baseline characteristics and outcomes among the probable-DALI, possible-DALI, conditional-DALI, and non-DALI groups are outlined in Table 3. Patients with and without drug exposure were similar with regards to age, sex, race, comorbidities (Charlson score), and traditional risk factors for ALI except for trauma, which was significantly higher in the non-DALI group. The DALI group had significantly higher severity of illness based on APACHE III scores and significantly higher SOFA scores on days 1 and 3. All malignancies were higher in the DALI group.
Table 3.
—Baseline Characteristics and Outcomes in (Probable, Possible, and Conditional) Patients With and Without DALI
| Characteristic | DALI |
No DALI (n = 457) |
||
| DALI (n = 12) | Possible (n = 37) | Conditional (n = 8) | ||
| Age, y | 67 (57-82) | 64 (54-73) | 45 (36-58) | 66 (49-79) |
| Sex, female | 3 (25) | 18 (49) | 4 (50) | 201 (44) |
| Race, white | 11 (92) | 31 (84) | 8 (100) | 386 (84) |
| Charlson score | 5 (4-7) | 4 (2-5) | 3 (2-6) | 3 (2-5) |
| APACHE III on admission | 81 (70-93) | 83 (65-110) | 90 (37-132) | 70 (52-94) |
| SOFA day 1 | 6 (4-8) | 8 (4-10) | 8 (3-10) | 5 (1-8)a |
| SOFA day 2 | 4 (1-7) | 6 (3-8) | 9 (3-11) | 4 (1-7) |
| SOFA day 3 | 5 (4-6) | 6 (3-9) | 11 (3-13) | 4 (1-6)a |
| Pancreatitis | 0 | 1 (3) | 0 | 18 (4) |
| Pneumonia | 6 (50) | 17 (46) | 3 (38) | 149 (33) |
| Sepsis | 4 (33) | 13 (35) | 1 (13) | 127 (28) |
| Trauma | 0 | 1 (3) | 1 (13) | 82 (18)b |
| Multiple transfusion | 4 (33) | 6 (16) | 1 (13) | 71 (16) |
| Shock | 6 (50) | 21 (57) | 2 (25) | 211 (46) |
| Solid cancer | 7 (58) | 12 (32) | 5 (63) | 71 (16)b |
| Metastatic cancer | 3 (25) | 6 (16) | 1 (13) | 12 (3)b |
| BMT | 0 | 6 (16) | 0 | 1 (0.22)b |
| Leukemia | 1 (8) | 5 (14) | 0 | 7 (2)b |
| Lymphoma | 0 | 6 (16) | 0 | 8 (2)b |
| Multiple myeloma | 0 | 3 (8) | 0 | 1 (0.22)b |
| Median time from drug exposure to development of ARDS, d | 3 (1-59) | 1 (1-58) | 15 (2-98) | |
| Outcomes | ||||
| ICU LOS, d | 11 (7-20) | 9 (3-25) | 12 (6-29) | 9 (5-17) |
| Hospital LOS, d | 26 (13-34) | 24 (6-37) | 19 (12-49) | 16 (9-29) |
| ICU mortality | 3 (25) | 14 (38) | 1 (14) | 88 (21)a |
| Hospital mortality | 5 (42) | 26 (70) | 1 (13) | 147 (32)b |
Data are presented as median (IQR) or No. (%). APACHE = Acute Physiology and Chronic Health Evaluation; BMT=bone marrow transplant; IQR = interquartile range; LOS = length of stay; SOFA = Sequential Organ Failure Assessment. See Table 1 for expansion of other abbreviation.
Non-DALI significantly different from probable and possible DALI (P < .05).
Non-DALI significantly different from probable and possible DALI (P < .001).
Among the outcome variables, ICU and hospital mortalities were both significantly higher in the DALI vs non-DALI group (35% vs 20%, P = .03 and 63% vs 32%, P < .001, respectively). ICU and hospital lengths of stay showed a similar trend without reaching statistical significance. When conditional cases were included in the DALI group, the results were similar (e-Table 3 (379.6KB, pdf) ).
After adjusting for the presence of malignancies and APACHE III score on admission in the logistic regression analysis, the development of DALI was independently associated with hospital mortality (OR, 2.8; 95% CI, 1.3-6.4; P = .009). Patients with DALI received significantly more steroids than did patients without DALI (42% vs 28%, P = .03), but a small sample size and limited data precluded meaningful efficacy analysis in these subgroups (for details, see e-Table 4 (379.6KB, pdf) ).
Discussion
In this population-based cohort of patients with ALI, exposure to potentially injurious drugs was common and was associated with a history of malignancy, higher severity of illness, and worse clinical outcomes. Few studies have been done to study the frequency or incidence of DALI. One study suggested that 10% of cases of diffuse alveolar damage diagnosed by surgical lung biopsy were caused by drugs.18 This is comparable to an incidence of 9.3% in our study. Other studies on the incidence of DALI are only in the form of case reports for individual drugs. Our study is unique in that it provides an overall estimate of the incidence of DALI. However, a significant limitation is that the possible-DALI group may not reflect the true frequency of DALI, thereby increasing the number of false-positives in our assessment of the frequency. This limitation remains a challenge because attributing causality is an unavoidable problem in drug-related studies because of the presence of multiple confounders, the absence of gold-standard diagnostic tests, and, in this case, the retrospective nature of our study. The sample size of the probable-DALI group, which more accurately reflects true DALI, was very small in our study (12 patients), thereby limiting meaningful analysis. Based on our results, further studies using a matched-case control design are warranted to further explore the relation between specific drugs and ALI.
In our study, only 14 of 514 patients (2.7%) had amiodarone-related ALI, of whom only five (1.1%) had probable amiodarone-induced ALI. We included all patients with ALI who had received amiodarone and not just the ones with a high risk of toxicity (postsurgical patients receiving supplemental oxygen, which may potentiate acute amiodarone toxicity).19 This may explain the low overall frequency of amiodarone-induced lung injury that was observed in our cohort.
We gathered data on chemotherapeutic/antiinflammatory agents that have not been reported in the past to study whether these drugs could be potential risk factors for DALI as well. However, most of these drugs were given as combination chemotherapy; hence, the role of individual drugs in the cause of ALI is difficult to ascertain. These agents may have a synergistic effect when used in combination that may further compound the risk of ALI, even if they may not cause ALI when used as monotherapy. Such synergism has been reported for 5-fluorouracil with mitomycin and oxaliplatin.20,21 5-Fluorouracil as monotherapy does not cause diffuse alveolar damage.7 Of note, platinum-based drugs, including cisplatin and carboplatin, were reported in 10 patients with ALI in our study. These agents may have a similar synergistic effect, but further studies are needed to ascertain whether they could be individual risk factors for ALI. Closer observation of patients receiving these newly found drugs (as mono- or combination therapy) is warranted.
Patients in the DALI group, both with the exclusion and the inclusion of the conditional group, were more severely ill and had higher cancer prevalence, SOFA scores, and mortality compared with the non-DALI group. ICU and hospital mortality remained significantly higher after adjusting for the presence of malignancy and severity of illness (APACHE III). However, residual confounding still remains an issue because of the discrepancy in the malignancy rates between the two groups, which cannot be completely adjusted by regression analysis. An alternative explanation is provided by a possible synergistic or additive effect of drugs in association with other risk factors for the development or outcome of ALI. The lungs of patients exposed to certain drugs may be “primed” and the addition of another risk factor, such as sepsis or shock, may accelerate the development of ALI (multiple hit hypothesis) so that these patients may have more severe lung damage. Previous studies have shown that the outcome of pure drug-induced ALI is variable and in many cases resolves quickly after discontinuation of the drug, although fatalities have been reported.3,7,13,22,23 In our cohort, mortality among patients with probable DALI was high (42%), but not as high as that in patients with additional ALI risk factors (possible DALI, 70%) (Table 3). The small sample size precluded adjusted mortality analysis in this important subgroup.
The higher mortality of the DALI group may be attributable to drugs such as bleomycin, cyclophosphamide, and amiodarone, which can directly damage lung tissue in the presence of high oxygen by producing reactive oxygen species.12 Patients with traditional risk factors for ALI/ARDS usually require high oxygen, which may exacerbate the drug-induced effect, thereby increasing mortality.
Corticosteroids are widely used in the setting of DALI but randomized control data as to their efficacy are lacking. In our cohort, patients with DALI did receive significantly more corticosteroids than did patients without DALI, but the response rate (Pao2/Fio2 ratio improvement) was the same in both groups (e-Table 3 (379.6KB, pdf) ). It is likely that the response to corticosteroids may be related to both the injurious drug as well as other confounding risk factors. Because of the retrospective nature of our study and a small sample size, no firm conclusions can be reached as to the efficacy of corticosteroids in DALI vs other forms of ALI.
The median time from last drug exposure to ALI development of 2 days, with the majority of patients progressing over 72 h, supports the predominant mechanism of ALI (acute NCPE) in the large proportion of our patients. However, in a significant minority of patients in our cohort (28%), the time from drug exposure to ALI development was more than 4 weeks, suggesting that at least some of those may represent a subacute drug-induced pneumonitis, rather than true ALI. The longer time frame for drug exposure in our study increases the sensitivity for DALI, yet the trade-off is potential overestimation of its true incidence in this cohort. On the other hand, by restricting drugs to include only chemotherapeutic agents, amiodarone, and nitrofurantoin, but not other agents, we may possibly be underestimating the true burden of DALI.
Data on cumulative dose, concurrent or previous radiotherapy, other cytotoxic drug therapy, or preexisting pulmonary disease were not collected or were unavailable. These are known risk factors for certain drugs and may affect outcome. Additional limitations are inherent to a single population retrospective study design with limited generalizability and high likelihood of unmeasured confounding.
Conclusions
Drugs are important risk factors for ALI and must be recognized as relevant causative agents, especially when used in combination and/or in the presence of other risk factors for ALI. This may be useful to identify patients at high risk of DALI who require close monitoring, to facilitate early discontinuation of offending agents, and to develop strategies for limiting lung damage. It may also have prognostic implications.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Dhokarh had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Dhokarh: contributed to study design and conduct and manuscript writing.
Dr Li: contributed to study design and conduct and manuscript writing.
Dr Schmickl: contributed to the conduct of the study and manuscript writing.
Dr Kashyap: contributed to conduct of the study and revision of the manuscript.
Dr Assudani: contributed to conduct of the study and revision of the manuscript.
Dr Limper: contributed to study design and revision of the manuscript.
Dr Gajic: contributed to supervision and was involved as senior author in all critical parts of the study.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributors: We thank all members of the METRIC group for their input and support. The study was performed at Mayo Clinic, Rochester, MN.
Additional information: The e-Tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- ALI
acute lung injury
- APACHE
Acute Physiology and Chronic Health Evaluation
- DALI
drug-associated acute lung injury
- IQR
interquartile range
- NCPE
noncardiogenic pulmonary edema
- SOFA
Sequential Organ Failure Assessment
Funding/Support: This work was supported in part by the National Institutes of Health [LM10468Z-01] and by the Mayo Clinic, Rochester, MN.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Lee-Chiong T, Jr, Matthay RA. Drug-induced pulmonary edema and acute respiratory distress syndrome. Clin Chest Med. 2004;25(1):95-104 [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulou I, Bamias A, Lyberopoulos P, Dimopoulos MA. Pulmonary toxicity from novel antineoplastic agents. Ann Oncol. 2006;17(3):372-379 [DOI] [PubMed] [Google Scholar]
- 3.Cooper JA, Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 1: cytotoxic drugs. Am Rev Respir Dis. 1986;133(2):321-340 [DOI] [PubMed] [Google Scholar]
- 4.Reed CR, Glauser FL. Drug-induced noncardiogenic pulmonary edema. Chest. 1991;100(4):1120-1124 [DOI] [PubMed] [Google Scholar]
- 5.Rosenow EC, III, Myers JL, Swensen SJ, Pisani RJ. Drug-induced pulmonary disease. An update. Chest. 1992;102(1):239-250 [DOI] [PubMed] [Google Scholar]
- 6.Limper AH. Chemotherapy-induced lung disease. Clin Chest Med. 2004;25(1):53-64 [DOI] [PubMed] [Google Scholar]
- 7.Rosenow EC, III, Limper AH. Drug-induced pulmonary disease. Semin Respir Infect. 1995;10(2):86-95 [PubMed] [Google Scholar]
- 8.Cooper JA, Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 2: noncytotoxic drugs. Am Rev Respir Dis. 1986;133(3):488-505 [DOI] [PubMed] [Google Scholar]
- 9. Pneumotox on line. The drug-induced lung diseases. http://www.pneumotox.com. Accessed August 2, 2011.
- 10.Irey NS. Teaching monograph. Tissue reactions to drugs. Am J Pathol. 1976;82(3):613-647 [PMC free article] [PubMed] [Google Scholar]
- 11.Silva CI, Müller NL. Drug-induced lung diseases: most common reaction patterns and corresponding high-resolution CT manifestations. Semin Ultrasound CT MR. 2006;27(2):111-116 [DOI] [PubMed] [Google Scholar]
- 12.Cooper JA, Jr, Matthay RA. Drug-induced pulmonary disease. Dis Mon. 1987;33(2):61-120 [DOI] [PubMed] [Google Scholar]
- 13.Erasmus JJ, McAdams HP, Rossi SE. Drug-induced lung injury. Semin Roentgenol. 2002;37(1):72-81 [DOI] [PubMed] [Google Scholar]
- 14.Leaver SK, Evans TW. Acute respiratory distress syndrome. BMJ. 2007;335(7616):389-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Malinchoc M, Cartin-Ceba R, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med. 2011;183(1):59-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herasevich V, Yilmaz M, Khan H, Hubmayr RD, Gajic O. Validation of an electronic surveillance system for acute lung injury. Intensive Care Med. 2009;35(6):1018-1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard GR, Artigas A, Brigham KL, et al. ; Consensus Committee Report of the American-European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. J Crit Care. 1994;9(1):72-81 [DOI] [PubMed] [Google Scholar]
- 18.Parambil JG, Myers JL, Aubry MC, Ryu JH. Causes and prognosis of diffuse alveolar damage diagnosed on surgical lung biopsy. Chest. 2007;132(1):50-57 [DOI] [PubMed] [Google Scholar]
- 19.Van Mieghem W, Coolen L, Malysse I, Lacquet LM, Deneffe GJ, Demedts MG. Amiodarone and the development of ARDS after lung surgery. Chest. 1994;105(6):1642-1645 [DOI] [PubMed] [Google Scholar]
- 20.Yamada K, Ikehara M, Tanaka G, Nomura I, Oshita F, Noda K. Dose escalation study of paclitaxel in combination with fixed-dose irinotecan in patients with advanced non-small cell lung cancer (JCOG 9807). Oncology. 2004;66(2):94-100 [DOI] [PubMed] [Google Scholar]
- 21.Trisolini R, Lazzari Agli L, Tassinari D, et al. Acute lung injury associated with 5-fluorouracil and oxaliplatinum combined chemotherapy. Eur Respir J. 2001;18(1):243-245 [PubMed] [Google Scholar]
- 22.Jehn U, Göldel N, Rienmüller R, Wilmanns W. Non-cardiogenic pulmonary edema complicating intermediate and high-dose Ara C treatment for relapsed acute leukemia. Med Oncol Tumor Pharmacother. 1988;5(1):41-47 [DOI] [PubMed] [Google Scholar]
- 23.Andersson BS, Luna MA, Yee C, Hui KK, Keating MJ, McCredie KB. Fatal pulmonary failure complicating high-dose cytosine arabinoside therapy in acute leukemia. Cancer. 1990;65(5):1079-1084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement