Abstract
Background:
Limited data are available on the effects of air travel in patients with pulmonary hypertension (PH), despite their risk of physiologic compromise. We sought to quantify the incidence and severity of hypoxemia experienced by people with PH during commercial air travel.
Methods:
We recruited 34 participants for a prospective observational study during which cabin pressure, oxygen saturation (Spo2), heart rate, and symptoms were documented serially at multiple predefined time points throughout commercial flights. Oxygen desaturation was defined as Spo2 < 85%.
Results:
Median flight duration was 3.6 h (range, 1.0-7.3 h). Mean ± SD cabin pressure at cruising altitude was equivalent to the pressure 1,968 ± 371 m (6,456 ± 1,218 ft) above sea level (ASL) (maximum altitude = 2,621 m [8,600 ft] ASL). Median change in Spo2 from sea level to cruising altitude was −4.9% (range, 2.0% to −15.8%). Nine subjects (26% [95% CI, 12%-38%]) experienced oxygen desaturation during flight (minimum Spo2 = 74%). Thirteen subjects (38%) reported symptoms during flight, of whom five also experienced desaturations. Oxygen desaturation was associated with cabin pressures equivalent to > 1,829 m (6,000 ft) ASL, ambulation, and flight duration (all P values < .05).
Conclusions:
Hypoxemia is common among people with PH traveling by air, occurring in one in four people studied. Hypoxemia was associated with lower cabin pressures, ambulation during flight, and longer flight duration. Patients with PH who will be traveling on flights of longer duration or who have a history of oxygen use, including nocturnal use only, should be evaluated for supplemental in-flight oxygen.
Each year, as many as 800 million people travel by commercial aircraft worldwide.1 To ensure passenger safety on US carriers, the Federal Aviation Administration requires that cabin pressure during flight be maintained at pressures equivalent to an altitude of ≤ 2,438 m (8,000 ft) above sea level (ASL). Passengers with chronic lung disease, however, may experience significant hypoxemia and physiologic stress resulting from changes in cabin pressure even within these mandated limits.2 Among those with lung disease, people with pulmonary hypertension (PH) are at particularly high risk of adverse effects due to hypoxic pulmonary vasoconstriction.3 Indeed, even modest levels of hypoxemia experienced during flight could result in further elevation of pulmonary artery pressures, thus leading to increased myocardial oxygen demand and hemodynamic compromise.
In contrast to the wealth of data available for healthy subjects, studies on the effects of altitude in people with lung disease are relatively sparse and are particularly lacking in PH. Limited studies are small (often < 20 subjects) and focus almost exclusively on patients with COPD.4,5 The extent to which such results can be extrapolated to other pulmonary conditions is unclear. A single study conducted among patients with restrictive lung disease suggests that they respond differently to air travel than do those with COPD.6,7 Not only are the data in patients with lung disease limited, but most studies have relied on laboratory-based approaches employing either hypoxia challenge or hypobaric chamber testing.4 Such controlled experimental conditions fail to reproduce the dynamic and often complex environmental changes to which passengers are exposed during commercial air travel.
To effectively advise patients with PH who anticipate air travel, health providers require a better understanding of the environmental exposures and physiologic stresses experienced by such patients during an actual flight. To address this knowledge gap, we administered a structured flight history survey among 60 patients diagnosed and treated for PH. Among those subjects, we recruited 34 subjects with prearranged travel plans to participate in a prospective observational study during which cabin pressure, oxygen saturation (Spo2), heart rate (HR), and symptoms were assessed at multiple predefined time points during commercial flights of varying duration.
Materials and Methods
Subject Recruitment and Survey
We invited 60 patients who had an established diagnosis of PH (World Health Organization [WHO] group I or IV) to participate in a survey assessing their personal flight history and eligibility for a prospective observational study on the effects of air travel in PH. Recruitment took place at an international PH conference (June 2010). Those completing the survey were invited to enroll in the prospective study if they had prearranged travel plans on a commercial airline within the next 90 days. Exclusion criteria were a resting Spo2 by pulse oximetry < 90% on room air or on oxygen as prescribed, resting HR > 110, inability to walk continuously for 6 min, or any history of a medical emergency occurring during air travel. All survey participants signed a written informed consent form as part of protocols approved by the institutional review board at the University of California San Francisco (IRB No. 10-00061).
Procedures and Measurements
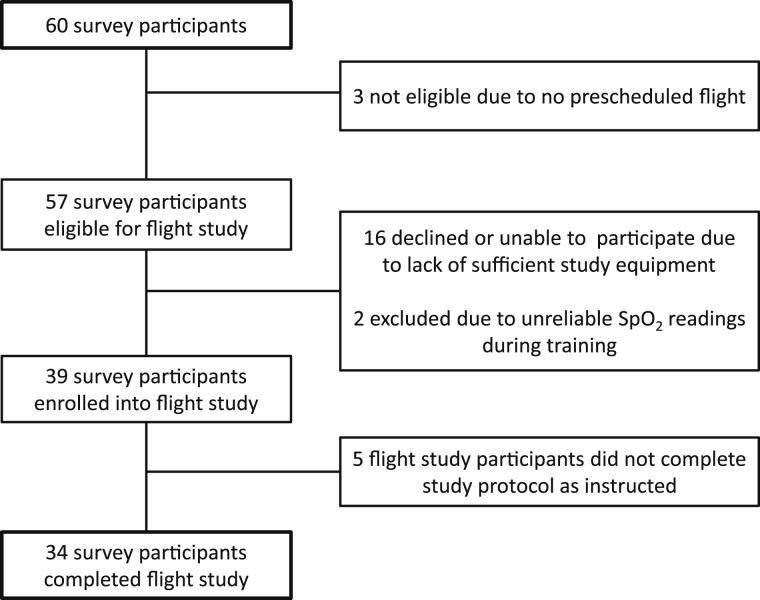
Among 60 subjects who completed the survey, three did not have a prescheduled flight (Fig 1). Of the 57 survey participants eligible to participate in the prospective flight study, 16 declined or were unable to participate because of lack of sufficient study equipment. Two participants with Raynaud phenomenon interfering with Spo2 readings were excluded. No subjects were precluded from participation based on the exclusion criteria noted previously. Prior to air travel, each subject was supplied with a CMS-50DL fingertip pulse oximeter (FaceLake) and a SUN-203F handheld analog altimeter (Sun Company). All eligible subjects completed a 15-min training session, during which they were instructed on how to use the equipment and then asked to perform a series of sample readings. During the training session, accuracy of the fingertip pulse oximeter was verified for each subject using a Nellcor N20P handheld pulse oximeter (Covidien-Nellcor).
Figure 1.
Flow of study enrollment. Spo2 = oxygen saturation.
Enrolled subjects were each given a flight log and instructed to perform 10 sets of recordings for Spo2, HR, and cabin pressure (measured as equivalent altitude ASL) at different stages during the flight: (1) preboarding, (2) seated before take-off, (3) during ascent, (4) at cruising altitude, (5) 15 min after reaching cruising altitude, (6) after walking to the lavatory (performed per protocol), (7) at initial descent, (8) prior to final descent, (9) immediately after landing, and (10) at final destination. Participants were also allowed to perform up to two additional discretionary sets of readings at cruising altitude. To avoid errant Spo2 readings, subjects were instructed to perform four replicate readings of HR and Spo2 at 20 s intervals for each set of recordings. In addition, participants were asked to record any symptoms occurring during flight in a corresponding diary. Information on supplemental oxygen use during flight was also noted by the participants. Five of the 39 subjects enrolled did not complete the flight protocol as instructed and, thus, were excluded from further analysis (Fig 1).
Retrospective Chart Review
From the 34 subjects who completed the flight study, we requested all relevant medical records from their treating physicians after participant consent. Clinical data, including hemodynamic measurements at the time of diagnostic right-sided heart catheterization, recent echocardiographic data, and 6-min walk distance, were abstracted from each participant’s medical record, when available.
Data Analysis
To calculate a single Spo2 and HR for each flight stage for each subject, we calculated the mean of four replicate readings. If, however, the most extreme value deviated by > 1 SD from the mean, we discarded that value, and recalculated the mean based on the remaining three measurements. We defined a meaningful desaturation as a mean Spo2 < 85% during any stage of flight. We calculated the 95% CI for the observed proportion of participants with desaturation using the standard sample-size-based equation. Comparisons among groups for continuous variables were performed using t tests or a Wilcoxon rank sum test. Comparisons among groups for categorical data were performed using Fisher exact tests. Relationships among continuous variables were modeled using general estimating equations with robust SEs to account for repeated measures. All P values presented are for two-tailed probabilities.
Results
The demographic and clinical characteristics of the participants are shown in Table 1. The study population was predominantly female, and the majority had idiopathic pulmonary arterial hypertension. Approximately 90% of the subjects were listed as WHO functional class II-III. All the participants were receiving PH-specific therapy; nearly one-third were receiving continuous prostacyclin infusion. There were no statistically significant differences in demographics or clinical characteristics between subjects who participated in the flight study (n = 34) and those who did not (survey-only participants, n = 26).
Table 1.
—Subject Characteristics
| Characteristic | Total (N = 60) | Flight Participants (n = 34) | Survey-Only Participants (n = 26) |
| Age, y | 50 ± 13 | 49 ± 11 | 51 ± 14 |
| Sex | |||
| Female | 48 (80) | 28 (82) | 20 (77) |
| Race/ethnicity | |||
| White, non-Hispanic | 47 (78) | 30 (88) | 17 (65) |
| Black | 4 (7) | 1 (3) | 3 (11) |
| Hispanic | 4 (7) | 1 (3) | 3 (11) |
| Asian | 3 (5) | 2 (6) | 1 (4) |
| Other | 2 (3) | 0 (0) | 2 (8) |
| Cause of pulmonary hypertension | |||
| Idiopathic/heritable pulmonary arterial hypertension | 36 (60) | 21 (62) | 15 (58) |
| Associated pulmonary arterial hypertension | |||
| Connective tissue disease | 10 (17) | 6 (18) | 4 (15) |
| Portal hypertension | 1 (2) | 1 (3) | 0 (0) |
| Congenital heart disease | 3 (5) | 0 (0) | 3 (11) |
| Drug induced | 1 (2) | 1 (3) | 0 (0) |
| Other/unsure | 4 (7) | 2 (6) | 2 (8) |
| Chronic thromboembolic pulmonary hypertension | 5 (8) | 3 (9) | 2 (8) |
| WHO functional class | |||
| Class I | 5 (8) | 2 (6) | 3 (11) |
| Class II | 30 (50) | 16 (47) | 14 (54) |
| Class III | 24 (40) | 15 (44) | 9 (35) |
| Class IV | 1 (2) | 1 (3) | 0 (0) |
| Clinical dataa | |||
| 6-min walk distance (n = 21), m | … | 453 ± 115 | … |
| Estimated RVSP by echocardiogram (n = 22), mm Hg | … | 72 ± 42 | … |
| MPAP by right-sided heart catheterization (n = 19), mm Hg | … | 49 ± 15 | … |
| Pulmonary hypertension therapyb | |||
| IV/SQ prostacyclin | 16 (27) | 11 (32) | 5 (19) |
| Inhaled prostacyclin | 6 (10) | 5 (15) | 1 (4) |
| Endothelin receptor antagonist | 27 (46) | 16 (47) | 11 (44) |
| Phosphodiesterase-5 inhibitor | 36 (61) | 21 (62) | 15 (60) |
| Combination therapy (≥ 2 classes) | 30 (51) | 20 (59) | 10 (40) |
Data are presented as No. (%) or mean ± SD. MPAP = mean pulmonary artery pressure; RVSP = right-sided ventricular systolic pressure; SQ = subcutaneous; WHO = World Health Organization.
Abstracted from subjects’ medical records.
Not reported for one subject.
Air travel statistics obtained by flight history survey are summarized in Table 2. The median number of round-trip flights in the previous year was two, ranging up to 12 flights. Approximately one-third of the subjects reported being evaluated for flight safety by their treating physician. There was substantial variability in what such safety evaluations entailed, and only one in 10 respondents reported having undergone an altitude simulation test. More than one-third of the subjects reported prior use of oxygen during air travel. Air travel variables for the flight study protocol participants did not differ significantly from those who completed the survey only.
Table 2.
—Flight History Survey Responses
| Characteristic | Total (N = 60) | Flight Participants (n = 34) | Survey-Only Participants (n = 26) |
| No. round trip flights/ya | 2 (0-12) | 2 (0-12) | 2 (0-5) |
| Duration of flight(s), h | 4 (1-10) | 4 (1-10) | 4 (2-9) |
| Evaluated for flight safetya,b | 22 (37) | 10 (29) | 12 (46) |
| Oxygen saturation | 15 (25) | 6 (18) | 9 (35) |
| Arterial blood gas | 9 (15) | 2 (6) | 7 (27) |
| Predictive equation | 2 (3) | 1 (3) | 1 (4) |
| Pulmonary function tests | 7 (12) | 2 (6) | 5 (19) |
| Altitude simulation test | 6 (10) | 4 (12) | 2 (8) |
| Not sure | 1 (2) | 0 (0) | 1 (4) |
| Other | 3 (5) | 2 (6) | 1 (4) |
| History of symptoms during flightb | 29 (48) | 19 (56) | 10 (38) |
| Chest pain | 11 (18) | 9 (26) | 2 (8) |
| Dyspnea | 19 (32) | 11 (32) | 8 (32) |
| Light-headedness | 9 (15) | 6 (18) | 3 (12) |
| Prescribed home oxygen | 32 (53) | 16 (47) | 16 (62) |
| Continuous oxygen use | 7 (12) | 2 (6) | 5 (19) |
| Oxygen with activity | 7 (12) | 2 (6) | 5 (19) |
| Oxygen use at night | 18 (30) | 12 (35) | 6 (23) |
| Liters of oxygen | 3 (2-4) | 3 (2-4) | 2 (2-3) |
| Prior use of in-flight oxygenb | 22 (37) | 11 (32) | 11 (42) |
| Compressed oxygen | 11 (18) | 6 (18) | 5 (19) |
| Portable oxygen concentrator | 20 (33) | 9 (26) | 11 (42) |
Data are presented as median (range) or No. (%).
Not reported for one subject.
Responses not mutually exclusive.
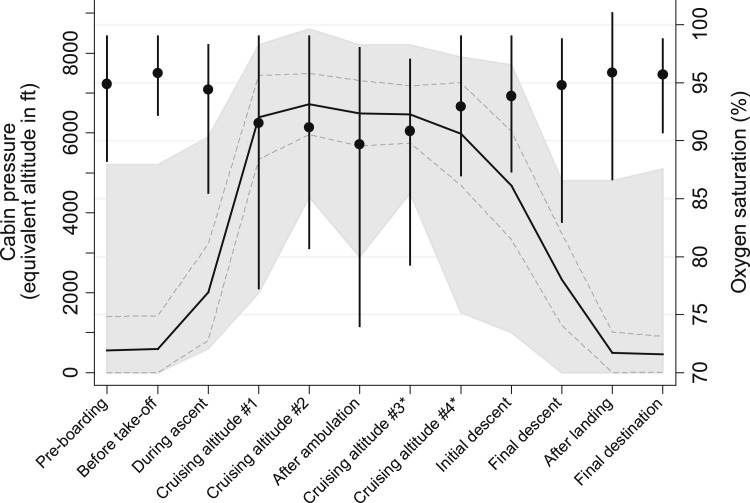
Mean changes in cabin pressure and Spo2 among the 34 who participated in the flight portion of the study are depicted in Figure 2. Median flight duration was approximately 3.6 h (range, 1.0-7.3 h). Mean cabin pressure at cruising altitude was 1,968 ± 371 m (6,456 ± 1,218 ft) ASL, but in some cases reached as high as 2,621 m (8,600 ft) ASL. Median change in resting Spo2 from sea level to cruising altitude was −4.9% (range, +2.0% to −15.8%). The lowest reported Spo2 values occurred after ambulation at cruising altitude, with participants documenting Spo2 values as low as 74%. Thirteen flight participants (38%) reported the development of symptoms during flight: six (18%), chest pressure/tightness; three (9%), increased dyspnea; four (12%), light-headedness; and two (6%), palpitations. Having any symptoms was not significantly associated with desaturation (P = .25).
Figure 2.
Cabin pressure and oxygen saturation measured at various time points during commercial air travel. Solid black line = mean cabin pressure (dashed gray line represents ± 1.0 SD and light gray shading represents range); ● = mean oxygen saturation (vertical bars represent range). *Optional readings.
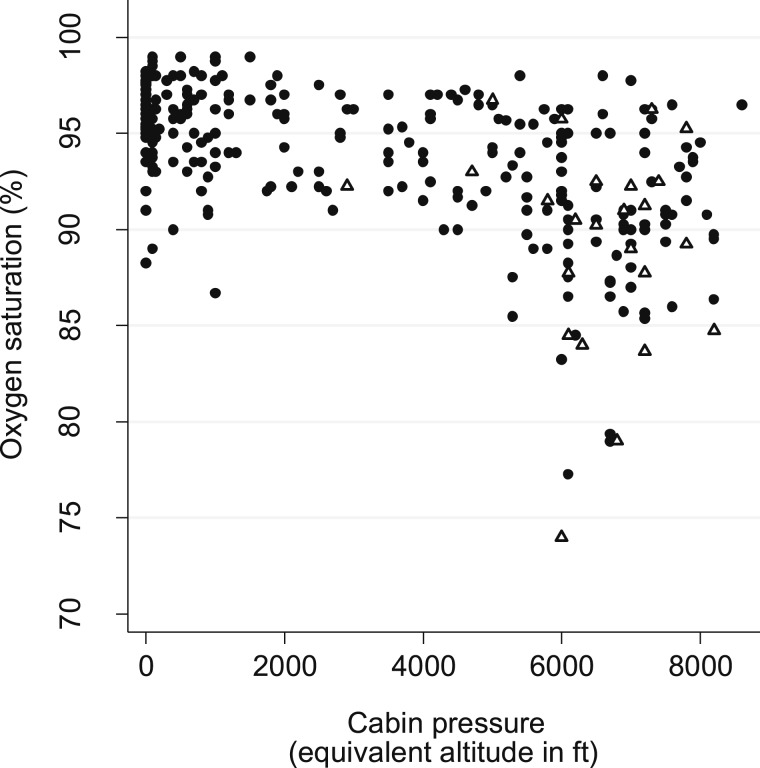
The relationship between cabin pressure and Spo2 is shown in Figure 3. Lower cabin pressure (higher altitude) was associated with lower Spo2 (r = 0.54, P < .0001) but was not associated with changes in HR (P = .28). The minimum altitude at which oxygen desaturation occurred was 1,829 m (6,000 ft), and, on average, oxygen desaturation occurred at 1,971 ± 73 m (6,467 ± 240 ft). Nine flight participants (26% [95% CI, 12%-38%]) experienced one or more oxygen desaturation events of < 85%. All desaturations were associated with higher elevation (> 1,829 m [6,000 ft] ASL; P < .001). Oxygen desaturations occurred more frequently after ambulation (Δ in Fig 3) than at rest (P = .01). Six of seven subjects with desaturation after ambulation also experienced low saturations during other flight stages. No medical emergencies or complications requiring intervention were reported by any study participant.
Figure 3.
Relationship between oxygen saturation and cabin pressure during commercial air travel. ● = measurements performed at rest; Δ = measurements performed immediately after ambulation at cruising altitude.
The differences between subjects who experienced an oxygen desaturation during flight and those who did not are shown in Table 3. No statistically significant differences in subject characteristics were observed. Neither resting Spo2 at sea level nor maximal estimated cabin altitude was predictive of desaturation during flight (P = = .75 and .61, respectively). Seven of the nine individuals with desaturation had a history of home oxygen use (P = .05). Five of these seven individuals had a history of nocturnal oxygen use only and did not use oxygen during their monitored flight. Prior use of in-flight oxygen was associated with the use of oxygen during the flight (P = .03, not shown in table) and was also associated with a decreased likelihood of desaturation (P = .02). Two subjects who used in-flight oxygen reported desaturations; in both cases, however, events occurred when the flow of oxygen was interrupted (eg, running out of battery supply). Flight duration was longer among those who desaturated compared with those who did not (median, 4.0 h vs 2.5 h; P = .03). There was no significant association between clinical variables and desaturation during flight.
Table 3.
—Factors Associated With Desaturation During Commercial Air Travel
| Subject Characteristics | Desaturationa (n = 9) | No Desaturation (n = 25) | P Value |
| Age, y | 48 ± 3 | 49 ± 3 | .95 |
| Sex, female | 8 (89) | 20 (80) | 1.00 |
| Idiopathic PAH | 6 (67) | 14 (56) | .70 |
| WHO functional class | .43 | ||
| I-II | 4 (44) | 14 (56) | |
| III | 4 (44) | 11 (44) | |
| IV | 1 (11) | 0 (0) | |
| PH therapy | |||
| IV/SC prostacyclin | 5 (56) | 6 (24) | .11 |
| Inhaled prostacyclin | 1 (11) | 4 (16) | 1.00 |
| Endothelin receptor antagonist | 4 (44) | 12 (48) | 1.00 |
| Phosphodiesterase-5 inhibitor | 8 (89) | 13 (52) | .11 |
| Combination therapy | 7 (78) | 13 (52) | .25 |
| Flight history | |||
| History of symptoms during flight | 3 (33) | 16 (64) | .14 |
| Prior use of home oxygen | 7 (78) | 9 (36) | .05 |
| Prior use of in-flight oxygen | 0 (0) | 11 (44) | .02 |
| Evaluated for flight safety | 3 (33) | 7 (28) | 1.00 |
| Clinical data | |||
| Resting oxygen saturation, % | 95 ± 2 | 95 ± 3 | .75 |
| 6-min walk distance,b m | 419 ± 65 | 463 ± 27 | .47 |
| Right ventricular systolic pressure,b mm Hg | 73 ± 32 | 71 ± 9 | .94 |
| Flight data | |||
| Duration of flight,c median (range), min | 240 (135-360) | 150 (60-440) | .03 |
| Symptoms during flight | 5 (56) | 8 (32) | .25 |
| Oxygen use during flight | 2 (22) | 7 (28) | 1.00 |
| Equivalent cabin altitude at cruising altitude, altitude ± SD | |||
| Maximum | 7098 ± 817 | 6914 ± 731 | .61 |
| Minimum | 5715 ± 409 | 5857 ± 504 | .85 |
| Average | 6467 ± 240 | 6602 ± 322 | .77 |
Data are presented as mean ± SD or No. (%), unless indicated otherwise. PAH = pulmonary arterial hypertension; PH = pulmonary hypertension. See Table 1 for expansion of other abbreviations.
The oxygen saturation nadir among those with a desaturation was 81% ± 4%.
Data from medical records; 6-min walk data available for 21 subjects (n = 5 and n = 16 with desaturation and without). Echocardiographic data available for 22 subjects (n = 4 and n = 18 with desaturation and without).
Not reported for two subjects (n = 9 and n = 23).
Discussion
Among people with PH, we found that hypoxemia with desaturation was relatively common during commercial air travel. Desaturations were associated with lower cabin pressure, ambulation, failure to use oxygen in people with home oxygen (predominantly nighttime-only users), and longer flight duration. In addition, we found that more than one-third of the participants reported symptoms during flight, including chest pressure/tightness, light-headedness, dyspnea, or palpitations. Only a minority of the participants reported being evaluated by their physician for flight safety prior to travel, and there was substantial variability in the type of safety evaluation performed. Despite these findings, all the flight participants arrived at their planned destination without any medical emergencies or complications. Overall, our results indicate that, even among selected patients with PH who choose to fly, oxygen desaturations and symptoms occur frequently and may go unrecognized by health providers.
Our results add to the existing literature on this subject in several ways. Most importantly, our study provides much needed data on the potential adverse effects of air travel among patients with PH, a condition that may be particularly vulnerable to the effects of altitude. Historically, such research has focused almost exclusively on men with COPD and has largely relied on methods to simulate altitude exposure, either by using 15.1% Fio2 to reproduce a hypoxic environment or by using a hypobaric chamber. Such laboratory-based studies, however, are often limited in duration (< 1 h) and exclude additional stressors encountered during air travel, such as exertion, dehydration, and sleep deprivation.8‐13 Our study contributes new data about an understudied condition that affects a distinctly different patient population composed predominantly of relatively younger women. Moreover, we adopted an underused, “real-world” approach by studying patients during actual flight.
Our study adds further evidence that desaturations and symptoms occur commonly during prolonged commercial flights and with associated activity. Prior research by Kelly et al14 assessed altitude and Spo2 among 14 patients with COPD during a commercial air flight. They reported a mean in-flight Spo2 of 86% ± 4% but noted that desaturations were common with activity such as walking to the lavatory, with a mean nadir of 78%. We found that desaturation events occurred variably in this study population, with little association with the clinical and functional measures often used to assess the severity of PH. We observed a mean in-flight Spo2 of 91% ± 4% while at cruising altitude and a mean nadir of 81% ± 4% in those that manifested desaturation events. The frequency and severity of oxygen desaturation was likely mitigated by the use of supplemental oxygen among some of our participants.
Our data also revealed that, although the mean altitude exposure during flight was only about 1,981 m (6,500 ft) ASL, participants were exposed to a wide range of altitudes, at times exceeding the Federal Aviation Administration limit of 2,438 m (8,000 ft) ASL (a finding also observed by Kelly et al14). Although lower cabin pressure (higher altitude) was associated with lower Spo2, the strength of this relationship was not sufficient to differentiate between those who desaturated during flight and those who did not. Finally, our study provides health providers with unique insight into the travel history of a larger cohort of patients with PH, including frequency of travel, evaluation for flight safety, and type of in-flight oxygen used, which, to our knowledge, has not been reported previously.
Several limitations must be considered when interpreting our data. In our cohort, resting Spo2 at sea level was not a good predictor of desaturation during flight, a finding that has been observed by others.6,15,16 As with other studies, our power to detect meaningful differences was limited by our modest sample size and the availability of complete medical records (recent right-sided heart catheterization, echocardiogram, and pulmonary function testing). Although no individual clinical factor was associated with oxygen desaturation during air travel, participants who used supplemental oxygen nocturnally and did not during air travel represented the majority of desaturation events during our study. Prior use of in-flight oxygen appeared to protect against desaturation. This observation is best explained by the fact that subjects who used oxygen during a prior flight were more likely to have used oxygen during the flight study. The lack of association between desaturation and oxygen use during flight in this study is further confounded by the fact that desaturation events recorded by two subjects using oxygen occurred specifically in settings in which oxygen delivery was temporarily interrupted. Excluding these events, the use of oxygen during flight protected against desaturation. Given the apparent benefit of oxygen among those who used it, individuals with any history of supplemental oxygen use should be evaluated for an oxygen prescription need prior to air travel.
Given the rare nature of the condition, we adopted a novel recruitment strategy by enrolling subjects at the time of an international conference on PH. As a result of this strategy, we recognize that our study cohort is only reflective of a select group of patients and may not be generalizable to all patients with PH. Despite having multiple exclusion criteria intended to maximize research participant safety, these criteria did not account for subject nonparticipation (see Fig 1), nor should they be taken as a basis to recommend against air travel for people with PH. Selection bias has the potential to skew our results insofar as patients attending a conference are likely to be less impaired than those who might not attend. Nonetheless, even in the face of such bias, we found that patients were at risk of desaturation and symptoms during the course of flight. In fact, it is worth noting that a substantial proportion of subjects studied were classified as WHO functional class III, one-third were being treated with prostacyclin infusion, and the majority were on combination PH therapies.
Conclusions
Considering the potential risks of air travel for patients with PH, we recommend that all patients with PH consult their physician prior to air travel. Based on our findings, we suggest that patients with PH who have a history of oxygen use, including nocturnal use only, be evaluated for supplemental in-flight oxygen. Furthermore, in view of the variability in aircraft cabin pressures, the statistically significant association of oxygen desaturation on longer flights, the increased likelihood of ambulation on longer flights, and the efficacy of oxygen in preventing in-flight desaturation, it is prudent to suggest that all patients with PH who will be traveling on flights of greater than 2.5 h in duration be evaluated for in-flight supplemental oxygen. Additional research is needed to assist health providers with more definitive guidance in identifying those patients with PH who are at greatest risk of developing clinically significant events during air travel and to assess their in-flight oxygen requirements.
Acknowledgments
Author contributions: Dr Roubinian: contributed to the study design; collection, analysis, and interpretation of data; critical review of the manuscript; and review and approval of the final version and is guarantor of the manuscript.
Dr Elliott: contributed to the study design, interpretation of data, critical review of the manuscript, and review and approval of the final version.
Dr Barnett: contributed to the interpretation of data, critical review of the manuscript, and review and approval of the final version.
Dr Blanc: contributed to the data analysis and its interpretation, critical review of the manuscript, and review and approval of the final version.
Ms Chen: contributed to the collection and analysis of data, critical review of the manuscript, and review and approval of the final version.
Dr De Marco: contributed to the interpretation of data, critical review of the manuscript, and review and approval of the final version.
Dr Chen: contributed to the study design; collection, analysis, and interpretation of data; drafting and critical review of the manuscript; and review and approval of the final version.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Abbreviations
- ASL
above sea level
- HR
heart rate
- PH
pulmonary hypertension
- Spo2
oxygen saturation
- WHO
World Health Organization
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Funding/Support: This study was funded by The CHEST Foundation and by the National Institutes of Health [Grants T32 HL007185 (Dr Roubinian) and K23 HL086585 (Dr Chen)].
References
- 1.U.S. Department of Transportation, Research and Innovative Technology Administration, Bureau of Transportation Statistics Transtats: passengers: all carriers-all airports. Research and Innovative Technology Administration, Bureau of Transportation Statistics website. http://www.transtats.bts.gov/Data_Elements.aspx?Data=1. Accessed July 27, 2010.
- 2.Dillard TA, Rosenberg AP, Berg BW. Hypoxemia during altitude exposure. A meta-analysis of chronic obstructive pulmonary disease. Chest. 1993;103(2):422-425 [DOI] [PubMed] [Google Scholar]
- 3.Luks AM. Can patients with pulmonary hypertension travel to high altitude?. High Alt Med Biol. 2009;10(3):215-219 [DOI] [PubMed] [Google Scholar]
- 4.British Thoracic Society Standards of Care Committee Managing passengers with respiratory disease planning air travel: British Thoracic Society recommendations. Thorax. 2002;57(4):289-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyle J. Medical guidelines for airline travel. Aviat Space Environ Med. 2003;74(9):1009-. [PubMed] [Google Scholar]
- 6.Seccombe LM, Kelly PT, Wong CK, Rogers PG, Lim S, Peters MJ. Effect of simulated commercial flight on oxygenation in patients with interstitial lung disease and chronic obstructive pulmonary disease. Thorax. 2004;59(11):966-970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong H, Jr, Tashkin DP, Lee EY, Simmons MS. Hypoxia-altitude simulation test. Evaluation of patients with chronic airway obstruction. Am Rev Respir Dis. 1984;130(6):980-986 [DOI] [PubMed] [Google Scholar]
- 8.Berg BW, Dillard TA, Derderian SS, Rajagopal KR. Hemodynamic effects of altitude exposure and oxygen administration in chronic obstructive pulmonary disease. Am J Med. 1993;94(4):407-412 [DOI] [PubMed] [Google Scholar]
- 9.Berg BW, Dillard TA, Rajagopal KR, Mehm WJ. Oxygen supplementation during air travel in patients with chronic obstructive lung disease. Chest. 1992;101(3):638-641 [DOI] [PubMed] [Google Scholar]
- 10.Dillard TA, Berg BW, Rajagopal KR, Dooley JW, Mehm WJ. Hypoxemia during air travel in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1989;111(5):362-367 [DOI] [PubMed] [Google Scholar]
- 11.Dillard TA, Moores LK, Bilello KL, Phillips YY. The preflight evaluation. A comparison of the hypoxia inhalation test with hypobaric exposure. Chest. 1995;107(2):352-357 [DOI] [PubMed] [Google Scholar]
- 12.Naughton MT, Rochford PD, Pretto JJ, Pierce RJ, Cain NF, Irving LB. Is normobaric simulation of hypobaric hypoxia accurate in chronic airflow limitation?. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1956-1960 [DOI] [PubMed] [Google Scholar]
- 13.Akerø A, Edvardsen A, Christensen CC, Owe JO, Ryg M, Skjønsberg OH. COPD and air travel: oxygen equipment and preflight titration of supplemental oxygen. Chest. 2011;140(1):84-90 [DOI] [PubMed] [Google Scholar]
- 14.Kelly PT, Swanney MP, Seccombe LM, Frampton C, Peters MJ, Beckert L. Air travel hypoxemia vs. the hypoxia inhalation test in passengers with COPD. Chest. 2008;133(4):920-926 [DOI] [PubMed] [Google Scholar]
- 15.Akerø A, Christensen CC, Edvardsen A, Skjønsberg OH. Hypoxaemia in chronic obstructive pulmonary disease patients during a commercial flight. Eur Respir J. 2005;25(4):725-730 [DOI] [PubMed] [Google Scholar]
- 16.Christensen CC, Ryg M, Refvem OK, Skjønsberg OH. Development of severe hypoxaemia in chronic obstructive pulmonary disease patients at 2,438 m (8,000 ft) altitude. Eur Respir J. 2000;15(4):635-639 [DOI] [PubMed] [Google Scholar]