Abstract
Aim:
Synthesis of superporous hydrogel with different concentrations of crosslinker was carried out using solution polymerization to study its effect on characteristics of superporous hydrogel. Methylene-bis-acrylamide was used as a crosslinker.
Materials and Methods:
The characterization studies were performed by measurement of apparent density, porosity, swelling studies, mechanical strength studies, and scanning electron microscopy analysis. Results and Discussion: As the concentration of crosslinker increased from 7.37% to 14.36 % the porosities decreased. In double distilled water, superporous hydrogels showed good increase in equilibrium swelling capacity compared to that in simulated gastric fluid. Scanning electron microscopic images clearly indicated the formation of interconnected pore and capillary channels. Characterization studies revealed that the increase in crosslinker concentration is beneficial from the mechanical stability point of view, but at the same time the decrease in porosity may lead to decrease in drug release rate by diffusion through these capillary channels. Batches B1 and B2 with low concentrations of crosslinker provide good porous structure, swelling characteristics, and mechanical strength appropriate for further applications of superporous hydrogel-based drug delivery systems.
Conclusion:
The concentration of crosslinker affects the porous structure, swelling characteristics and mechanical strength. By setting appropriate degree of crosslinking it is possible to prepare superporous hydrogel having desired characteristics, which will provide a platform to design the drug delivery systems based on it.
Keywords: Crosslinker, penetration pressure, superporous hydrogel, swelling
INTRODUCTION
Hydrogels are cross-linked hydrophilic polymers with a network structure consisting of acidic, basic, or neutral monomers, which are able to imbibe large amounts of water. Superporous hydrogels (SPHs) are porous hydrophilic crosslinked structures with the ability of absorbing aqueous fluids up to a few hundred times their own weight.[1–7] SPHs are generally prepared by copolymerization/ crosslinking of co-monomers or crosslinking of linear polymers by irradiation or by chemical compounds.[8] Gas blowing techniques are used to synthesize SPHs. Acrylamide (AM), generally used as monomer, is prepared on an industrial scale by the hydrolysis of acrylonitrile by nitrile hydratase. Most AM is used to synthesize polyacrylamides, which find many uses as water-soluble thickeners. AM has various pharmaceutical[9–12] and biomedical[13–15] applications in the form of hydrogel or SPHs.
Control of foaming and polymerization is necessary to form homogeneous open capillary channels in SPHs. The commonly used foaming agents are inorganic carbonates such as sodium carbonate and sodium bicarbonate; co-monomers are other polymerizable groups compatible with the polymer such as acrylate and methacrylate; and crosslinkers are methylenebisacrylamide (BIS), diacrylate, and higher molecular weight acrylate, which have been safely applied in drug delivery systems. Solvent treatments with SPHs affect its characteristics.
Fast swelling and large swelling ratio make SPHs an excellent candidate material to develop gastric retention devices.[16] As reported, increase in the concentration of crosslinker decreases gelation times for super absorbent polymer formation,[17] as well as it decreases the water absorbency of the hydrogel composite.[18]
In this investigation, SPHs with different BIS concentration were prepared in order to set the suitable BIS concentration that provides SPH with good characteristics for SPH-based drug delivery systems.
MATERIALS AND METHODS
Materials
AM was purchased from Burgoyne Burbidges and Co. Pvt. Ltd., Mumbai, India. Acrylic acid (AA), BIS, Span 80, ammonium persulphate (APS), and N,N,N’,N’-tetramethylethylenediamine (TEMED) were purchased from SD Fine Chem Ltd., Mumbai, India. Double distilled water (DDW) was prepared in laboratory. Simulated gastric fluid (SGF) with pH of about 1.2 was prepared in laboratory by dissolving 2 g of NaCl, 3.2 g pepsin, and 6.8 ml of HCl in DDW to a final volume of 1000 ml.[19] NaCl, pepsin (1:3000), HCl, and all other chemicals used were purchased from SD Fine Chem, Mumbai and used as obtained.
Synthesis of SPH of poly (acrylamide-co-acrylic acid (P(AM-co-AA))
All ingredients except sodium bicarbonate were used as solution in DDW. The pH of the monomer solutions was adjusted to 5.5[20] with 5 M sodium hydroxide.
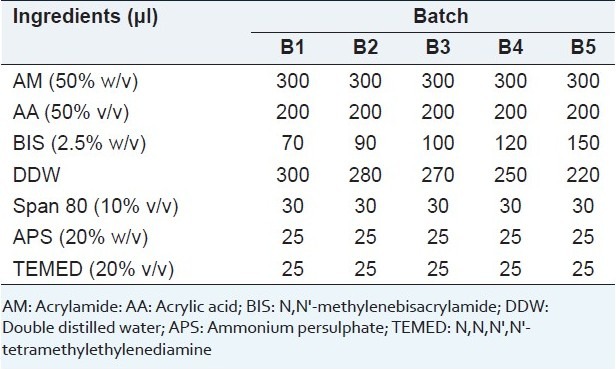
For the synthesis of SPH of poly (acrylamide-co-acrylic acid, P(AM-co-AA)) the following substances were added subsequently into a test tube at room temperature (25°C): AM 50%; AA 50%; BIS 2.5%; DDW; Span 80 10%; APS 20%; TEMED 20%; and 200 mg of sodium bicarbonate as shown in Table 1. The amount of BIS as a crosslinker was set from 7.37–14.36 % to study its effect on characteristics of SPHs. In this procedure, polymerization was allowed to continue for approximately 10 min. After adding each substance to the test tube, the reaction mixture was vigorously shaken. Finally, sodium bicarbonate was added very quickly to the solution and mixed with a spatula. If sodium bicarbonate was not added quickly enough, since polymerization had already been started by APS as initiator, under this condi-tion, some clumps were formed additionally and homogenous SPH polymer was not obtained.
Table 1.
Formulation of SPHs using 200 mg of sodium bicarbonate in each batch
After synthesis of SPHs, they were removed with forceps, allowed to dry in an oven at 60°C for 48 h, and cut into pieces of required size. SPH was submerged in hexane.[20] This treatment dehydrates SPHs quickly as well as provides drying. Thereafter, SPHs were removed with forceps and were put in an oven at 60°C for 48 h in order to be sure that SPHs have been dried completely. These SPHs were stored in airtight container until further use.
Scanning electron microscopy analysis
Dried SPHs were cut to expose their inner structure and used for scanning electron microscopy (SEM) studies. The morphology and porous structures of SPHs were examined using the ESEM EDAX XL-30 Scanning Electron Microscope (Philips, Netherlands), with an operating voltage of 30 kV.
Density and porosity measurement
For density measurement, the solvent displacement method was used. Dried SPHs, which were treated with different solvents, were used for density measurements, which actually show the apparent densities of SPHs. Pieces of SPHs were taken and weighed in order to determine the mass of each piece. A hydrophobic solvent such as hexane that is not absorbed by SPHs was used for this purpose. By the use of forceps, a piece of the polymer was immersed in a predetermined volume of hexane in a graduated cylinder, and the increase in the hexane volume was measured as the volume of the polymer. The density was calculated[21] from the following eq. 1:
Density = MSPH / VSPH, (1)
where VSPH is the volume of SPH displaced by solvent and MSPH is the mass of SPH.
For porosity measurement, dried hydrogels were immersed in hexane over night and weighed after excess hexane on the surface was blotted. The porosity was calculated from the following eq. 2:
Porosity = VP / VT, (2)
where VT is the total volume of SPH and VP (= VT - VSPH) is the pore volume of SPH. Total volume of SPH can be measured from its dimensions, as it is cylindrical in shape.
Swelling studies
The dried SPHs were used to determine their swelling ratio in DDW and SGF. The swelling ratio can be calculated from the following eq. 3:
Q = (Ms – Md) / Md, (3)
where Q is the swelling ratio, Ms the mass in the swollen state, and Md the mass in the dried state. At the beginning of each experiment, Md of a piece of polymer was measured by weight and then it was immersed in an excess of DDW for swelling. The swollen SPHs were put on a grid boat like mesh arrangement for easy handling of SPHs during pull up and down without breaking, with a mesh size of 1 mm. This technique allows to put the polymer in water and to weigh it without breaking. Each time the grid boat with the polymer was removed from water, it was gently dried by tissue paper in order to remove adhering water. At specific time intervals the polymer was removed from the water and weighed in order to measure Ms.[22] When the weight became constant it was considered as Ms and the time was considered as swelling time.
Mechanical strength studies
The penetration pressure (PP) of SPHs was measured using a bench comparator as described by Chen et al. with modifications.[23] The fully swollen hydrogel was put longitudinally under the lower touch and weights were successively applied to the upper touch until the polymer completely fractured. The compressive force could be read from the gauge and the penetration pressure[24] could be calculated from eq. 4:
PP = Fu / S, ....(4)
where Fu is the ultimate compressive force at complete breakage of the polymer and S is the area of the lower touch.
RESULTS AND DISCUSSION
Synthesis of SPH of P(AM-co-AA)
To obtain homogeneous SPHs with as many pores as possible, polymerization should take place when the foam was stabilized. In the synthesis procedure of SPH, AA and AM are the monomers. BIS is used as a crosslinker, and span 80 is used as a foam stabilizer of the foam, which is formed by carbon dioxide originating from sodium bicarbonate. Here span 80 was tried instead of Pluronic F127 as reported by Dorkoosh et al.[20] Span 80 does not contribute to the chemical structure of the polymer, but is very important as a surface-active agent to create the highly porous polymer structure. APS is used as a polymerization initiator and TEMED as a catalyst.
APS and TEMED combination initiated the radical polymerization. The radicals formed will then attack the double bonds of AA and AM, and to the double bond of BIS. After that, the double bonds will be opened; the monomers will covalently bind to each other and form a long aliphatic chain, which are subsequently crosslinked by the added crosslinker.[20]
One of the important factors that influence the synthesis of the SPHs was the pH of the AA monomer solution. At pH 5.0 and low concentration of crosslinker, SPHs with well-distributed pores of 50 – 150 μm approximately in size, were produced because of the stability and the proper formation rate of the foam.
Scanning electron microscopy analysis
Figure 1 shows the SEM image of SPH. SPH possessed large numbers of interconnected pores, indicating that formation hydrogel with the superporous structure. SPH possesses large numbers of pores, indicating that formation of SPH would not destroy the superporous structure. In the structure of SPH the inner surface contained large numbers of the pores connected to each other. It can be observed in the SEM image which shows structures with a great penetration of the medium into the system with pores that form connections (channels) with the interior of the structure. The capillary channels were clearly observed from SEM image and this may enable water to enter into the hydrogel networks or drug molecules to diffuse out of them.
Figure 1.
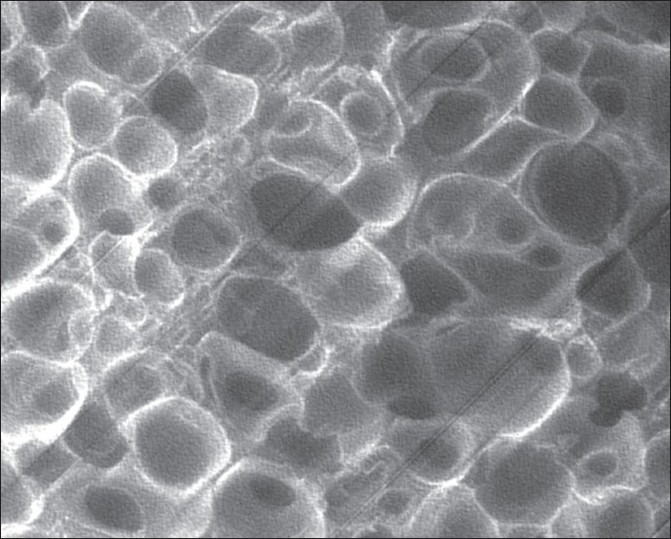
SEM image of SPH at magnifi cation ×24
Density and porosity measurement
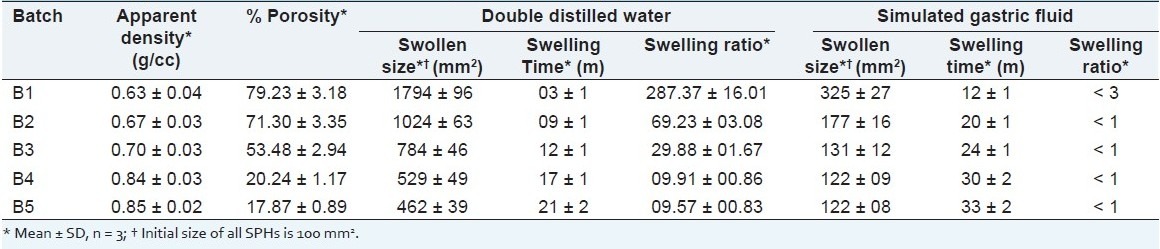
Apparent densities and porosities of SPHs are shown in Table 2. SPHs showed increase in density from 0.63 ± 0.04 g/cc to 0.85 ± 0.02 g/cc indicating higher the concentration of BIS higher will be the density. SPHs have density lower than that of the swelling media, DDW and SGF, and so all float on the swelling medium. SPHs showed porosities ranging from 79.23 ± 3.18% to 17.87 ± 0.89%, reflecting the decrease in porosity with increase in BIS concentration, which may be due to increase in crosslink density. The decrease in porosity of the polymer is seen due to the formation of strong crosslink, tends to decrease the size of interconnecting pores and the capillary structure. SEM images support the formation of interconnected pore and capillary channels. A higher concentration of BIS produces a larger degree of polymer chains branching and generates an additional network. Thereby, with the BIS content increasing, the crosslinking density increases. As a result, the network space gets diminished, and less water enters the hydrogel.
Table 2.
Apparent densities, porosities, and swelling parameters of SPHs
Swelling studies
Table 2 shows swelling parameters in DDW and in SGF. Figure 2 shows swelling ratio curves for SPHs in DDW. In DDW SPHs showed decrease in swelling capacity with increase in BIS concentration. This is because water molecules are taken up into SPHs by capillary forces, and making water uptake much faster than the diffusion. When the same SPHs were placed into SGF showed too less swelling capacity. This may be due to the change in pH of swelling medium as DDW has higher pH than SGF. It revealed that the swelling capacity of SPHs depend on the pH of the medium. SPHs swollen in DDW showed very short swelling time to reach its equilibrium size compared to that of swollen in SGF. Swelling ratio of SPHs swollen in descending order with BIS concentration. While in case of SPHs swollen in SGF it was less than 3 which reflects too poor swelling in SGF. In general, increase in BIS concentration tends to poor swelling characteristics in both swelling media. Due to increase in crosslinking density, the media absorbency of the SPH could have been decreased.
Figure 2.
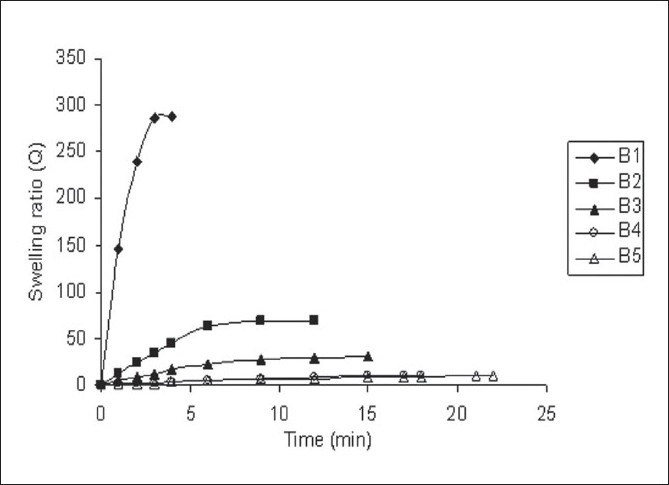
Swelling ratio curves for superporous hydrogels in double distilled water
Mechanical properties
The penetration pressures of SPHs are shown in Figure 3. One of the most important requirements for a gastric retention superporous hydrogel is its structural integrity. A superporous hydrogel should be able to withstand the pressure expected in the stomach during repeated gastric contractions, especially the housekeeper waves. Formulation variables, such as the amount of crosslinker, type of monomer, amount of blowing agent as well as process variables all affect the mechanical properties of SPHs. SPHs when swollen in DDW showed mechanical strength that can withstand the pressure during gastric contraction, but when compared to that of in SGF it was less. SPHs showed too good penetration pressure more than 400 cm water , when swollen in SGF. The maximum pressure during the gastric contraction was reported to range from 50–70 cm water.[25]
Figure 3.
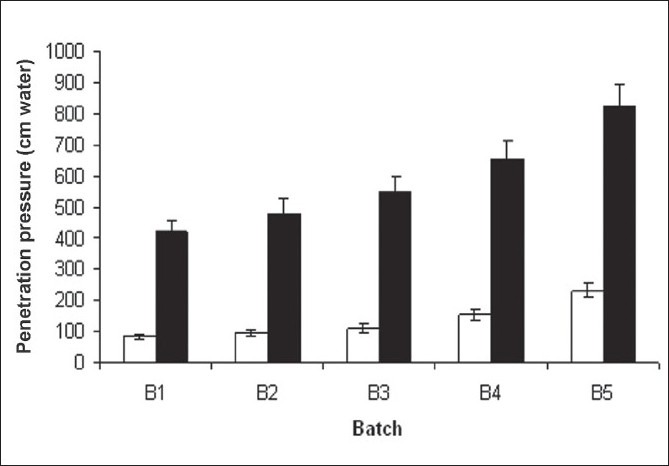
Penetration pressure of superporous hydrogels in Double distilled water (□) and in SGF (■); Mean ± SD, n = 3
CONCLUSIONS
Increasing the BIS concentration in SPH showed negative effects on swelling characteristics. Higher the concentration of BIS higher will be the density and lower will be the porosity. The increase in BIS concentration is beneficial from mechanical stability point of view, but at the same time the decrease in porosity may lead to decrease in drug release rate by diffusion through these capillary channels. Batch B1 and B2 with good porous structure, swelling characteristics, and mechanical strength are more appropriate for further applications of SPH-based drug delivery systems.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chen J, Park H, Park K. Synthesis of superporous hydrogels: Hydrogels with fast swelling and superabsorbent properties. J Biomed Mater Res. 1999;44:53–62. doi: 10.1002/(sici)1097-4636(199901)44:1<53::aid-jbm6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Van Dijk-Wolthuis WN, Tsang SK, Kettenes-van den Bosch JJ, Hennink WE. A new class of polymerizable dextrans with hydrolyzable groups: Hydroxyethyl methacrylated dextran with and without oligolactate spacer. Polymer. 1997;38:6235–42. [Google Scholar]
- 3.Peniche C, Cohen ME, Vazquez B, Sanroman J. Water sorption of flexible networks based on 2-hydroxyethyl methacrylate-triethylenglycol dimethacrylate copolymers. Polymer. 1997;38:5977–82. [Google Scholar]
- 4.Badiger MV, McNeill ME, Graham NB. Porogens in the preparation of microporous hydrogels based on poly(ethylene oxides) Biomaterials. 1993;14:1059–63. doi: 10.1016/0142-9612(93)90206-h. [DOI] [PubMed] [Google Scholar]
- 5.Lee YM, Kim SS. Hydrogels of poly(ethylene glycol)-co-poly(lactones) diacrylate macromers and β-chitin. Polymer. 1997;38:2415–20. [Google Scholar]
- 6.Omidian H, Park K, Rocca JG. Recent developments in superporous hydrogels. J Pharm Phamacol. 2007;59:317–27. doi: 10.1211/jpp.59.3.0001. [DOI] [PubMed] [Google Scholar]
- 7.Park K, Omidian H. Experimental design for the synthesis of polyacrylamide superporous hydrogels. J Bioact Compat Polymers. 2002;17:433–50. [Google Scholar]
- 8.Satish CS, Satish KP, Shivakumar HG. Hydrogels as controlled drug delivery systems: Synthesis, crosslinking, water and drug transport mechanism. Indian J Pharm Sci. 2006;68:133–40. [Google Scholar]
- 9.Brazel CS, Peppas NA. Pulsatile local delivery of thrombolytic and antithrombotic agents using poly(N-isopropylacrylamide-co-methacrylic acid) hydrogels. J Control Release. 1996;39:57–64. [Google Scholar]
- 10.Andersson M, Axelsson A, Zacchi G. Diffusion of glucose and insulin in a swelling N-isopropylacrylamide gel. Int J Pharm. 1997;157:199–208. doi: 10.1016/s0378-5173(97)00243-3. [DOI] [PubMed] [Google Scholar]
- 11.Chavda HV, Patel CN. Chitosan superporous hydrogel composite-based floating drug- delivery system: A newer formulation approach. J Pharm Bioall Sci. 2010;2:124–31. doi: 10.4103/0975-7406.67010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavda HV, Patel CN, Prajapati ST, Patel CV. A newer formulation approach based on superporous hydrogel particles for gastroretentive drug-delivery system: Preparation and in vitro evaluation. Inventi Impact: NDDS. 2010;1 Article ID: 2010nd70a. [Google Scholar]
- 13.Blanco MD, Garcia O, Trigo RM, Teijon JM, Katime I. 5-Fluorouracil release from copolymeric hydrogels of itaconic acid monoester, I: Acrylamide-co-monomethyl itaconate. Biomaterials. 1996;17:1061–7. doi: 10.1016/0142-9612(96)85906-0. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Sheardown H. Glucose permeable poly (dimethyl siloxane) poly (Nisopropyl acrylamide) interpenetrating networks as ophthalmic biomaterials. Biomaterials. 2005;26:233–44. doi: 10.1016/j.biomaterials.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Chavda HV, Patel CN. Preparation and characterization of swellable polymer-based superporous hydrogel composite of poly (acrylamide-co-acrylic acid) Trends Biomat Artif Organs. 2010;24:83–9. [Google Scholar]
- 16.Qiu Y, Park K. Superporous IPN hydrogels having enhanced mechanical properties. AAPS PharmSciTech. 2003;4:4. doi: 10.1208/pt040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabiri K, Omidian H, Hashemi SA, Zohuriaan-Mehr MJ. Synthesis of fast-swelling superabsorbent hydrogels: Effect of crosslinker type and concentration on porosity and absorption rate. Eur Polym J. 2003;39:1341–8. [Google Scholar]
- 18.Mahdavinia GR, Mousavi SB, Karimi F, Marandi GB, Garabaghi H, Shahabvand S. Synthesis of porous poly(acrylamide) hydrogels using calcium carbonate and its application for slow release of potassium nitrate. Exp Polymer Lett. 2009;3:279–85. [Google Scholar]
- 19.Lee SS, Lim CB, Pai CM, Lee S, Park IS, Moon G, et al. Composition and pharmaceutical dosage form for colonic drug delivery using polysaccharides. 1999 US Patent 6413494. [Google Scholar]
- 20.Dorkoosh FA, Brussee J, Verhoef JC, Borchard G, Rafiee-Tehrani M, Junginger HE. Preparation and NMR characterization of superporous hydrogels (SPH) and SPH composites. Polymer. 2000;41:8213–20. [Google Scholar]
- 21.Park K, Chen J, Park H. Hydrogel composites and superporous hydrogel composites having fast swelling, high mechanical strength, and superabsorbent properties. 2001 US Patent 6271278. [Google Scholar]
- 22.Chen J, Park H, Park K. Synthesis of superporous hydrogels: Hydrogels with fast swelling and superabsorbent properties. J Biomed Mater Res. 1999;44:53–62. doi: 10.1002/(sici)1097-4636(199901)44:1<53::aid-jbm6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Park K. Synthesis and characterization of superporous hydrogel composites. J Control Release. 2000;65:73–82. doi: 10.1016/s0168-3659(99)00238-2. [DOI] [PubMed] [Google Scholar]
- 24.Polnok A, Verhoef JC, Borchard G, Sarisuta N, Junginger HE. In vitro evaluation of intestinal absorption of desmopressin using drug delivery systems based on superporous hydrogels. Int J Pharm. 2004;269:303–10. doi: 10.1016/j.ijpharm.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 25.Guyton AC. Vol. 2. Philadelphia: W.B. Saunders Co; 1977. Basic human physiology: Normal function and mechanisms of disease; pp. 662–4. [Google Scholar]


