Abstract
Vaccines are the preparations given to patients to evoke immune responses leading to the production of antibodies (humoral) or cell-mediated responses that will combat infectious agents or noninfectious conditions such as malignancies. Alarming safety profile of live vaccines, weak immunogenicity of sub-unit vaccines and immunization, failure due to poor patient compliance to booster doses which should potentiate prime doses are few strong reasons, which necessitated the development of new generation of prophylactic and therapeutic vaccines to promote effective immunization. Attempts are being made to deliver vaccines through carriers as they control the spatial and temporal presentation of antigens to immune system thus leading to their sustained release and targeting. Hence, lower doses of weak immunogens can be effectively directed to stimulate immune responses and eliminate the need for the administration of prime and booster doses as a part of conventional vaccination regimen. This paper reviews carrier systems such as liposomes, microspheres, nanoparticles, dendrimers, micellar systems, ISCOMs, plant-derived viruses which are now being investigated and developed as vaccine delivery systems. This paper also describes various aspects of “needle-free technologies” used to administer the vaccine delivery systems through different routes into the human body.
Keywords: Edible vaccines, microneedles, microparticulates, needle-free delivery, TLRs, vaccine delivery systems, vaccine
INTRODUCTION
Vaccine is a material that induces an immunologically mediated resistance to a disease but not necessarily an infection. Vaccines are generally composed of killed or attenuated organisms or subunits of organisms or DNA encoding antigenic proteins of pathogens. Sub-unit vaccines though exceptionally selective and specific in reacting with antibodies often fail to show such reactions in circumstances such as shifts in epitopic identification center of antibody and are poorly immunogenic. However, the selectivity and specificity of sub-units of the causative organism like proteins, carbohydrates can be exploited for producing strong and prolonged immune responses by catering them to the immune system in such a way that a specific and strong immune response is induced. These epitopes may also allow the generation of vaccines not only against infectious diseases, but also against chronic diseases such as hepatitis C or cancer.
In order to induce an effective protective immunity, these vaccines require boosting with agents called “adjuvants.” Adjuvants are believed to act by forming complexes with the agent to be delivered from which immunogens are slowly released.
Vaccine delivery systems (e.g., emulsions, microparticles, immune-stimulating complexes ISCOMs, liposomes).
Immunostimulatory adjuvants: Conserved molecular patterns of pathogen stimulate immunity as they are identified by pattern recognition receptors like “Toll” receptors located mainly on B-cells, dendritic cells of mammals (e.g., unmethylated CpG containing DNA).
Adjuvants potentiate the immunostimulatory property of the antigen while being non-immunogenic, nontoxic, and biodegradable by themselves.
Aluminium salts such as aluminium hydroxide, aluminium phosphate; oil emulsions such as Freunds incomplete adjuvant; particulate matter such as ISCOMs; synthetic polynucleotides are other types of adjuvants.
VACCINE DELIVERY SYSTEMS
Delivery of antigens from oil-based adjuvants such as Freunds[1] adjuvant lead to a reduction in the number of doses of vaccine to be administered but due to toxicity concerns like inductions of granulomas at the injection site, such adjuvants are not widely used. FDA approved adjuvants for human uses are aluminium hydroxide and aluminium phosphate in the form of alum. Hence, search for safer and potent adjuvants resulted in the formulation of antigen into delivery systems that administer antigen in particulate form rather than solution form.
Other reasons driving the development of vaccines as controlled drug delivery systems are as follows:
-
Immunization failure with conventional immunization regimen involving prime doses and booster doses, as patients neglect the latter.
Vaccines delivery systems on the other hand:
Allow for the incorporation of doses of antigens so that booster doses are no longer necessary as antigens are released slowly in a controlled manner.
Control the spatial and temporal presentation of antigens to the immune system there by promoting their targeting straight to the immune cells.
Vaccine delivery systems can be classified as follows
Solid particulates: Solid particulate systems such as microspheres and lipospheres are being exploited for vaccine delivery [Table 1] based on the fact that intestine is an imperfect barrier to small particulates. Antigens entrapped in such particulates when taken up by M-cells can generate immunity.
Table 1.
Delivery of vaccines by polymeric microparticles through different routes

Methods such as light microscopy, confocal microscopy, electron microscopy, extraction of polymer from tissue followed by quantification by gel permeation chromatography, flow cytometry[2] indicated that microparticulates of <10 μm in diameter can enter gut associated lymphoid tissue (GALT) within 1 h of oral administration and can be used as antigen carriers for controlled release vaccine applications.
Particle size[3] is an important consideration while formulating microparticulate systems as it influences their uptake and release and hence immune responses. Small (<10 μm) microspheres due to their large surface to mass ratio, are capable of facilitating extracellular delivery of antigen to the phagocytic accessor cells leading to faster release and increased antigen processing. Larger particles could not be phagocytosed by macrophages until they have disintegrated into smaller debris. A combination of larger and smaller particles might produce a pulsatile pattern for antigen release thus mimicking an immunization process involving prime and booster shots.
Polymers in solid particulate vaccine delivery
Biodegaradable polymers such as PLGA,[4] previously used as surgical implant and suture material is now being exploited for matrix antigen delivery. PLGA microspheres are rapidly taken up by M-cells and translocated towards the underlying lymphatic tissue within 1 h.
Shi et al.,[5] developed spray dried PLGA microspheres loaded with recombinant tuberculosis (TB) antigen, TB10.4-Ag85B for pulmonary administration against tuberculosis infection. Particles were of 3.3 μm in size and, hence, were respirable. Results have shown intial burst release of antigens followed by a sustained release up to 10 days. Interleukin-2 secretion in a T-lymphocyte assay due the microspheres was found to be stronger than antigen solutions.
However, the use of PLGA can be limited by acid hydrolytic degradation products detrimental to the entrapped protein and loss of immunogenicity on storage. Also organic solvents used to load the antigen onto the polymer can be detrimental to the antigen.
Domb et al.[6] entrapped a recombinant malaria antigen, R32NS1, derived from the circumsporozoite protein of Plasmodium falciparum in biodegradable polymers like polylactide (PLD) or polycaprolactone (PCL) in the absence or presence of lipid A as an adjuvant. PCL lipospheres without immunomodulators have shown a superior sustained immunogenic response over PLD lipospheres, the reason being in the different biodegradation rate of polymers.
Chitosan, a mucoadhesive linear polysaccharide derived from partial deacetytlation of chitin is safer over PLGA as there is no need to use organic solvents because of the ability of positively charged chitosan to bind with negatively charged immunogenic DNA. Dinesh kumar et al.,[7] prepared chitosan microspheres (1%, 2%, 3%) loaded with tetanus toxoid which constituted 1%, 2%, 3% of chitosan micropheres. In vitro studies have shown cumulative percentage release of tetanus toxoid from microspheres as 74.09%, 89.31%, and 80.23%, respectively, for 50 days.
Sexton et al. designed a layer-by-layer (LBL)[8] hydrogel capsule made up of poly (methacrylic acid) modified with thiol groups (PMA)SH as a carrier for ovalbumin as model antigen in transgenic mice models. Interacting polymers are assembled layer-by-layer onto a template that is removed later in the process. Conversion of the thiol groups into disulfide linkages during the deposition process ensures that layer-by-layer structure remains stable in the oxidizing extracellular environment. Further, when inside the cell, due to the reducing environment, capsule is degraded thereby releasing the vaccine to the target thus conferring bio deconstructible feature to the delivery system. Also due to the particulate nature of the capsule, phagocytosis by antigen presenting cells and dendritic cells is promoted. The reported results by the scientists suggest that immune responses in the transgenic mice in vivo were significant when compared to those generated when antigen is administered alone.
Other particulate systems in use are crosslinked albumin, gelatin. Empty gelatin microparticles produce only a mild inflammatory response at injection site suggesting minimal immunogenic activity.
Poly (phosphazenes)[4] are class of polymers with a simple –P = N– backbone with physicochemical properties strongly influenced by side chain attachments to phosphorous atom. Antigen is entrapped into such polymers in aqueous state at reduced temperatures. The system is then rendered insoluble by the addition of crosslinking agents such as calcium thus promoting sustained release from the precipitated solid. Further control is obtained by coating the solid surface with cationic polymer poly (lysine) and this approach has been used in the release of antibacterial drugs in CR string made from Ca Alginate designed for impaction into dental periodontal cavities.
Polyanhydrides such as poly (fumaric-co-sebacic)[4] anhydride fabricated into microspheres of 0.5-5 μm in diameter were seen as early as 1 h post feeding and were observed in the Peyer's patch at 3, 6, 12, 24 h following oral administration.
LIPOSOMAL DELIVERY SYSTEMS
Liposomes and their derivatives “lipoplexes” (liposome/DNA complexes) are hollow spherical constructs of phospholipid bilayers capable of entrapping hydrophilic moieties in the aqueous compartment and hydrophobic moieties in the lipid bilayers with cholesterol imparting rigidity to the bilayer. However, lipoplexes tend to aggregate during storage, due to neutralization of positive charge on liposomes by negative charge on DNA. This drawback is overcome by formulating liposomes/protamine/DNA (LPD). Protamine is an arginine rich peptide. It condenses with DNA before DNA can complex with positive lipids there by conferring stability to the preparation.
Viruses, proteins, glycoproteins, nucleic acids, carbohydrates, and lipids can be entrapped and targeted at cellular and subcellular level for evoking immune responses [Table 2].
Table 2.
Current research in liposomes as vaccine delivery systems
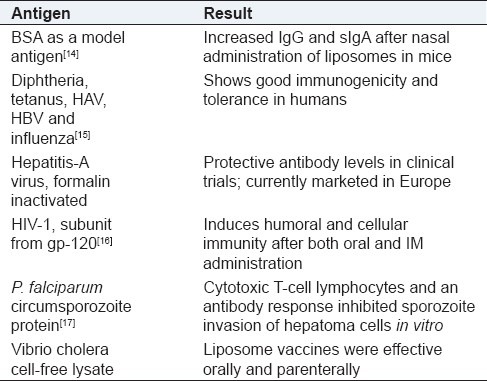
As vaccine adjuvants these systems exert immunomodulatory effects by virtue of their particulate nature and their ability to bind with cell surface lipid receptors such as CD1a after complement activation. The phospholipid bilayer fuses with cell wall hence tend to get incorporated into elements of reticuloendothelial system (RES) rapidly. The development of polymerized liposomes, which have shown enhanced stability in the gastrointestinal tract, also offers potential for use in mucosal vaccination. Polymerized liposomes coated with targeting molecules such as antibodies, antibody fragments, antigens and molecules are capable of binding to specific cell surface receptors found in the mucosal tissues. Stealth liposomes or sterically stabilized liposomes contain hydrophilic surfaces due to coating of liposomes with PEG and this covalently binds with the polyethylene found in the lipid bilayer there by reducing the opsonization by serum proteins and increasing circulation half lives.
Purified and isolated nucleic acid molecules encoding a basal body rod protein of a strain of Campylobacter,[12] particularly Campylobacter jejuni, encapsulated in liposomes along with adjuvants like aluminum phosphate, aluminum hydroxide, QS21, Quil A, calcium phosphate, calcium hydroxide, zinc hydroxide, a glycolipid analog, an octadecyl ester of an amino acid, a muramyl dipeptide and a lipoprotein served many purposes. Proteins expressed by nucleic acids are found to be immunogenic against the disease caused by Campylobacter, in the diagnosis of infection by Campylobacter, and as tools for the generation of immunological reagents. Monoclonal antibodies or antisera raised against these peptides are useful for the diagnosis of infection by Campylobacter, specific detection of Campylobacter in in-vitro and in-vivo assays, and for use in passive immunization for prevention and treatment of diseases caused by Campylobacter.
Oral liposomes encapsulated with recombinant H. pylori heat shock protein 60 (rHsp60)[13] vaccine were prepared and their activity against H. pylori infection in mice was investigated. Results with rHsp60 plus Cholera Toxin, liposome-encapsulated rHsp60, liposome encapsulated rHsp60 plus Cholera Toxin showed 73.3%, 66.7%, and 86.7%, respectively, immune responses against H. pylori infection.
Liposomal vaccines based on viral membrane proteins (virosomes) have been approved as products in Europe for hepatitis A and influenza.
Niosomes are non-ionic surfactant vesicles and are now being exploited ad carrier systems for vaccine delivery. For example, encapsulation of ovalbumin into Wasag®7[18] (70% stearate sucrose ester, 30% palmitate sucrose ester (40% mono-, 60% di/tri-ester) niosomes resulted in a significant increase in antibody titres when compared to empty niosomes or ovalbumin alone or control formulation when evaluated in BALB/c mice.
VIROSOMES
Virosomes are small spherical unilamellar lipid membranes vesicles (150 nm) embedded with viral membrane proteins such as hemagglutin and neuraminidase of influenza virus but devoid of nucleocapsid including the genetic material of the source virus. These proteins enable the virosome membranes to fuse with cells of the immune system and thus deliver their contents—the specific antigens—directly to their target cells, eliciting a specific immune response even with weak-immunogenic antigens. Once they have delivered the antigens, the virosomes are completely degraded within the cells. A Viral protein intercalated into the phospholipid bilayer not only confers structural stability and homogeneity to virosomal formulations, but it significantly contributes to the immunological properties of virosomes, which are clearly distinct from other liposomal and proteoliposomal carrier systems. It has been shown that a physical association between the virosome and the antigen of interest is a prerequisite for the full adjuvant effect of virosomes. Hence, virosomes represent vesicular systems into which antigens can be loaded into virosomes or adsorbed onto the virosomal surface through hydrophobic interactions.
Virosome immunopotentiation mechanisms
The nature of the elicited immune response to virosome formulations is dependent on whether the epitopes of the antigen are located on the surface of the virosome (PeviPRO™)[19] or inside the virosome (PeviTER™).[19] PeviPRO™ elicits a humoral immune response. The antigen is degraded in endosomes of the cell and, therefore, generates predominantly an MHC II antigen presentation. PeviTER™ formulated antigens generate in vivo not only a CD4+ and CD8+ positive response but are also able to induce a strong cytotoxic T-cell response (CTL). Virosomal encapsulation ensures a proper presentation of the antigens through the MHC I pathway because the antigen is delivered in a natural way into the cytosol of the antigen presenting cell.
Registered vaccines against hepatitis A (Epaxal®) and influenza (Inflexal®V)[20] have validated the excellent characteristics of virosomes as an adjuvant and carrier system. Together, these two vaccines are approved in over 45 countries, and more than 10 million patients have been immunized to date.
This new generation of vaccines offers additional benefits because the vaccines are effective even in immune-suppressed patients and in infants. Furthermore, they have a high safety profile as embedded viruses do not replicate.
EMULSION DELIVERY SYSTEMS
Emulsions are heterogenous liquid systems may be water-in-oil emulsions, oil-in-water emulsions, or more complex systems such as water-in-oil-in-water multiple emulsions, microemulsions, or nanoemulsions. Antigens are dissolved in a water phase and emulsified in the oil in the presence of an appropriate emulsifier. The controlled release characteristics of an emulsion are determined by factors such as viscosity of oil phase, oil-to-water phase ratio and emulsion droplet size. For example, high oil content can cause unnecessary injection site irritation and too large a droplet size can result in a physically unstable product there by reducing its shelf life. Squalene O/W[21] emulsion containing influenza vaccine was approved in Italy in 1997 and in several additional countries in 2000.
Huang et al.,[22] developed a novel emulsion-type vaccine delivery systems of the amphiphilic bioresorbable polymer poly(ethylene glycol)-block-poly(lactide-co-epsilon-caprolactone) (PEG-b-PLACL) using ovalbumin as model antigen. Results from physicochemical characterization studies and in vitro release studies showed that PEG-b-PLACL-emulsified formulations are composed of homogenous fine particles and are stable, reproducible and hence are advantageous over vaccines prepared with conventional adjuvants. In vivo studies in mice have shown that antigen-specific antibody titers and T-cell proliferative responses, as well as the secretion of IFN-gamma, were significantly enhanced for ovalbumin- PEG-b-PLACL-based emulsions.
POLYMERIC NANOPARTICLE DELIVERY SYSTEMS
Polymeric nanoparticles because of their size are preferentially taken up by the mucosa associated lymphoid tissue. They are extensively reviewed for nasal and oral delivery of vaccines. Limited doses of antigen are sufficient to induce effective immunization. Hence, the use of nanoparticles for oral delivery of antigens is suitable because of their ability to release proteins and to protect them from enzymatic degradation in the GIT.
Biodegradable PACA[23] nanoparticles have been shown to enhance the secretory immune response after their oral administration in association with ovalbumin in rats. PMMA nanoparticles[23] being very slowly degradable (30%-40% per year) appear to be particularly suitable for vaccine purposes because prolonged contact between antigen and immunocompetent cells favors persistent immunity. Nanoparticles labeled with MAb specific to M-cells increase the level of absorption of nanoparticulate vaccines and hence immune response.
Metal chelating polymers such as polyaminocarboxylic acids such as EDTA (ethylenediamine tetra-acetic acid), DTPA (diethylenetriamine-pentaacetic acid) form non-covalent complexes with antigenic epitopes and are useful in their controlled delivery in vivo.[24] The existence of at least one Histidine residue at the amino- or carboxyl-terminus of a biologic molecule (e.g., protein, peptidic antigen, or fusion construct with His tag) is an important factor contributing to binding of the biologic to the polymer as it results in improved specificity of binding of the biologic molecule to the metal ion in the metal affinity complex.
An effective prophylactic mucosal gene expression vaccine (GXV)[25] is made up of at least four different plasmid DNAs encoding corresponding RSV antigens, coacervated with chitosan to formulate nanospheres. When given by intranasal route in a murine model of RSV infection, nanospheres resulted in significant induction of RSV-specific antibodies, nasal IgA antibodies, cytotoxic T lymphocytes, and IFN-gamma production in the lung and splenocytes resulting in the reduction of viral titres. Other nanocarrier types that have been used as multivalent vaccine constructs include metallic oxide particles, polysaccharide-based spermine, alginate capsules (which are natural polymers) and synthetic biocompatible and biodegradable poly (D,L-lactide-co-glycolide) copolymer.
MICELLAR DELIVERY SYSTEMS
Micelles have been well investigated as potential antigen carriers. Micelles are self-aggregated clusters of amphiphilic surfactant molecules. Surfactants above critical micellar concentration orient themselves into micellar structures in order to avoid contact with incompatible external phase and can enclose lipophilic cavity or hydrophilic cavity (reverse micelle) thus promoting entrapment of antigens for their delivery into the body.
The invention[26] by Moyer describes methods and systems for generating a safe and effective oral smallpox vaccine for humans using a genetically defective strain of vaccinia virus to confer immunity following oral delivery of the vaccine. The vaccine invention can be delivered as a live virus with the ability to express viral proteins but unable to achieve complete lytic virus replication, or be delivered as viral antigens. Under the claimed methods, micelles, microstarch particles, omega-3 fatty acids, and other nanoparticles and immuno-potentiators are methods of preparing the vaccine for use.
Quay[27] describes system in which biologically active agent (genetically defective strain of vaccinia virus) and permeabilizing peptide are administered in combination with one or more mucosal delivery-enhancing agents such as mixed micelle as carrier leading to increased bioavailability and production of immunity following oral administration.
Berzofsky[28] et al. reported a method in which the mucosal tissue of the subject is contacted with a preparation comprising of a purified soluble antigen (cytokine) and adjuvants like cholera toxin (CT), mutant cholera toxin (MCT) or mutant-E. coli heat labile enterotoxin (MLT) for inducing a protective mucosal cytotoxic T-lymphocyte response in the human subject. The absorption-promoting agent is selected from a surfactant, mixed micelle, clyclodextrin or beta-cyclodextrin derivative.
Formulations and methods for transmucosal delivery of a beneficial agent use a combination of a pH-responsive component and a temperature-responsive component. The temperature-responsive component in aqueous solutions is capable of undergoing a temperature-dependent sol to gel phase transition. The temperature-responsive compound is an alkylene oxide copolymer capable of forming micelles in aqueous solutions. These formulations were found to have bioadhesive properties and hence are suitable for delivering wide variety of beneficial agents.
DENDRIMER-BASED DELIVERY SYSTEMS
Dendrimers are branched, synthetic polymers with layered architectures. By combining the multifunctional polymeric material with a biologically active substance in an aqueous loading environment, the carrier system can be administered as a drug delivery vehicle to a human subject. Radially layered poly (amidoamine-organosilicon) dendrimers (PAMAMOS)[29] are reverse unimolecular micelles that consist of hydrophilic, nucleophilic polyamidoamine (PAMAM) interiors and hydrophobic organosilicon (OS) exteriors. PAMAM or PPI [poly (propylene imine)] dendrimers, available under the trademark name of “Starburst” serve as nonviral gene transfer agents, enhancing the transfection of DNA by endocytosis and, ultimately, into the cell nucleus. A transfection reagent namely SuperFect™ consisting of activated dendrimers is available for commercial purposes.
Invention by Wright[30] features an Influenza vaccine having a dendrimer as an adjuvant. Vaccine contains an influenza antigen and a dendrimer in a physiologically compatible carrier. The use of the dendrimer makes it possible to adjuvant Influenza without producing a toxic complex since even a small amount of the dendrimer acts as an effective adjuvant. As a result the dose of influenza antigen necessary to yield a compatible antigenic response is substantially reduced compared to the dose of antigen given without the dendrimer.
A novel approach for the treatment of renal cell carcinomas uses a chimeric molecule comprising a granulocyte macrophage colony stimulating factor (GM-CSF)[31] attached to a G250 kidney cancer specific antigen which is transfected in to the cancerous cell by the use of dendrimer, there by providing a highly effective “vaccine” that raises an immune response directed against renal cell cancers. A dendritic structure consisting of a poly (N-isopropylacrylamide) segment, a poly (L-lysine) segment and a poly (lactic acid) segment or their respective derivatives has been disclosed.
ISCOMS—IMMUNOSTIMULATORY COMPLEXES
ISCOMs are spontaneously formed spherical open cage-like complexes when saponin, cholesterol, phospholipid, and immunogen, usually protein are mixed together and have typically a diameter of 30-80 nm. ISCOMs combine certain aspects of virus particles such as their size and orientation of surface proteins, with the powerful immunostimulatory activity of saponins. Unlike other vaccine adjuvants, ISCOMs have shown to promote a broad immune response by simultaneously promoting high levels of antibody and strong T cell responses, including enhanced cytokine secretion and activation of cytotoxic T lymphocyte responses in a variety of experimental animal models and has now progressed to phase I and II human trials.
Brunham and Murdin[32] describe a two-step immunization procedure against chlamydia infection by initial administration of Chlamydia protein followed by administration of a chlamydia protein in ISCOMs. Such Immunogenic compositions have utility as chlamydial vaccines and in diagnostic applications. Other ISCOM based vaccines are invented for infections by Moraxella, Helicobacter infections, Campylobacter infections.
ISCOM-based veterinary vaccine against equine influenza is commercially available.
EDIBLE VACCINES
Subunit vaccines contain specific macromolecules, i.e., one specific epitope from many epitopes present on the antigen. Subunit vaccines are thus safer over conventional vaccines as they eliminate the use of live viruses or microbes to stimulate immunity. But subunit vaccines involve expensive manufacturing procedures and are thermo labile necessitating cold chain storage from point of manufacture until vaccination which aggravates the expenses in providing costlier facilities like refrigeration to render stability to the preparation.
Production of vaccines in “plants” offer attractive benefits and overcome many of the above-mentioned limitations.
Plant vaccines serve as an inexpensive means of processing and expressing proteins that can be quite complex to handle as plants require only sunlight, water, and minerals to carry out the process.
Avoidance of contamination with animal pathogens, improved stability of heat labile vaccine components and oral delivery of resulting vaccines are few of many advantages obtained when plants are used for the expression of vaccines.
Both mucosal and systemic immune responses can be produced by the mucosal administration of a plant derived vaccine.
Production of edible vaccines
Edible vaccines[33] are produced by integrating gene cloning, tissue culture and plant transformation techniques. The first step in the process of creating an edible vaccine is the selection of a suitable immunogen. The gene encoding the immunogen is cloned into an expression vector that contains plant regulatory sequences capable of driving gene expression and indicating the gene's terminus. This vector is then used in plant transformation. For example, Agrobacterium[34] is a plant pathogen which during the process of infecting plants, transfers a portion of its DNA (t-DNA) into plant's genome by a process similar to conjugation. Scientists have exploited this property of Agrobacterium to transfer desired sequences through it into plant genome. Plant tissues are cultured and transformed cells are positively selected and regenerated into transgenic plants. It approximately takes 6 weeks to 18 months to produce a transgenic plant and depends on the type of species.
The expression of the Streptococcus mutants surface protein antigen A (SpaA) in tobacco has been demonstrated by Curtiss and Cardineau after incorporating transgenic tobacco tissue into the diet of mice.
Animal trials[35] demonstrating antigenicity of plant-derived vaccinogens include tobacco- and lettuce-derived hepatitis B surface antigen, a tobacco- and potato-derived bacterial diarrhea antigen, a potato-derived Norwalk virus antigen, and an Arabidopsis-derived foot-and-mouth disease antigen.
Plant viruses
Self-replicating plant viruses can also express foreign genes in plants but cannot pass on the genes to the future generation of plants. Thus, an additional step of inoculation of plant with chimeric virus is necessary for the production of plant-virus derived vaccines. However the high level of foreign protein expression (up to 2g/kg of plant tissue) within a short period (1–2 weeks after inoculation) makes this an attractive alternative for vaccine production. Before administration, chimeric virus particles are purified from plant tissues which are unpalatable, contain toxins and are not suitable for direct consumption. Intranasal immunization has been the only delivery mechanism to attain immune responses both mucosal and systemic for purified chimeric plant virus expressing a vaccinogen. However, immunogen should be protected from being digested if it is being given by oral route.
The use of plant viruses as carrier molecules for immunogens has started with the finding that an epitope from the foot-and-mouth disease virus (FMDV) was expressed on the surface of cowpea mosaic virus (CPMV). Chimeric plant viruses[35] were proven effective as carrier proteins for vaccinogens in 1994 after rabbits raised an immune response against purified chimeric CPMV particles expressing epitopes derived from human rhinovirus 14 (HRV-14) and HIV-1. Since then numerous reports have examined plant viruses as effective alternative vaccinogen expression vectors. Antibodies have been stimulated in mice after injection with plant-virus-derived HIV-1 epitopes, mouse zona pellucida epitope and rabies virus epitope, whereas complete protection was conferred by a plant-virus-derived canine parvovirus epitope in a mink challenge trial.
DNA VACCINES
DNA vaccines consist of bacterial plasmids into which specific sequences are incorporated. Gene expression is promoted by the cytomegalovirus promoter and its adjacent intron A sequence (ensures high transcription efficiency) and elements like a transcription termination signal and a prokaryotic antibiotic resistance gene.
DNA inserted in the plasmid stimulates immunity by acting as a pathogen-associated molecular pattern (PAMP) which has high affinity for Toll-like receptors (TLRs). TLRs are “pattern recognition receptors” with an ability to identify the conserved molecular patterns of the DNA associated with pathogens. One such sequence that is common in bacterial DNA but rare in mammalian DNA is the hypomethylated CpG dinucleotide that mainly binds to TLR-9. Stimulation of a range of TLR9-expressing cells, including B cells and dendritic cells (DC) leads to a cascade of activation, proliferation and differentiation of natural killer cells, T cells and monocytes/macrophages. Attempts are now being made by the industry to use synthetic CpG phosphorothioate oligonucleotides as adjuvants for a range of different vaccines. However one reason for which DNA vaccines may not be effective for human application is that, TLR9 is not expressed by myeloid dendritic cells but only on plasmacytoid dendritic cells of the mammals. Interestingly, it was found that DNA vaccines perform well in Tlr9–/– mice, which indicates that there are alternate pathways apart from TLR-9 stimulation for inducing immune responses.
A DNA fusion vaccine designed to activate immunity against B-cell Lymphoma[36]
Genes encoding for variable regions (Vh, Vl) of tumor specific antigens (Idiotypic determinants) expressed by B-cell lymphoma were assembled as single chain Fv (scFv). But this fragment is weakly immunogenic. The fusion of 3′ position of scFv with a gene encoding the fragment C portion of tetanus toxin gave a DNA fusion vaccine and lead to the amplification of the immune response and suppression of lymphoma growth. Polyclonal and monoclonal anti-Id antibodies showed clinical effects but raising patient-specific antibodies is practically difficult. DNA vaccines avoid this problem as Id determinants can be expressed by using the variable region genes, either as whole or as single chain Fv (scFv) fused with a sequence derived from tetanus toxin (fragment C (FrC)) to the scFv.
DNA VACCINE DELIVERY STRATEGIES
Physical methods
Techniques such as tattooing, gene gun, electroporation, ultrasound, and laser provide energy (electrical, ultrasonic, laser beam) that brings about a transient change in permeability of cell membrane thereby promoting the entry of immunogenic DNA into the cells. The cell permeability is restored on the removal of applied energy after a short time period.
Tattooing
It is a physical method for injecting DNA into skin cells.
The effect of two adjuvants, cardiotoxin and plasmid DNA carrying the mouse granulocyte macrophage colony stimulating factor (GM-CSF)[37] when given by tattooing and as intramuscular injections has been determined. Model antigen used in this study was gene encoding the capsid protein of the human papillomavirus type 16 (HPV16). From the results, it is concluded that the delivery of the HPV16 L1 DNA alone using a tattoo device elicited a stronger and more rapid humoral and cellular immune responses than intramuscular needle delivery together with molecular adjuvants.
Gene gun
Gene gun is a biolistic device that enables the DNA to directly enter into the cell following bombardment of target DNA in the gene gun chamber kept against the target site.
In a study carried out by Jane McAllister and David Proll,[38] four groups of mice were immunized with plasmid DNA containing the LacZ gene encoding β-galactosidase. 3 groups of mice received shots of 1 μg of DNA coated onto gold microcarriers through gene gun intradermally (ID). Gold microcarriers of 0.6, 1.0, and 1.6 μm size were used respectively. The fourth group received three doses of 100 μg DNA in saline as intramuscular (IM) injection. Antigen-specific IgG titres were found to be higher in mice receiving intradermal vaccination than IM vaccinated mice. From this it is concluded that gene gun immunization is more effective over IM injection as DNA from former is directly shot into the target cells where as DNA from the latter must enter the cell before protein (antigen) synthesis. As a result though the dose of DNA administered via gene gun is only 1/100th of dose injected intramuscularly, a greater proportion of the administered DNA is used for antigen synthesis.
Electroporation
This technique involves application of electrical pulses to the skin thereby creating transient pores in the skin promoting the entry of DNA into the cell. On removal of electrical energy, skin regains its structure holding the entrapped immunogenic agent due to closure of pores.
Chron Vac-C,[39] a therapeutic DNA vaccine given to patients already infected with the virus in order clear the infection by boosting immune response, showed acceptable safety when delivered by electroporation in phase I/II clinical study at Karolinska University Hospital. This clinical study was carried out at the Infectious Disease Clinic and Center for Gastroenterology at the Karolinska University Hospital in Sweden. This was among the first infectious disease DNA vaccine to be delivered in humans using electroporation-based DNA delivery.
In addition DNA vaccine delivery by electroporation is being investigated in many cancers such as prostrate cancer, metastatic melanoma and is under clinical trials.
Ultrasound
In this ultrasonic energy is used to disrupt the cell membrane temporarily.
In a phase II study, repeated intranodal injections of adenovirus- CD 154 (Ad-ISF35)[39] are being given by ultrasound, in subjects with chronic lymphocytic leukemia/small lymphocytic lymphoma.
Ultrasound and laser are emerging techniques for the delivery of DNA vaccines.
Viral and nonviral methods of DNA vaccine delivery
Viral vectors such as retrovirus, adeno virus, herpes simplex virus, vaccinia virus are efficient in DNA transfer due to their nanoscale dimensions, well characterized surface properties allowing the incorporation of immunogenic components (e.g., virosomes). But drawbacks such as the limited DNA carrying capacity, toxicity, immunogenicity, the possibility of insertional mutations in host DNA and high cost warrants their use.
Nonviral carriers include microspheres, nanospheres, liposomes as discussed in the above sections find potential application as carriers for DNA vaccines.
MUCOSAL DELIVERY OF VACCINES
Mucosal vaccination offers protection against microorganisms which gain access to body via mucosal membranes. Patient compliance, ease of administration, reduction in possibility of needle-borne injections, stimulation of both systemic and mucosal immunity are some of the advantages.
Coadministration of antigens with adjuvants like aluminium hydroxide, complete Freunds adjuvant, incomplete freunds adjuvant, cholera toxin, heat labile enterotoxin of E. coli, etc., potentiated immune response of antigen. For example, Freunds adjuvant when administered subcutaneously to neonatal mice-induced mixed T helper1 and 2 responses with interferon-ψ component against Helicobacter pylori infection.
Delivery systems like PLG microspheres, PLGA microparticles carrying immunogenic agents etc are taken up by Peyers patches. Particles of <5 μm further move into lymph nodes and spleen-stimulating-specific IgG, IgM responses. Chitosan, a bioadhesive polysaccharide discussed earlier is suitable for mucosal vaccination due to its ability to open up tight junctions and promote paracellular transport of antigen across mucosa.
Nasal mucosa delivery
nasal mucosa[40] is the first contact site for antigens being inhaled, systemic and local immunity can be stimulated by activation of T-cells, B-cells, and dendritic cells present in nasal associated lymphoid tissue located beneath nasal epithelium in the form of IgG and secretory IgA. Hence, nasal delivery of vaccines can be used to treat upper respiratory tract infections and also to produce systemic immunity.
Intranasal vaccines include those against influenza A and B virus, proteosoma-influenza, adenovirus-vectored influenza, group B meningococcal native, attenuated respiratory syncytial virus and parainfluenza 3virus.
NEEDLE-FREE DELIVERY
Needle-free vaccine delivery[41] is gaining popularity these days due to the following reasons:
Patient's concern about pain associated with the injections; disposal issues and potential for cross contamination of blood borne diseases is eliminated.
Differentiate their products from the existing products as the pharmaceutical industry faces massive losses in revenues from the expired patents and to withstand pressure from generic companies.
Search for alternative ways to deliver growing list of new biopharmaceutical and molecular entities like vaccines, DNA, peptides and proteins that cannot be delivered orally.
Urge to evolve into specialty pharmaceutical companies developing their own branded pharmaceutical products, based on off-patented drugs.
Following are some needle-free delivery strategies
Jet injectors
Liquid jet injectors
Solid dose jet injectors
Microneedles
Melt in mouth strips
JET INJECTORS
Jet injectors uses either a spring mechanism or pressurized gases—generally carbon dioxide, nitrogen, helium contained in small cartridge or large canister form to force the aerosolized drug solution or suspension through the skin, either directly into the muscles or into the subcutaneous or intradermal layers.
Liquid jet injectors
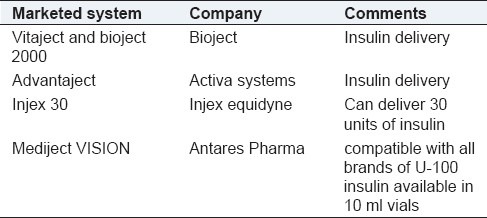
Liquid jet injectors are being developed as single use and multiuse systems. Reusable systems are for chronic conditions like diabetes where dose can be given once daily for prolonged release. Disposable units are prefilled with drug, once used can be discarded. These are used in emergency situations like treating allergies, intermittent conditions like pain and migrane attacks, for office based vaccinations and mass vaccination (spread of diseases due to reuse of needles can be prevented). These systems use a power source which can be compressed gas or a spring forcing the liquid under high pressures resulting in the formation of pores in the skin without the use of a needle. This is followed by reduced pressure profile which forces the rest of liquid into the skin. Since the drug is in liquid form there is no need of reformulation. Table 3 gives some of the examples of marketed liquid jet injectors.
Table 3.
Marketed liquid jet injectors
Walther[42] et al. showed that the needle-free, low-volume jet injection of small amounts of naked plasmid DNA for β-galactosidase (LacZ) and green fluorescence protein reporter gene constructs in different preclinical tumor models is successfully and safely employed for nonviral gene transfer. Qualitative and quantitative analysis of jet-injected tumors revealed efficient gene expression which indicates deep penetration of DNA and improved intratumoral dispersion. Based on these strong preclinical data, they conducted a phase I gene transfer trial using jet injection for intratumoral application of small amounts of plasmid DNA. Gene transfer efficiency was evaluated by analyzing LacZ expression quantitatively and qualitatively at the mRNA and protein levels. The results of this gene transfer trial support its great potential for therapeutic applications to locally treat accessible metastases from breast cancer or melanoma and other solid tumors. Further, preclinical studies with small interfering RNA delivered through jet injectors indicate that this technique can be employed in other areas such as cancer gene therapy approaches such as DNA vaccination, immunogene therapy, or gene suppression strategies. Also, therapeutic in vivo jet-injection transfer of the cytosine deaminase suicide gene or the human tumor necrosis factor-a gene showed significant tumor growth inhibition.
Limitations
Careful control over power source is necessary for accurate and reliable delivery of vaccine to different skin types or different skin areas of same person.
Bleeding and pain caused when the high speed jet bombards with blood vessel and nerves is the other major limitation that can compromise patient compliance.
Solid dose injectors
Delivering vaccines in solid form ensures that the therapeutic or immunogenic agent is more stable and avoids any need of cold chain storage. Both the prime dose and booster shots can be combined in a single administration there by increasing patient compliance.
Powderject technology[43] used for the delivery of solid formulations fires the drug at supersonic velocities into outer layers of skin using a helium powered devices. In practice, the device is held against the skin and when the helium microcylinder is actuated, the pressurized gas entrains the drug particles and accelerates them to velocities which enable them to penetrate the skin. This technology is in development by Corgentech to develop a local anesthetic agent and by Pfizer for the delivery of DNA vaccines on gold carrier particles.
Glide solid dose injector technology[44] pushes the pharmaceutical material (formulated as tiny solid rod with drug and excipients) by means of spring powered hand actuator device. When preset spring force is achieved the actuator triggers and automatically delivers the drug. The pushing action is important as it delivers the drug in a controlled manner at the depth of skin every time irrespective of the skin type and location. In this way, it is advantageous over powderject where in it is difficult to fix a velocity that will work accurately and reliably for all patients. The actuator can be fabricated as disposable unit or reusable unit incase if multiple doses are to be delivered. In the latter case actuator is retained and preloaded drug cassettes are supplied.
MICRONEEDLES
Microneedle consists of an array of microstructured projections coated with a drug or vaccine that is applied to the skin to provide intradermal delivery of active agents, which otherwise would not cross the stratum corneum. The delivery of vaccines or drugs is not by diffusion as in transdermal delivery systems but by a temporary mechanical disruption of the skin leading to the placement of the drug or vaccine within the epidermis, where it can more readily reach its site of action. Microneedles are fabricated on the micro scale (1 μm in diameter and range from 1 to 100 μm in length) and this differentiates them from conventional needles. They are made up of various materials such as: metals, silicon, silicon dioxide, polymers, glass and other materials. They can be designed to be long enough to penetrate the stratum corneum, but short enough not to puncture nerve endings. This reduces the chances of pain, infection, or injury.
Types of microneedles
Solid (straight, bent, filtered)
Hollow needles: Hollow needle designs include arrays of hollow needles with tapered and beveled tips that can contain and deliver microliter quantities of drugs/vaccines using simple diffusion or a pump system to very specific locations thus enabling their targeting.
Applications of microneedles
Solid microneedles could be used with drug patches to increase diffusion rates; increase permeability by poking holes in skin, rub drug over area, or coat needles with agent to be delivered.
Hollow needles could be used with drug patches and timed pumps to deliver drugs at specific times.
Also, these microneedles could be used to remove fluid from the body for analysis – such as blood glucose measurements – and to then supply microliter volumes of insulin or other drug as required.
These are capable of very accurate dosing, complex release patterns, promote local delivery and biological drug stability enhancement by storing in a micro volume that can be precisely controlled.
Recent works on microneedles
Gill et al. studied on uniformity of coating of compounds like calcein, vitamin B, bovine serum albumin and plasmid DNA, modified vaccinia virus and microparticles (1--20 μm diameter) on both individual and arrays of microneedles by using a novel micron-scale dip-coating process.
Matriano et al.[45] examined the use of Microneedles coated with a dry-film of antigen to deliver ovalbumin as a model protein antigen by inserting them into the skin of hairless guinea pigs in vivo using a high-velocity injector.
Lee et al.[46] has studied on microneedles encapsulated with proteins, DNA etc that dissolvke within the skin for bolus or sustained delivery without leaving any biohazardous sharp medical waste for transdermal drug delivery. Such dissolving microneedles can be fabricated with polymers like PVP (generally used as plasma expander and hence safe) to deliver inactivated influenza virus in lyophilized form for influenza vaccination which targeted the delivery of vaccine to skin's antigen presenting cells and generated robust immune responses. Polylactic acid, polyglycolic acid, and their copolymers are widely used in the fabrication of biodegradable polymer microneedles.[47]
MELT IN MOUTH STRIPS[48]
0As the name indicates, these strips containing immunogens are meant to dissolve in child's mouth. Undergraduate students at John Hopkins in collaboration with Aridis Pharmaceuticals developed these strips for protection against rotavirus infection. Rotavirus is a common cause of severe diarrhea and vomiting in children, leading to about 600 000 deaths annually. Rotavirus vaccine at present is available in a liquid or freeze-dried form that must be chilled for transport and storage, making it very expensive for use in impoverished areas. In addition, newborns sometimes spit out the liquid, a problem that is less likely to occur with a strip that sticks to and dissolves on the tongue in less than a minute.
CONCLUSION
Vaccine drug delivery systems are gaining popularity these days due to the benefits they offer. Vaccine drug delivery systems are now being proven to be patient friendly as they avoid the need to administer booster doses and provide a long term therapy in small doses. Their use is further encouraged by administering them via needle-free technologies. Edible vaccines on the other hand open an attractive avenue for the oral delivery of vaccines.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Elgert KD. Immunology: Understanding the immune system. 2nd ed. United States: Wiley-Blackwell; 2009. p. 629. [Google Scholar]
- 2.Carino GP. Vaccine Delivery. In: Mathiowitz E, editor. Encyclopedia of Controlled Drug Delivery. Vol. 2. United States: Wiley Interscience; 1999. p. 996. [Google Scholar]
- 3.Oyewumi MO, Kumar A, Cui Z. Nano-microparticles as immune adjuvants: Correlating particle sizes and the resultant immune responses. Expert Rev Vaccines. 2010;9:1095–107. doi: 10.1586/erv.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grooves MJ. Parenteral Drug Delivery. In: Mathiowitz E, editor. Encyclopedia of Controlled Drug Delivery. Vol. 2. United States: Wiley Interscience; 1999. p. 764. [Google Scholar]
- 5.Shi S, Hickey AJ. PLGA microparticles in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen TB10.4-Ag85B. Pharm Res. 2010;27:350–60. doi: 10.1007/s11095-009-0028-7. [DOI] [PubMed] [Google Scholar]
- 6.Amselem S, Alving CR, Domb AJ. Polymeric biodegradable lipospheres™ as vaccine delivery systems. [Last accessed on 2010 Jan 25];Polym Adv Technol. 1992 3:351–7. Available from: http://www.onlinelibrary.wiley.com/doi/10.1002/pat.1992.220030611/abstract . [Google Scholar]
- 7.Dineshkumar B, Dhanaraj SA, Santhi K, Vijayan P, Raghu Chandrasekhar. Single dose vaccine delivery system of tetanus toxoid formulation based on chitosan microspheres. [Last accessed on 2010 Jan 25];Int J Advances in Pharm Sci. 2010 1:42–9. Available from: http://www.arjournals.org/ijaps1/ijaps.2010.0976.1055.01004.pdf . [Google Scholar]
- 8.Sexton A, Whitney PG, Chong SF, Zelikin AN, Johnston AP, De Rose R, et al. A protective vaccine delivery system for in vivo T cell stimulation using nanoengineered polymer hydrogel capsules. ACS Nano. 2009;3:3391–400. doi: 10.1021/nn900715g. [DOI] [PubMed] [Google Scholar]
- 9.Jones DH, McBride BW, Thornton C, O’Hagan DT, Robinson A, Farrar GH. Orally administered microencapsulated Bordetella pertussis fimbriae protect mice from B. pertussis respiratory infection. Infect Immun. 1996;64:489–94. doi: 10.1128/iai.64.2.489-494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh J, Pandit S, Bramwell VW, Alpar HO. Diphtheria toxoid loaded poly-(epsilon-caprolactone) nanoparticles as mucosal vaccine delivery systems. Methods. 2006;38:96–105. doi: 10.1016/j.ymeth.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Raghuvanshi RS, Katare YK, Lalwani K, Ali MM, Singh O, Panda AK. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. Int J Pharm. 2002;245:109–21. doi: 10.1016/s0378-5173(02)00342-3. [DOI] [PubMed] [Google Scholar]
- 12.Shahiwala A, Vyas TK, Amiji MM. Nanocarriers for Systemic and Mucosal Vaccine Delivery. Recent Pat Drug Deliv Formul. 2007. [Last accessed on 2010 Jan 25]. pp. 11–9. Available from: http://www.bentham.org/ddf/samples/ddf%201-1/Amiji.pdf . [DOI] [PubMed]
- 13.Sijun H, Yong X. Helicobacter pylori vaccine: Mucosal adjuvant and delivery systems. Indian J Med Res. 2009;130:115–24. [PubMed] [Google Scholar]
- 14.Saluja V, Amorij JP, Van Roosmalen ML, Leenhouts K, Huckriede A, Hinrichs WL, et al. Intranasal Delivery of Influenza Subunit Vaccine formulated with GEM particles as an Adjuvant. AAPS J. 2010;12:109–16. doi: 10.1208/s12248-009-9168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mengiardi B, Berger R, Just M, Glück R. Virosomes as carriers for combined vaccines. Vaccine. 1995;13:1306–15. doi: 10.1016/0264-410x(95)00028-y. [DOI] [PubMed] [Google Scholar]
- 16.Gorse GJ, Corey L, Patel GB, Mandava M, Hsieh RH, Matthews TJ, et al. HIV-1MN recombinant glycoprotein 160 vaccine-induced cellular and humoral immunity boosted by HIV-1MN recombinant glycoprotein 120 vaccine. National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. AIDS Res Hum Retroviruses. 1999;15:115–32. doi: 10.1089/088922299311547. [DOI] [PubMed] [Google Scholar]
- 17.Rathore D, McCutchan TF. The cytotoxic T-lymphocyte epitope of the Plasmodium falciparum circumsporozoite protein also modulates the efficiency of receptor-ligand interaction with hepatocytes. Infect Immun. 2000;68:740–3. doi: 10.1128/iai.68.2.740-743.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rentel CO, Bouwstra JA, Naisbett B, Junginger HE. Niosomes as a novel peroral vaccine delivery system. Int J Pharm. 1999;186:161–7. doi: 10.1016/s0378-5173(99)00167-2. [DOI] [PubMed] [Google Scholar]
- 19.Moser C, Amacker M, Kammer AR, Rasi S, Westerfeld N, Zurbriggen R. Influenza virosomes as a combined vaccine carrier and adjuvant system for prophylactic and therapeutic immunizations. Expert Rev Vaccines. 2007;6:711–21. doi: 10.1586/14760584.6.5.711. [DOI] [PubMed] [Google Scholar]
- 20.Herzog C, Hartmann K, Künzi V, Kürsteiner O, Mischler R, Lazar H, et al. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27:4381–7. doi: 10.1016/j.vaccine.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 21.O’Hagan DT, Tsai T, Reed S. Emulsion-based adjuvants for improved influenza vaccines. In: Rappuoli R, Giudice GD, editors. Influenza vaccines for the future. Vol. 2. Basel: Springer; 2010. [Last accessed on 2010 Jan 25]. pp. 327–57. Available from: http://www.springerlink.com/content/r532776816880001/ [Google Scholar]
- 22.Huang MH, Chou AH, Lien SP, Chen HW, Huang CY, Chen WW, et al. Formulation and immunological evaluation of novel vaccine delivery systems based on bioresorbable poly(ethylene glycol)-block-poly(lactide-co-epsilon-caprolactone) J Biomed Mater Res B Appl Biomater. 2009;90:832–41. doi: 10.1002/jbm.b.31352. [DOI] [PubMed] [Google Scholar]
- 23.De Jaeghere F, Doeker E, Gurny R. Nanoparticles. In: Mathiowitz E, editor. Encyclopedia of Controlled Drug Delivery. Vol. 2. United States: Wiley Interscience; 1999. p. 660. [Google Scholar]
- 24.Turnell W, Gomurashvill Z, Parcher B, Hughes J, Anderl J. Biodegradable metal-chelating polymers and vaccines. Google Patents. 2009. Patent application number: 201000043902010. Available from: http://www.faqs.org/patents/app/20100004390#ixzz0y6Sf8jdT .
- 25.Mohapatra SS. Mucosal gene expression vaccine: A novel strategy for respiratory syncytial virus. Pediatr Infect Dis J. 2003;22(2 Suppl):S100–3. doi: 10.1097/01.inf.0000053894.31944.26. [DOI] [PubMed] [Google Scholar]
- 26.Moyer MP. Oral smallpox vaccine production and methods to evaluate safety, efficacy, and potency of orally delivered vaccine. Google Patents. 2003. [Last accessed on 2010 Jan 25]. Available from: http://www.freepatentsonline.com/y2004/0175398.html .
- 27.Shahiwala A, Vyas TK, Amiji MM. Nanocarriers for Systemic and Mucosal Vaccine Delivery. [Last accessed on 2010 Jan 25];Recent Pat Drug Deliv Formul. 2007 1:1–9. doi: 10.2174/187221107779814140. Available from: http://www.bentham.org/ddf/samples/ddf%201-1/Amiji.pdf . [DOI] [PubMed] [Google Scholar]
- 28.Belyakov IM, Ahlers JD, Clements JD, Strober W, Berzofsky JA. Interplay of cytokines and adjuvants in the regulation of mucosal and systemic HIV-specific CTL. J Immunol. 2000;165:6454–62. doi: 10.4049/jimmunol.165.11.6454. [DOI] [PubMed] [Google Scholar]
- 29.Heegaard PM, Boas U, Sorensen NS. Dendrimers for Vaccine and Immunostimulatory Uses. A Review. [Last accessed on 2010 Dec 27];Bioconjug Chem. 2009 21:405–18. doi: 10.1021/bc900290d. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19886668 . [DOI] [PubMed] [Google Scholar]
- 30.Wright, Craig D. Adjuvant properties of poly (amidoamine) dendrimers. Google Patents. 1998. [Last accessed on 2010 Dec 27]. Available from: http://www.freepatentsonline.com/5795582.html .
- 31.Esuvaranathan K, Mahendran R, Lawrencia C. Methods and compositions for delivery of pharmaceutical agents. Google Patents. 2010. [Last accessed on 2010 Dec 27]. Available from: http://www.freepatentsonline.com/7709457.html .
- 32.Dong-Ji Z, Yang X, Shen C, Lu H, Murdin A, Brunham RC. Priming with Chlamydia trachomatis major outer membrane protein (MOMP) DNA followed by MOMP ISCOM boosting enhances protection and is associated with increased immunoglobulin A and Th1 cellular immune responses. Infect Immun. 2000;68:3074–8. doi: 10.1128/iai.68.6.3074-3078.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gómez E, Zoth SC, Berinstein A. Plant-based vaccines for potential human application: A review. Hum Vaccin. 2009;5:738–44. doi: 10.4161/hv.5.11.9879. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt G, Gadermaier G, Pertl H, Siegert M, Oksman-Caldentey KM, Ritala A, et al. Production of recombinant allergens in plants. Phytochem Rev. 2008;7:539–52. doi: 10.1007/s11101-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walmsley AM, Arntzen CJ. Plants for delivery of edible vaccines. Curr Opin Biotechnol. 2000;11:126–9. doi: 10.1016/s0958-1669(00)00070-7. [DOI] [PubMed] [Google Scholar]
- 36.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: Precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8:108–20. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 37.Pokorna D, Rubio I, Müller M. DNA-Vaccination via tattoing induces stronger humoral and cellular immune responses than intramuscular delivery supported by molecular adjuvants. Genet Vaccines Ther. 2008;6:4. doi: 10.1186/1479-0556-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McAllister J, Proll D. Comparison of DNA vaccine delivery systems: intramuscular injection versus gene gun administration. CBRN Defense Centre, DSTO Platforms Sciences Laboratory, Australia. 2004. [Last accessed on 2010 Dec 27]. Available from: http://www.hdl.handle.net/1947/3727 .
- 39.Bolhassani A, Safaiyan S, Rafati S. Improvement of different vaccine delivery systems for cancer therapy. Mol Cancer. 2011;10:3. doi: 10.1186/1476-4598-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pires A, Fortuna A, Alves G, Falcão A. Intranasal Drug Delivery: How, Why and What for? [Last accessed on 2010 Dec 27];J Pharm Pharm Sci. 2009 12:288–311. doi: 10.18433/j3nc79. Available from: http://www.ejournals.library.ualberta.ca/index.php/JPPS/article/view/6188/5624 . [DOI] [PubMed] [Google Scholar]
- 41.Benette S, Potter C. When shove comes to push for needle free injections. [Last accessed on 2010 Dec 27];Drug Delivery Report Autumn/Winter. 2006 22:78. Available from: http://www.iptonline.com/articles/public/page78nonprint.pdf . [Google Scholar]
- 42.Walther W, Siegel R, Kobelt D, Knösel T, Dietel M, Bembenek A, et al. Novel Jet-Injection Technology for Nonviral Intratumoral Gene Transfer in Patients with Melanoma and Breast Cancer. Clin Cancer Res. 2008;14:7545–53. doi: 10.1158/1078-0432.CCR-08-0412. [DOI] [PubMed] [Google Scholar]
- 43.Burkoth TL, Bellhouse BJ, Hewson G, Longridge DJ, Muddle AG, Sarphie DF. Transdermal and transmucosal powdered drug delivery. Crit Rev Ther Drug Carrier Syst. 1999;16:331–84. doi: 10.1615/critrevtherdrugcarriersyst.v16.i4.10. [DOI] [PubMed] [Google Scholar]
- 44.Schiffelers R. IDrugs. Vol. 12. London, UK: 2009. Sep 2-4, Drug Delivery--Select Biosciences Inaugural Summit; pp. 679–82. [PubMed] [Google Scholar]
- 45.Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, et al. Macroflux microprojection array patch technology: A new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19:63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16:915–20. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104:51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 48.John Hopkins University. Department of Biomedical Engineering. Students Devise Oral Quick Dissolve Strips for Rotavirus Vaccine. News Release. 2007. May 14, [Last accessed on 2010 Dec 27]. Available from: http://www.jhu.edu/news_info/news/home07/may07/rotaviru.html .


