Abstract
In the present study we investigated the effect of polyamidoamine (PAMAM) dendrimers on the aqueous solubility of aceclofenac. The aqueous solubility of aceclofenac was measured in the presence of dendrimers in distilled water. The effect of variables, such as pH condition, concentration, temperature and generation (molecule size) of dendrimer, has been investigated. Results showed that the solubility of aceclofenac in the dendrimer solutions was proportional to dendrimer concentration. The order in which the dendrimers increased the solubility at a constant pH condition was G3 > G0. The influence of dendrimer solution pH on the solubility enhancement of aceclofenac suggests that it involves an electrostatic interaction between the carboxyl group of the aceclofenac molecule and the amine groups of the dendrimer molecule. The solubility of aceclofenac was inversely proportional to the temperature of dendrimer solution.Different generation (G0 and G3) PAMAM dendrimers have the potential to significantly enhance the solubility of poor water-soluble drugs.
Keywords: Aceclofenac, dendrimer, polyamidoamine dendrimers, solubility enhancement
INTRODUCTION
Drug delivery systems have revolutionized medicine by significantly improving the therapeutic efficacy and minimizing the side-effects of clinically established drugs.[1–3] Dendrimers have attracted much interest because their unique structures and properties make them promising new scaffolds for drug delivery.[4,5] Dendrimer, also called arborols molecules[6] or artificial proteins,[7] is derived from the Greek words dendron (tree) and meros (part).[8] They are monodisperse, well-defined artificial macromolecules which have highly branched, three-dimensional features that resemble the architecture of a tree, having defined molecular weight and host-guest entrapment properties.[9] The highly branched molecular structure of dendrimers resembles Christmas stars, ice crystals, or treetops.[10] A typical dendrimer consists of three basic components: a central core from which the polymeric branches emanate; repeat units, the nature of which determines the microenvironment of the interior and in turn the solubilization ability of the dendrimer; and the terminal surface groups, the nature and number of these groups are mostly responsible for the performance of dendrimers in solution.[11] The branched units are prearranged in layers called “generations” and correspond to the repeating monomer unit of these macromolecules.[8]
Dendrimers are divided into two families, compact and extended dendrimers.[12] They are prepared through divergent or convergent iterative procedures to obtained different sizes or generations.[13] Polyamidoamine (PAMAM) dendrimers are founded on an ethylenediamine core, and branched units are constructed from methyl acrylate and ethylenediamine.[11] PAMAM dendrimers are biocompatible, nonimmunogenic, water-soluble[14] and possess empty internal cavities and have a much higher amino group density compared with conventional macromolecules, e.g., a third-generation PAMAM prepared from ammonia core has 1.24 × 10–4 amine moieties per unit volume (cubic Angstrom units) in contrast to the 1.58 × 10–6 amine moieties per unit volume of a conventional star polymer which are responsible for high solubility and reactivity.[15,16] These specific properties make dendrimers suitable for drug delivery systems.[17–19] As poor solubility is generally related to a low bioavailability, this presents a major challenge during drug formulation.[20] Hydrophobic drugs can be interacted with the dendrimer to make them water-soluble.[21] Drugs can either be attached to dendrimers’ end groups or encapsulated in the macromolecule interior.[22] The high density of amino groups and the special structure in PAMAM dendrimers may be projected to have potential applications in enhancing the solubility of the low aqueous soluble drugs.[23] Water-soluble polymer drug conjugates prolong the half-life of drugs.[24] Duncan and co-workers[25,26] have prepared conjugates of PAMAM dendrimers with cisplatin, a potent anticancer drug with nonspecific toxicity and poor water solubility. The conjugates show increased solubility and decreased systemic toxicity.
Aceclofenac, a phenylacetic acid derivative, non-steroidal anti-inflammatory drug (NSAID), is used in the management of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis. Though the use of such drugs is restricted by their significant toxicity the commonest adverse effects of NSAIDs are usually gastrointestinal disturbances,[27] such as gastrointestinal discomfort, nausea, and diarrhea. It is clear that usually most NSAIDs can damage the esophagus, stomach, duodenum, small intestine and large intestine.[28] It was suggested that the parenteral use of NSAIDs could control these proven side-effects. But due to poor aqueous solubility of NSAIDs, their use in topical and parenteral formulations has also been limited. The poor aqueous solubility of the drug is generally related to a low bioavailability, which requires extensive efforts to enhance aqueous solubility.[20] The solubility of Nifedipine,[14] Ibuprofen,[29] nicotinic acid,[30] ketoprofen,[31] and furosemide[32] has been tested with dendrimer. In the present study we investigated the effect of the concentration of G3 PAMAM dendrimer, generation of dendrimer, pH condition and temperature on increasing the solubility of hydrophobic drug, aceclofenac.
MATERIALS AND METHODS
Materials
Aceclofenac was a gift sample from Rantus Pharma Pvt. Ltd. (Hyderabad, India). G0 and G3 PAMAM dendrimer was a gift sample from Dendritech Inc. (Midland, USA). All other ingredients used were of pharmaceutical grade.
Methods
The solubility of aceclofenac in PAMAM dendrimer solutions in the range 0–10 mg/ml was determined in phosphate buffers (0.05 M NaH2PO4). Solubility studies were carried out using the Higuchi rotating bottle method.[33] The method used for sample preparation was similar for each system, i.e. excess aceclofenac was added to 5-ml vials containing 4 ml of each test solution. The vials were then incubated in an orbital shaking incubator for 24 h to provide sufficient time to reach equilibrium. After 24 h, samples were filtered through 0.45μ cellulose acetate filters (Sartorious Inc., Minnetonka, MN), and the absorbance of the aceclofenac test solutions at the characteristic wavelength 245 nm was measured using the UV spectrophotometer. The effect of pH on solubility was examined at pH 4, 7 and 10, which was achieved by drop-wise addition of either 1 M NaOH or 0.1 M HCl. The effect of temperature was examined at 25, 30, 35, 40, 45 and 50°C.
RESULTS AND DISCUSSION
Effect of PAMAM dendrimer concentration on the solubility of aceclofenac
Various trials of solubility experiments of aceclofenac were carried out using G0 PAMAM dendrimer (of molecular weight 517 Dalton and 4 amine groups on the exterior of molecules) and G3 PAMAM dendrimer (of molecular weight 6,909 Dalton and 32 amine groups on the exterior of molecules) and the results are shown in Figures 1 and 2. It was observed that the solubility of aceclofenac increased considerably with PAMAM dendrimer concentrations. In the presence of PAMAM dendrimer at a fixed pH condition, the solubility of aceclofenac in the dendrimer solutions increased in an almost linear manner with an increase in concentration of the PAMAM dendrimer. This was most probably due to the increase in the number of the surface amines group and internal cavities that are available to interact with aceclofenac molecules. Owing to this specific and interesting property of PAMAM dendrimers, the cavities in PAMAM dendrimers can keep small guest molecules inside and make dendrimers suitable for enhancing the solubility of hydrophobic drug molecules such as aceclofenac in aqueous solutions. Also, there are tertiary amines in these internal cavities, which could interact with the atoms of the aceclofenac molecules by hydrogen bond formation. Moreover, PAMAM dendrimers have primary amines on the surface, which could interact electrostatically with the carboxyl group in the aceclofenac molecules. G3 PAMAM dendrimers possess open and internal cavities and many functional terminal groups that are responsible for high solubility and reactivity. These specific properties make dendrimers suitable for drug delivery systems.[17]
Figure 1.
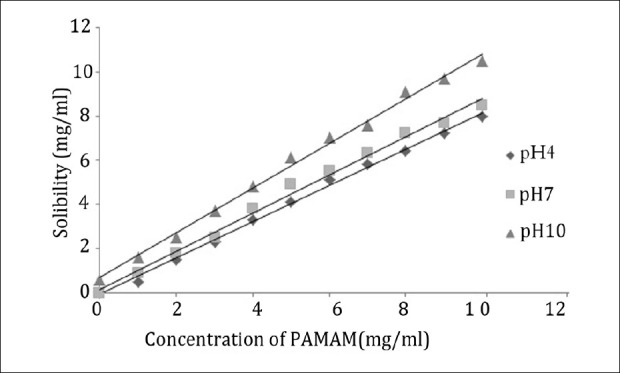
Solubility of aceclofenac in the presence of increasing concentration of G0 PAMAM dendrimer
Figure 2.
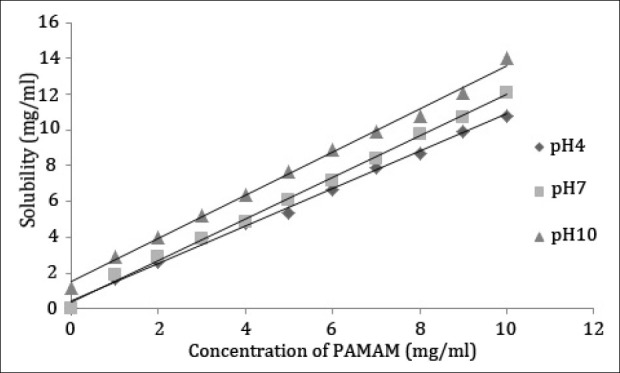
Solubility of aceclofenac in the presence of increasing concentration of G3 PAMAM dendrimer
Effect of different generations of PAMAM dendrimers on the solubility of aceclofenac
The effect of different generations of PAMAM dendrimers (G0 and G3) on the solubility of aceclofenac was investigated at pH 4, 7 and 10, respectively. The results are shown in Figure 1 and Figure 2, from which it is obvious that the solubility of aceclofenac was affected by the generation of PAMAM dendrimer. The solubility of aceclofenac in G3 PAMAM dendrimer solution was in fact higher than that in G0 PAMAM dendrimer solution. The solubility of hydrophobic compounds in dendrimer solutions likely depends on the dendrimer generation.[34] The solubility of aceclofenac in PAMAM dendrimer solutions, at a given pH condition, depends on the surface area and amino groups of PAMAM dendrimer particles, therefore a molecule of a higher generation PAMAM dendrimer particle has a privileged ability to absorb and interact with the aceclofenac molecule than that of a lower generation one.
Effect of pH condition on the solubility of aceclofenac in PAMAM dendrimer solutions
Solubility profiles of aceclofenac measured in the presence of the amine-terminated full-generation dendrimers at pH 4, 7, and 10 are shown in Figures 1 and 2 and the solubility was pH-dependent. The solubility of aceclofenac in PAMAM dendrimer solutions was highest at pH 10, less at pH 7, and least at pH 4. To determine the most effectual pH condition on the solubility of aceclofenac using PAMAM dendrimers, the solubility of aceclofenac in PAMAM dendrimer solution was investigated at a range of pH values, the concentration of G0 and G3 PAMAM dendrimer being constant.
The enhancement in solubility is might be attributed to an electrostatic interaction between the surface amine groups of dendrimer molecule and the carboxyl group of aceclofenac. Evidence for this is seen from the solubility of aceclofenac in dendrimer solutions over a range of pH values [Figures 1 and 2]. The weakly acidic aceclofenac molecule (pKa 4.7) is not fully ionized at low pH value and hence cannot freely interact electrostatically with the dendrimer molecule. The highest solubility of aceclofenac in dendrimer solutions at high pH was because of complete ionization of aceclofenac at pH 10, as a result, aceclofenac freely interacted electrostatically with the dendrimer molecule. It reveals that at low pH value there is lower significant improve of solubility of aceclofenac in dendrimer solution compared to that of high pH value.
Effect of temperature on the solubility of aceclofenac in PAMAM dendrimer solutions
Figure 3 shows that the amount of aceclofenac dissolved in G0 and G3 PAMAM dendrimers was inversely proportional to temperature. The reason of this effect is under further research.
Figure 3.
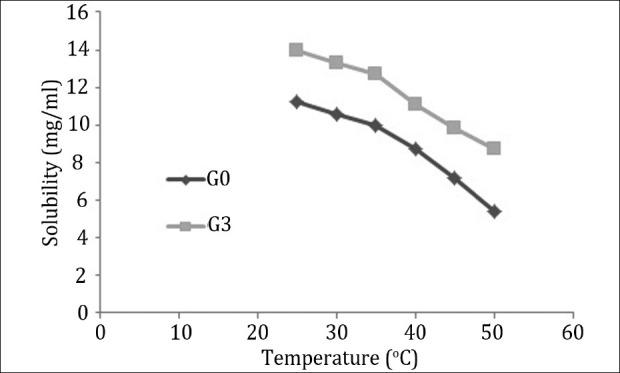
Solubility of aceclofenac in PAMAM dendrimer solution at various temperatures
CONCLUSION
Different generation (G0 and G3) PAMAM dendrimers have the potential to significantly enhance the solubility of poor water-soluble drugs such as aceclofenac. The aceclofenac solubility depends on the concentration of the dendrimer, the pH value of the solution, and the generation of the dendrimer. All observations are evidence of interactions between the carboxyl group of the aceclofenac molecule and the surface amine groups of the dendrimer molecule. The solubility of aceclofenac in dendrimer solution was inversely proportional to the temperature under the experiment performed.
ACKNOWLEDGMENT
We are grateful to Dendritech Inc. (USA) for the gift sample of dendrimer. We are thankful to Rantus Pharma Pvt. Ltd. (Hyderabad, India) for providing gift sample of aceclofenac.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–60. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 2.Twaites B, Alarcon CD, Alexander C. Synthetic polymers as drugs and therapeutics. J Mater Chem. 2005;15:441–55. [Google Scholar]
- 3.Henck JO, Byrn SR. Designing a molecular delivery system within a preclinical time frame. Drug Discov Today. 2007;12:189–99. doi: 10.1016/j.drudis.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Bosman AW, Janssen HM, Meijer EW. About dendrimers: Structure, physical properties and applications. Chem Rev. 1999;99:1665–88. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]
- 5.Tomalia DA, Frechet JMJ. Discovery of dendrimers and dendritic polymers: A brief historical prospective. J Polym Sci APolym Chem. 2002;40:2719–28. [Google Scholar]
- 6.Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–37. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Svenson S, Tomalia DA. Dendrimers in biomedical applications-reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–29. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Aulenta F, Hayes W, Rannard S. Dendrimers: A new class of nanoscopic containers and delivery devices. Eur Polym J. 2003;39:1741–71. [Google Scholar]
- 9.Yiyun C, Tongwen X. Dendrimers as potential drug carriers. Part I. solubilization of non-steroidal anti-inflammatory drugs in the presence of polyamidoaminedendrimers. Eur J Med Chem. 2005;40:1188–92. doi: 10.1016/j.ejmech.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Vogtle F, Gestermann S, Hesse R, Schwierz H, Windisch B. Functional dendrimers. Prog Polym Sci. 2000;25:987–1041. [Google Scholar]
- 11.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, et al. A new class of polymers: starbust-dendrimtic macromolecules. Polym J. 1985;17:117–32. [Google Scholar]
- 12.Supritz C, Engelmann A, Reineker P. Optical absorption in dendrimers. J Lumin. 2004;110:122–3. [Google Scholar]
- 13.Maes W, Verstappen B, Dehaen W. Synthesis of 1, 2, 4-triazole dendrimers. Tetrahedron. 2006;62:2677–83. [Google Scholar]
- 14.Devarakonda B, Hill RA, de Villiers MM. The effect of PAMAM dendrimer generation size and surface functional group on the aqueous solubility of nifedipine. Int J Pharm. 2004;284:133–40. doi: 10.1016/j.ijpharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Tomalia DA, Naylor AM, Goddard WA. Starburst dendrimers: Molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew ChemInt Ed Engl. 2003;29:138–75. [Google Scholar]
- 16.Neerman MF, Zhang W, Parrish AR, Simanek EE. In vitro and in vivo evaluation of a melamine dendrimer as a vehicle for drug delivery. Int J Pharm. 2004;281:129–32. doi: 10.1016/j.ijpharm.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Esfand R, Tomalia DA. Poly (amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6:427–36. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 18.Schiavon O, Pasut G, Moro S, Orsolini P, Guiotto A, Veronese FM. PEG–Ara–C conjugates for controlled release. Eur J Med Chem. 2004;39:123–33. doi: 10.1016/j.ejmech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Brana MF, Dominguez G, Saez B, Romerdahl C, Robinson S, Barlozzari T. Synthesis and anti-tumor activity of new dendritic polyamines–(imide–DNA–intercalator) conjugates: Potent Lck inhibitors. Eur J Med Chem. 2002;37:541–51. doi: 10.1016/s0223-5234(02)01362-4. [DOI] [PubMed] [Google Scholar]
- 20.Lagrange F, Pénhourcq F, Matoga M, Bannwarth B. Binding of ketoprofen Enantiomers in various human albumin preparations. J Pharm Biomed Anal. 2000;23:793–802. doi: 10.1016/s0731-7085(00)00380-0. [DOI] [PubMed] [Google Scholar]
- 21.Patri AK, Kukowska-Latallo JF, Baker JR. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliv Rev. 2005;57:2203–14. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Furuta P, Frechet JM. Controlling solubility and modulating peripheral function in dendrimer encapsulated dyes. J Am ChemSoc. 2003;125:13173–81. doi: 10.1021/ja037133f. [DOI] [PubMed] [Google Scholar]
- 23.Newkome GR, Moorefield CN, Baker GR. Alkane cascade polymers processing micellar topology: tderivatives. Angew ChemInt Ed Engl. 2003;30:1178–80. [Google Scholar]
- 24.Kono K, Kojima C, Hayashi N, Nishisaka E, Kiura K, Watarai S, et al. Preparation and cytotoxic activity of poly (ethylene glycol)- modified poly(amidoamine) dendrimers bearing adriamycin. Biomaterials. 2008;29:1664–75. doi: 10.1016/j.biomaterials.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Malik N, Evagorou EG, Duncan R. Dendrimer–platinate: Anovel approach to cancer chemotherapy. Anticancer Drugs. 1999;1:767–76. [PubMed] [Google Scholar]
- 26.Duncan R, Malik N. Dendrimers: Biocompatibility and potential for delivery of anticancer agents. Proc Int Symp Control Release Bioact Mater. 1996;23:105–6. [Google Scholar]
- 27.Polisson R. Nonsteroidal anti-inflammatory drugs: Practical and theoretical considerations in their selection. Am J Med. 1996;100:31S–36S. doi: 10.1016/s0002-9343(97)89544-7. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy DM. Comparative toxicity of nonsteroidal anti-inflammatory drugs. Am J Med. 1999;107:37S–47S. doi: 10.1016/s0002-9343(99)00366-6. [DOI] [PubMed] [Google Scholar]
- 29.Milhem OM, Myles C, McKeown NB, Attwood D, D’Emanuele A. Polyamidoamine Starburst® dendrimers as solubility enhancers. Int J Pharm. 2000;197:239–41. doi: 10.1016/s0378-5173(99)00463-9. [DOI] [PubMed] [Google Scholar]
- 30.Yiyun C, Tongwen X. Solubility of nicotinic acid in polyamidoamine dendrimer solutions. Eur J Med Chem. 2005;40:1384–9. doi: 10.1016/j.ejmech.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Yiyun C, Tongwen X, Rongqiang F. Polyamidoamine dendrimers used as solubility enhancers of ketoprofen. Eur J Med Chem. 2005;40:1390–3. doi: 10.1016/j.ejmech.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Devarakonda B, Otto DP, Judefeind A, Hill RA, de Villiers MM. Effect of pH on the solubility and release of furosemide from polyamidoamine (PAMAM) dendrimer complexes. IntJ Pharm. 2007;345:142–53. doi: 10.1016/j.ijpharm.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 33.Higuchi T, Conners KA. Phase-solubility techniques. In: Reilly CN, editor. Advances in Analytical Chemistry and Instrumentation. New York: John Wiley; 1965. pp. 117–212. [Google Scholar]
- 34.Chie K, Kenji K, Kazuo M. Synthesis of polyamidoamine dendrimers having poly(ethylene glycol) grafts and their ability to encapsulate anticancer drugs. Bioconjug Chem. 2000;17:910–7. doi: 10.1021/bc0000583. [DOI] [PubMed] [Google Scholar]


