Abstract
Introduction:
Betamethasone dipropionate (BD) has anti-inflammatory, immunomodulatory, and antiproliferative activity. The aim of the current work was to test the hypothesis that the addition of corticosteroid such as BD and a keratolytic agent such as salicylic acid in nanocarrier based microemulsions formulation would result in enhancement and sustaining of corticosteroid delivery rate leading to better anti-psoriatic activity. Clinical use of BD is restricted to some extent due to its poor permeability across the skin. So to increase its permeation across the skin, microemulsion-based gel formulations were prepared and characterised.
Materials and Methods:
Microemulsions were prepared by aqueous phase titration method, using oleic acid:sefsol (1.5:1), Tween 20, isopropyl alcohol, and distilled water as the oil phase, surfactant, cosurfactant and aqueous phase, respectively. Selected formulations were subjected to physical stability studies and consequently in vitro skin permeation studies. Surface studies of optimized formulation were done by transmission electron microscopy. In vivo anti-inflammatory activity was done by carageenan-induced raw paw edema method.
Results:
The droplet size of microemulsions ranged from 60 to 190 nm. The optimized formulation exhibited viscosity 28.55 ± 2.03 mP, refractive index 1.409, pH 6.4, and conductivity 10-4 scm-1. The optimized microemulsion was converted into hydrogel using carbopol 934, and salicylic acid was incorporated into it. Drug deposition in skin was found to be 29.73 μg/mg. Assessment of skin permeation was done by histopathology studies which indicated changes in the structure of epidermal membrane of skin. In vivo anti-inflammatory activity indicated 72.11% and 43.96% inhibition of inflammation in case of developed microemulsion gel and marketed gel, respectively.
Conclusions:
The developed microemulsion gel containing BD and salicylic acid provided sustained and good anti-inflammatory activity for the treatment of psoriasis.
Keywords: Betamethasone dipropionate, microemulsions, salicylic acid, transmission electron microscopy
INTRODUCTION
Psoriasis is a common dermatological condition affecting 2% of the population. It is a chronic (prolonged) inflammation of the skin characterized by erythematous scaly plaques. Corticosteroid such as betamethasone has been used extensively in topical therapy for the treatment of mild to moderate psoriasis.[1] Salicylic acid exhibits keratolytic action and is helpful in psoriasis as it softens and removes the hyperkeratinized scales formed due to the deposition of the dead cells on the skin. These scales act as a major barrier for drug delivery across the skin. Moreover, salicylic acid also possesses anti-inflammatory activity.
Betamethasone dipropionate (BD) is a highly potent glucocorticoid receptor agonist which possesses immunosuppressive, anti-inflammatory, and anti-proliferative effects. It exerts its action by inhibition of phospholipase A2 which leads to the inhibition of synthesis of arachidonic acid and controls the biosynthesis of prostaglandins and leukotrienes. Its various dosage forms such as ointment, cream, lotion, and foam are in use for the treatment of mild to moderate psoriasis.[2,3] However, the clinical use of BD has some practical disadvantages such as poor permeability through skin which reduces its therapeutic effectiveness at the target site. The main limitation lies in the barrier function of the skin, which is considered one of the most impermeable epithelia of the human body to exogenous substances. Therefore, the major challenges for a topical formulation are to provide a sufficient increase in drug penetration into the skin, without inducing any significant irreversible alteration to the skin barrier function.[4]
There has been increased interest during recent years in the use of topical vehicle systems that could modify drug permeation through the skin. Many of the dermal vehicles contain chemical enhancers and solvents to achieve these goals. But use of these chemical enhancers may be harmful, especially in chronic application, as many of them are irritants. Therefore, it is desirable to develop a topical vehicle system that does not require the use of chemical enhancers to facilitate drug permeation through the skin. One of the most promising techniques for enhancement of skin permeation of drugs is microemulsion. Microemulsions offer several significant advantages including low skin irritation, powerful permeation ability, and high drug-loading capacity for topical delivery when compared with the other carriers such as liposomes or solid lipid nanoparticles.[5,6]
The main aim of this study was to determine whether the addition of salicylic acid which is a keratolytic agent and BD into microemulsion would result in enhancement and sustaining of corticosteroid delivery rate leading to better anti-psoriatic activity. The low viscosity of microemulsion restrains its clinical application due to inconvenient use, and therefore hydrogel-thickened microemulsion systems were formulated with good stability, powerful permeation ability, and suitable viscosity for the topical delivery which provided longer contact with skin. The present investigation was focused on the preparation and characterization of microemulsion with BD and salicylic acid, in vitro permeation studies, and in vivo anti-inflammatory activity of the optimized formulation. Furthermore, assessment of permeation of drug into skin was done by histopathological studies. The long-term goal of this work was to develop topical BD formulations for clinical use to increase therapeutic index.
MATERIALS AND METHODS
Materials
BD was obtained as a gift sample from Ranbaxy Research Laboratory (Gurgaon, India). Salicylic acid, isopropyl alcohol, and oleic acid were purchased from S.D. Fine chemicals (New Delhi). Propylene glycol mono caprylic ester (Sefsol 218®) was obtained as a gift sample from Nikko Chemicals Co., Ltd, Japan. PEG 400, Tween 80, Tween 20, and ethanol were purchased from Merck (Merck, India). Caprylo caproyl macrogol-6 glycerides (Labrasol), diethyleneglycol monoethyl ether (Transcutol P), and Plurol Oleique were obtained as a kind gift samples from Gattefosse (Mumbai, India). All other chemicals used were of analytical grade.
Screening of excipients
The most important criterion for screening of components is the solubility of poorly soluble drug in oils, surfactants, and cosurfactants.
Screening of oil for microemulsion
To find out the suitable oil that can also provide excellent skin permeation rate of BD, the solubility of BD was determined in different oils, viz. oleic acid, isopropyl myristate, sefsol oil, castor oil, labrafil, sefsol:oleic acid (1:1), and oleic acid:sefsol (1.5:1) mixture. One milliliter of different oils was taken in small vials and excess amount of the drug was added. The vials were tightly stoppered and were continuously stirred for 72 h at 25°C, and after 72 h, samples were centrifuged at 5000 rpm for 20 min. The supernatant was separated, filtered through a 0.45-μm membrane filter, and after appropriate dilution with methanol, solubility of drug in different oils was determined by UV method at 240 nm.
Screening of surfactant and cosurfactant for microemulsion
To find out the suitable surfactant and cosurfactant, the solubility of BD was determined in various surfactants including labrasol, Tween 80, Tween 20, and a combination of Tween 80:labrasol. The solubility of BD was also checked in cosurfactants including isopropyl alcohol, transcutol P, PEG 400, and ethanol.
Phase studies
On the basis of solubility studies, oleic acid:sefsol (1.5:1) was selected as an oil phase. Tween 20 and isopropyl alcohol were chosen as surfactant and cosurfactant, respectively. Distilled water was used as an aqueous phase. For the determination of existence zone of microemulsion, pseudoternary phase diagrams were constructed using water titration method (spontaneous emulsification method).[7] Surfactant and cosurfactant (Smix) were mixed in different weight ratios (1:0, 1:1, 1:2, 1:3, and 1:4). These Smix were chosen in increasing concentration of cosurfactant with respect to surfactant. For each phase diagram, oil and specific Smix were mixed well in different ratios. Sixteen different combinations of oil and Smix (1:9, 1:8, 1:7, 1:6, 1:5 1:4, 1:3.5, 1:3, 3:7, 1:2, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1) were made so that maximum ratio could be covered for the study to delineate the boundaries of the phases formed precisely in the phase diagrams. Slow titration with aqueous phase was done for each weight ratio of oil and Smix under moderate stirring, and visual observation was used for transparent and easily flowable microemulsion. Gels were claimed for those clear and highly viscous mixtures that did not show a change in the meniscus after being tilted to an angle of 90°. The physical state of microemulsion was marked on a pseudo three component phase diagram with one axis representing the aqueous phase, second representing oil, and the third representing a mixture of surfactant and cosurfactant at fixed weight ratio (Smix ratio).
Selection of formulations
From the pseudoternary phase diagrams showing maximum microemulsion area, a number of microemulsions with different formulas were selected covering the entire range of microemulsion occurrence in the phase diagrams with minimum surfactant and maximum water concentration. 0.05% w/w of BD, which was kept constant in all the selected formulations, was added to the oil phase during the formulation of microemulsions. Selected formulations were subjected to various physical stability tests.
Physical stability studies
To overcome the problem of metastable formulations, physical stability tests were performed.[8] The selected microemulsions were subjected to centrifugation at 5000 rpm for 30 min. The formulations that did not show any phase separations were taken for the heating and cooling cycle. Six cycles between refrigerator temperature (4°C) and (45°C) with storage at each temperature of not less than 48 were done. The formulations that were found stable were subjected to a freeze–thaw cycle test. Formulations were kept in deep freezer (Vestfrost, Delhi, India) at -20°C for 24 h. After 24 h the microemulsions were removed and kept at room temperature. The physically stable microemulsions returned to their original form within 2–3 min. Three such cycles were repeated.
Characterization of microemulsions
In vitro skin permeation studies
In vitro skin permeation studies were performed on a fabricated Franz diffusion cell with an effective diffusional area of 3.14 cm2 and 5 ml of receiver chamber capacity using rat abdominal skin. The full-thickness rat skin was excised from the abdominal region, and hair were removed with an electric clipper. The subcutaneous tissue was removed surgically, and the dermis side was wiped with isopropyl alcohol to remove adhering fat.[9] The cleaned skin was washed with distilled water and stored in the deep freezer at -21°C until further use. The skin was brought to room temperature and mounted between the donor and receiver compartment of the Franz diffusion cell, where the stratum corneum side faced the donor compartment and the dermal side faced the receiver compartment. Initially, the donor compartment was empty and the receiver chamber was filled with phosphate buffer (pH 7.4). The receiver fluid was stirred with a magnetic rotor at a speed of 100 rpm, and the assembled apparatus was placed in the oven and the temperature was maintained at 37 ± 1°C. All the receiver fluid was replaced every 30 min to stabilize the skin. It was found that the receiver fluid showed negligible absorbance after 4.5 h and beyond indicating complete stabilization of the skin. After complete stabilization of the skin, 1 ml of microemulsion formulation (0.5 mg/ml BD) was placed into each donor compartment and sealed with paraffin film to provide occlusive conditions. Samples were withdrawn at regular intervals (0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 20, 22, and 24 h), filtered through a 0.45-membrane filter, and analyzed for drug content by UV spectrophotometer at λmax of 237 nm.
Permeation and distribution data analysis
The cumulative amount of BD permeated through the albino rat skin (Q, μg/cm2) was plotted as a function of time (t, h) for each formulation. The permeation rate (flux) at the steady state (Jss , μg/cm2/h) and lag time were calculated from the slope and intercept of the straight line obtained by plotting the cumulative amount of BD permeated per unit area of skin versus time at steady-state condition, respectively. Permeability coefficient (Kp) was calculated by dividing the flux by initial drug concentration (Co) in the donor portion of cell as given below:
Kp = Jss/Co.
Enhancement ration (Er) was calculated by dividing the Jss of the respective formulation by the Jss of the control formulation as given below:
Er = Jss of formulation/Jss of control
In order to determine the drug disposition in the skin, it was weighed, cut into small pieces, and sonicated for 15 min with methanol in order to extract the BD content. The resulting solution was centrifuged and passed through 0.25 μm filter and drug content (μg/mg of skin) was determined spectrophotometerically.
Particle size and polydispersity index
The average size and polydispersity index of the microemulsion droplets were determined by photon correlation spectroscopy (Nano ZS90, Malvern Instrument, Worcestershire, UK) at 633 nm which is based on the principle of dynamic light scattering. The measurements were performed using a He-Ne laser at 633 nm by using Avalanche photo diode detector. Light scattering was monitored at 25°C at a 90° angle. Droplet size distribution studies were performed at refractive index of 1.40 because the refractive index for all formulation were in this range. The viscosity and dielectric constant of the medium were set at 4.55 mPas and 79.4, respectively. Zeta potential was determined by using second-generation PALS (Phase Analysis Light Scattering), called M3PALS which measures the particle velocity.
Viscosity, refractive index, conductivity, and pH
Viscosity of microemulsion was determined by using Brookfield DV III ultra V6.0 RV cone and plate rheometer (Brookfield Engineering Laboratories, Middleboro, MA, USA). Refractive index was determined for different microemulsion formulations by using Abbe's refractometer (Nirmal International, Delhi, India) at 25°C in triplicate. The pH was determined for the optimized microemulsion by using a calibrated digital pH meter (Mettler Toledo MP 220, Greifensee, Switzerland) in triplicate at room temperature. Conductivity was measured by using a digital thermo conductivity meter (1152, Emcee Electronics, Venice, FL, USA) and current flow was observed.
Surface morphology by transmission electron microscopy
Morphology and structure of the microemulsion were studied using Morgagni 268D transmission electron microscopy (TEM) (FEI, Netherland) operating at 70 KV and capable of point-to-point resolution. Combination of bright field imaging at increasing magnification and diffraction modes were used to reveal the form and size of microemulsion droplets. In order to perform the TEM observations, a drop of microemulsion was applied on carbon-coated grid with 2% phosphotungstic acid and was left for 30 s. The dried coated grid was taken on a slide and covered with a cover slip. The slide was observed under the electron microscope.
Hydrogel-thickened microemulsion
The very low viscosity often exhibited by microemulsion is inappropriate for topical use. The viscosity can be increased by adding thickening agents, which also change the appearance of the system, usually influencing drug release. Recently, the gel matrices such as carbopol 934, xanthum gum, carrageennan, sodium alginate, ethyl cellulose, and HPMC have been used to prepare the microemulsion-based gel for improving the viscosity of microemulsion.[10] The selection of polymer for preparing gel is normally based on the character of external phase (oil for w/o type and water for o/w type). Because BD microemulsion is a type of o/w type, so carbopol 934 was selected for preparation of microemulsion gel. For preparation of microemulsion gel, initially 3 g of carbopol 934 and 5 g salicylic acid were added in 87.68 ml of the water and stirred with magnetic stirrer till homogenous mixture was obtained. Then 12.32 ml of already prepared microemulsion was added drop by drop to make a total volume of 100 ml till homogeneous mixture was obtained. The clear hydrogel-thickened microemulsion was evaluated for viscosity, pH, extrudability, homogenecity, and spreadability. Content uniformity was carried out to ascertain that concentration of drug in each portion was uniform. For that an accurately weighed quantity of gel (6 g) from three different portions was taken and extracted with methanol. The extracted drug was analyzed by using UV spectrophotometer. Hydrogel-thickened microemulsion was applied gently on stratum corneum and in vitro permeation study was performed.
In vivo anti-inflammatory study
The protocol to carry out in vivo anti-inflammatory efficacy studies was approved by the Institutional Animal Ethics Committee Jamia Hamdard (New Delhi, India). The committee's guidelines were followed for the studies. The anti-inflammatory and sustaining action of the optimized formulation was evaluated by the carrageenan induced hind paw edema method by using digital plethysmometer (Ugo Basile, Italy) in Wistar rats of either sex weighing 180–200 g.[11] A left hind paw of each rat was marked, just below tibiotarsal junction, so that every time the paw was dipped up to the fixed mark to ensure constant paw volume. Animals were randomly divided into three groups (control, optimized microemulsion gel A 2 , and marketed formulation) each containing six rats. Both optimized and marketed formulations were applied on the dorsal area of 9 cm2 gently with the help of micropore adhesive, 0.5 h before carrageenan injection. No formulation was applied to the control. Acute inflammation was produced by injecting 0.1 ml of 1% (w/v) carrageenan suspension in the subplantar region of the left hind paw 0.5 h after treatment with drug. The paw volume was measured at 0, 1, 2, 3, 6, 12, and 24 h. The amount of paw swelling was determined for 24 h and expressed as percent edema relative to the initial hind paw volume. Percent inhibition of edema was calculated for placebo and drug loaded group with respect to control group using the following formula:
![]()
Histopathology studies
Abdominal skin of Wistar rats was treated with the optimized BD microemulsion gel. After 24 h, the rats were killed and skin samples were taken from untreated (control) and treated areas. Each specimen was stored in 10% formalin solution in phosphate buffer saline (pH 7.4). The specimens were cut into sections vertically. Each section was dehydrated using ethanol embedded in paraffin wax for fixing and stained with hematoxylin and eosin. These samples were then observed under light microscope (Motic, Japan) and compared with control samples.
RESULTS AND DISCUSSION
Criteria for excipient selection
The excipients selected were needed to be pharmaceutically acceptable, nonirritating, and nonsensitizing to the skin and to fall into the GRAS (generally regarded as safe) category. Higher solubility of the drug in the oil phase was another important criterion, as it would help the microemulsion to maintain the drug in solubilized form. Safety is a major determining factor in choosing a surfactant, as a large amount of surfactants may cause skin irritation. Nonionic surfactants are considered to be less toxic than ionic surfactants and therefore tweens were selected. Another important criterion for selection of the surfactants is that the required hydrophilic lipophilic balance value to form the o/w microemulsion should be greater than 10. The right blend of low and high hydrophilic lipophilic balance surfactants leads to the formation of a stable microemulsion formulation. The presence of cosurfactant decreases the bending stress of interface and allows the interfacial film sufficient flexibility to take up different curvatures required to form microemulsion over a wide range of composition.
Screening of excipients
Drug loading per formulation is a very critical design factor in the development of microemulsion systems for poorly soluble drugs, which is dependent on the drug solubility in oil phase. Solubility of BD was seen maximum in case of oleic acid:sefsol mixture (1.5:1; Table 1). Moreover, oleic acid is a powerful enhancer for transdermal delivery,[12] as it increases the fluidity of the intercellular lipid barriers in the stratum corneum by forming separate domains that interfere with the continuity of the multilamellar stratum corneum and induce highly permeable pathways in the stratum corneum. Nonionic surfactants are widely used in topical formulations as solubilizing agents, but some recent results indicate that they may affect the skin barrier function. Among the various surfactants evaluated, the maximum solubility of BD was found in Tween 20 [Table 2] so it was selected as surfactant. The maximum solubility of BD in cosurfactant was found with isopropyl alcohol [Table 2]. Isopropyl alcohol was selected as cosurfactant because it is very good penetration enhancer and solubilizing agent. The penetration enhancement of lipophilic drugs by alcohols is due to the higher solubility of the drug substance in the lipophilic area of the stratum corneum because of the presence of alcoholic enhancers.[13] The effect of alcohols on the phase behavior of nonionic microemulsion depends on the number of carbons of alcohol. The presence of alcohol overcomes the need for any additional input of energy. These properties make the components useful as vehicles for drug delivery. Alcohols can influence the formation of microemulsion by both interfacial and bulk effects.[13] So for the development of pseudoternary phase diagram oleic acid:sefsol (1.5:1) was selected as the oil phase, Tween 20 as surfactant, isopropyl alcohol as cosurfactant, and distilled water as aqueous phase.
Table 1.
Solubility of BD in different oils alone and in combination
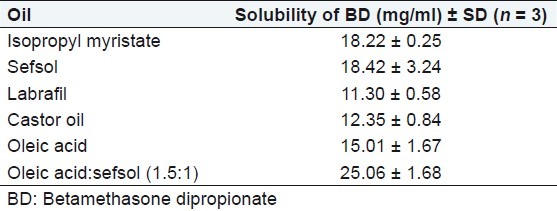
Table 2.
Solubility of BD in different surfactants and cosurfactants

Phase studies
The relationship between the phase behavior of a mixture and its composition can be captured with the aid of a phase diagram.[7] The aim of the construction of pseudoternary phase diagram was to find out the existence range of microemulsion. Care was taken to ensure that observations are not made on metastable system. Pseudoternary phase diagrams were constructed separately for each Smix ratio [Figure 1] for getting o/w microemulsion regions. The area of microemulsion isotropic region changed slightly as the ratio of surfactant in Smix was increased. In the phase diagrams, the existence of large or small microemulsion region depends on the capability of the particular Smix to solubilize the oil phase. The extent of solubilization results in a greater area with the formation of more clear and homogenous solution.
Figure 1(a–d):
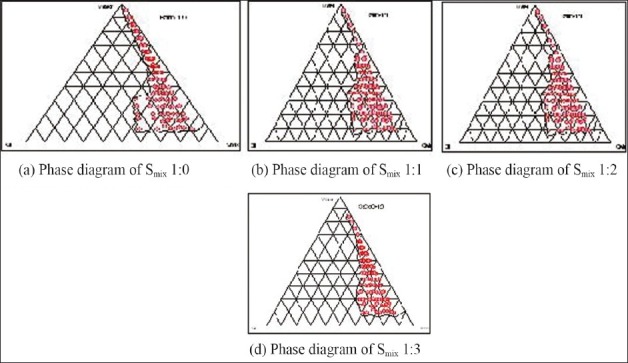
Pseudoternary phase diagram indicating o/w microemulsion region using oleic acid + sefsol (1.5:1), Tween 20 (surfactant), and isopropyl alcohol (cosurfactant)
The construction of pseudoternary phase diagrams was started using surfactant, i.e., Tween 20 alone (1:0). It was found that the region of microemulsion existence was very less and most of the region was composed of emulsions. Therefore, with surfactant Tween 20, cosurfactant isopropyl alcohol was also incorporated in the ratio 1:1 and it was found that region of microemulsion existence increased greatly. Increase in the concentration of cosurfactant to (1:2) resulted in even larger area of microemulsion existence, along with some emulsion, gels, or microemulsion gels area. Increasing cosurfactant concentration further from 1:2 to 1:3, and 1:4 resulted in the reduction of the microemulsion existence area and more area was composed of emulsion and gels.
The existence of microemulsion region whether large or small depends on the capability of that particular surfactant or surfactant mixture to solubilize the oil phase. The extent of solubilization results in a greater area with more of clear, homogenous solution. It was seen that when the surfactant (Tween 20) was used alone, oil phase was solubilized to a lesser extent implying that surfactant alone was not able to reduce the interfacial tension of the oil droplets to sufficiently low level and thus was not able to reduce the free energy of the system to ultra low level desired to produce microemulsions. When a cosurfactant was added, the interfacial tension was reduced to much low level and very small free energy was achieved which helped in larger microemulsion area existence in phase diagram. With a further increase in cosurfactant from 1:1 to 1:2, a further drop in interfacial tension and free energy was achieved resulting in maximum area of microemulsion formation. With a further increase in cosurfactant concentration (1:3), the interfacial tension of interfacial film increased when compared with above, and more of gel area and less of microemulsion area was observed.
Selection of formulation from phase diagram
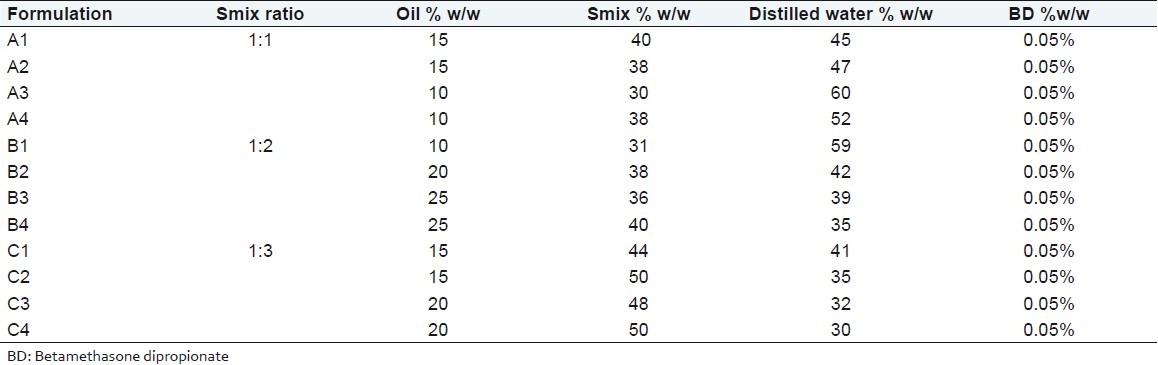
It is reported that large amount of surfactant causes skin irritation and toxicity-related issues, and therefore it is important to use the minimum amount of surfactant and cosurfactant in the formulation. However, for topical delivery, where enhanced skin permeation is the aim, it is not purposeful to select the lowest surfactant concentration. The surfactant concentration should be chosen so that it gives the maximum flux, which is an important criterion but its level should not be toxic to cause any irritation to the skin. This is usually not obtained with formulations that contain the highest amount of surfactant because high surfactant concentration decreases the thermodynamic activity of the drug in the vehicle, and the affinity of the drug to the vehicle becomes greater. While going through pseudoternary phase diagram, oil could be solubilized up to the extent of 40% w/w, but in such cases the Smix concentration was very high. For the preparation of drug-loaded microemulsions, 0.05% BD was dissolved in oil phase. Therefore, from each phase diagram, microemulsion formulations containing different concentration of oil were selected which contained minimum to maximum Smix concentration [Table 3].
Table 3.
Composition of various BD loaded microemulsions
Physical stability studies
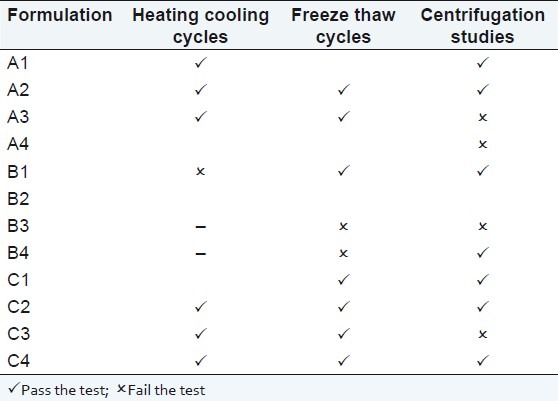
Microemulsions are considered to be thermodynamically stable systems that are formed at a particular concentration of oil, surfactant, and water, with no phase separation, creaming, or cracking. Selected formulations from phase diagram were subjected to different stress stability testing like heating cooling cycle, centrifugation, and freeze–thaw cycle. During physical stability testing, some formulations became turbid and in some phase separation occurred. One reason of this instability in microemulsions may be due to the ostwald ripening in which molecules move as a monomer and coalescence of small droplets takes place, resulting in the formation of large droplets by diffusion processes driven by the gain in surface free energy. The other reason may be that when temperature quench occurs during stress stability study, instability of microemulsion occurs due to separation of oil phase and droplet distribution of smaller size is favored by the change in curvature free energy.[14] Only those formulations, which showed no phase separation, creaming, cracking, coalescence, and phase inversion during stress stability tests, were selected for further studies [Table 4].
Table 4.
Physical stability studies of drug loaded formulations
Characterization of microemulsions
The formulations that passed physical stability test were evaluated for droplet size, polydispersity index, viscosity, pH, and refractive index.
In vitro skin permeation studies
The permeation ability of various BD loaded microemulsions was evaluated using the in vitro permeation experiments. A steady increase of BD in the receptor chamber with time was observed. The permeation profiles of microemulsions were in accordance with the Fick's diffusion equation. On the basis of permeation studies, it was found that the formulation A2 consisting of 15% oil phase, 38% (Smix 1:1) and 47% distilled water exhibited 42.33% of cumulative amount of drug permeated (μg/cm2) with the flux of 1.18 (μg/cm2/h) after 24 h and highest amount of drug was deposited in skin 30.563 μg/mg.
Particle size and polydispersity index
The average size and polydispersity index of the microemulsion droplets were determined by photon correlation spectroscopy (Nano ZS90, Malvern Instrument, Worcestershire, UK). The droplets size of all microemulsions ranged from 60 to 190 nm. The polydispersity index showed that all the microemulsions had narrow size distribution. The average particle size and polydispersity index of the formulation A2 were found to be 114 nm [Figure 2] and 0.121, respectively, indicating micro range of droplets with minimum variation in particle size.
Figure 2.
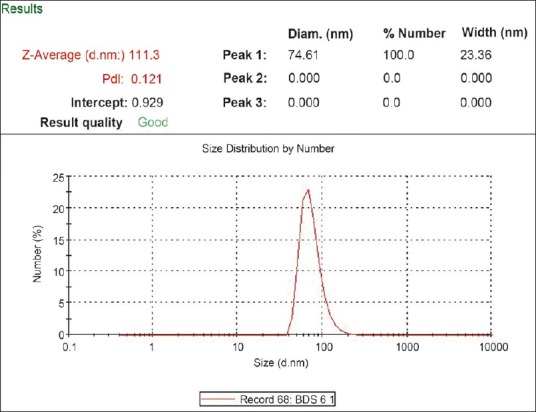
Droplet size and size distribution of microemulsion formulation (A2)
Refractive index, pH, conductivity, and viscosity of microemulsion
Viscosity of the microemulsion (A2) formulation was very low (28.55 ± 2.03mP) as expected for o/w emulsion [Table 5]. The low viscosity may be due to presence of low amount of Tween 20 (a fatty acid polyhydric alcohol ester having high intrinsic viscosity) compared with isopropyl alcohol (a short chain alcohol having low intrinsic viscosity) and also the low concentration of oil. Refractive index is the net value of the components of microemulsion and indicates isotropic nature of formulation. Refractive index of microemulsion was determined using an Abbes type refractometer (Nirmal International, New Delhi, India) at 25 ± 0.5 °C. The mean value of the refractive index for the formulation A2 was found to be 1.409. The specific conductivity of microemulsion A2 was found to be 10-4 s cm-1. The apparent pH of the formulation was measured by pH meter (AccumentAB 15, Fisher scientific, USA) in triplicate at 25 ± 1°C and found to be around 6.4 [Table 5].
Table 5.
Parameters for the optimized formulation A2

Surface morphology of particle
The TEM studies were carried out to get more insight about the morphology of the microemulsion systems. From the results of TEM it was concluded that the particles of optimized formulation were spherical in shape and finely distributed with micron size range between 95 and 150 nm [Figure 3].
Figure 3.
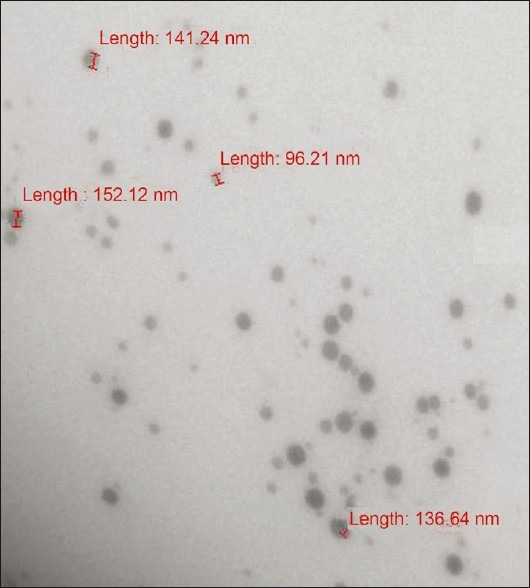
TEM photograph of particle size of microemulsion (A2) drug-loaded BD
Hydrogel-thickened microemulsion
To convert o/w BD microemulsion into a gel, microemulsion gel was prepared using carbopol 934 (3%) and salicylic acid (5%). A small quantity of gel was pressed between the thumb and index finger, and the consistency and homogeneity of the gel were observed. It was found that there were no coarse particles in the optimized gel formulation. The spreading of carbopol gel was found to be more uniform and the gel spreaded in a circular pattern equally on all sides and it almost reached to 1.44 times of the initial diameter upon application of 44.6 g weight. During the in vitro studies [Figure 4], it was found that the optimized formulation (A2) gel had cumulative amount of drug permeated/area and drug deposition in the skin of 29.77 μg/cm2 and 29.73 μg/mg respectively [Table 6]. A decrease in the permeation from microemulsion-based gel was seen due to high viscosity of gel. pH of the formulation was found to be 5.9 which is compatible with skin pH. The spreadibility of the formulation was found to be 1.44 times greater than marketed formulation, and drug uniformity was found to be 98.69 ± 1.03% [Table 7]. The gel possessed good spreading properties, comparable to that of the marketed product and suggested that the gel would result in a more uniform and wider spread upon topical application.
Figure 4.
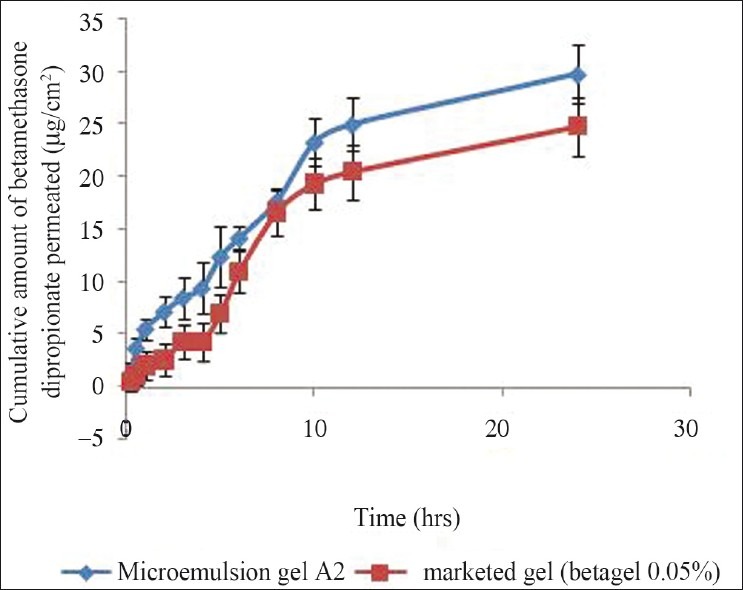
Comparison of in vitro skin permeation of BD from microemulsion gel (A2) with salicylic acid and marketed formulation gel in phosphate buffer (pH 7.4)
Table 6.
In vitro permeation profile of prepared microemulsion gel and marketed gel
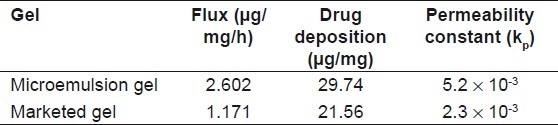
Table 7.
Evaluation of microemulsion gel parameters

Anti-inflammatory studies
The anti-inflammatory effects of optimized gel formulation containing salicylic acid were compared with the control and marketed gel formulation.[10] The anti-inflammatory activity of optimized formulation of microemulsion gel was evaluated using the carrageenan-induced hind paw edema method using digital Plethysmometer. The rat's left footpad became edematous soon after injection of carrageenan and reached its peak at 3 h (75.2%). Mean percent edema and % inhibition of inflammation of all the three groups were calculated [Figure 5]. Inhibition of edema was found to be highest in the groups in which microemulsion gel was applied. The microemulsion gel inhibited edema (P < 0.05) 72.11% up to 24 h. Marketed formulation inhibited the edema 43.96% up to 24 h. Based on the anti-inflammatory studies, it can be concluded that BD microemulsion gel formulation containing salicylic acid gives maximum inhibition of edema than the marketed formulation and the control.
Figure 5.
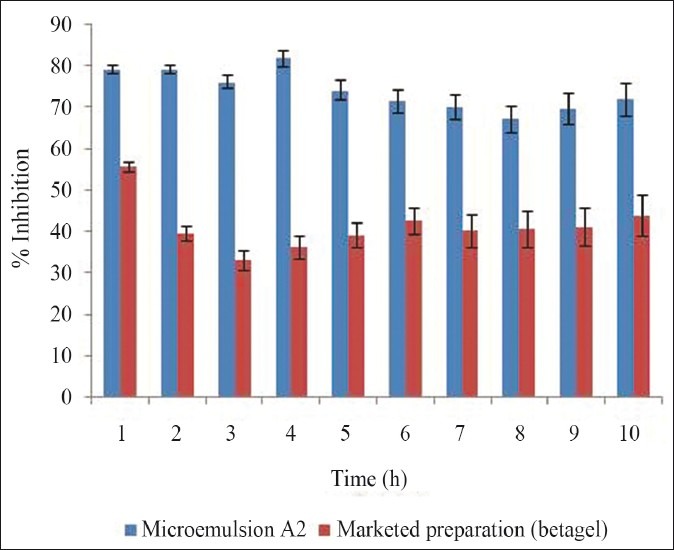
Comparison of anti-inflammatory activity of marketed product and microemulsion gel (A2)
Histopathology studies
The influence of BD and salicylic acid-loaded microemulsion gel on anatomical structure of the rat skin was assessed with the help of histopathological studies. The photomicrographs of untreated rat skin (control) showed normal skin [Figure 6] with well-defined epidermal and dermal layers. Keratin layer was well formed and lied just adjacent to the topmost layer of the epidermis. Dermis was devoid of any inflammatory cells. When the skin was treated with microemulsion formulation, definite changes were observed in the skin morphology. The disruption and extraction of lipid bilayers were clearly evident as distinct voids and empty spaces visible in the epidermal region. The disruption of epidermal layer indicated permeation of BD through stratum corneum. The extraction of subcutaneous lipids might cause dehydration of SC leading to significant loss of moisture.
Figure 6.
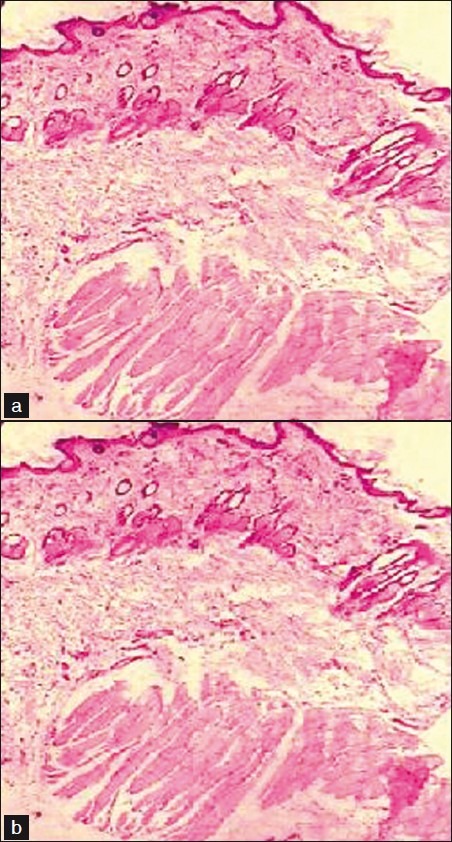
Light power photomicrograph of (a) untreated and (b) treated skin
CONCLUSION
A safe and effective microemulsion gel formulation of BD with salicylic acid for the treatment psoriasis was developed, which provided enhanced permeation of the drug, reduced dosing frequency, and sustained the drug release for the desired period of time.
ACKNOWLEDGMENT
The authors are thankful to All India Council of Technical Education (AICTE), Govt. of India for financial assistance to this project. The authors are also thankful to Nikko Chemicals Co., Ltd, Japan for providing sefsol as gift samples.
Footnotes
Source of Support: All India Council of Technical Education (AICTE), Govt. of India.
Conflict of Interest: None declared.
REFERENCES
- 1.Glickman FS. Lepra, psora, psoriasis. J Am Acad Dermatol. 1986;14:863–6. doi: 10.1016/s0190-9622(86)70101-1. [DOI] [PubMed] [Google Scholar]
- 2.Zulfakar H, Abdelouahab N, Heard CM. Enhanced topical delivery and ex-vivo anti inflammatory activity from a betamethasone dipropionate formulation containing fish oil. Inflamm Res. 2010;59:23–30. doi: 10.1007/s00011-009-0065-z. [DOI] [PubMed] [Google Scholar]
- 3.Simonsen L, Høy G, Didriksen E, Persson J, Melchior N, Hansen J. Development of a new formulation combining calcipotriol and betamethasone dipropionate in an ointment vehicle. Drug Dev Ind Pharm. 2004;30:1095–102. doi: 10.1081/ddc-200040297. [DOI] [PubMed] [Google Scholar]
- 4.Baboota S, Al-Azaki A, Kohli K, Ali J, Dixit N, Shakeel F. Development and evaluation of a microemulsion formulation for transdermal delivery of terbenafine. PDA J Pharm Sci Technol. 2007;61:276–85. [PubMed] [Google Scholar]
- 5.Dixit N, Kohli K, Baboota S. Nanoemulsion system for the transdermal delivery of a poorly water soluble cardiovascular Drug. PDA J Pharm Sci Technol. 2008;62:46–55. [PubMed] [Google Scholar]
- 6.Date AA, Naik B, Nagarsenkar MS. Novel drug delivery system: Potential in improving topical delivery of antiacne agents. Skin Pharmacol Physiol. 2006;19:2–16. doi: 10.1159/000089138. [DOI] [PubMed] [Google Scholar]
- 7.Shafiq-un-Nabi S, Shakeel F, Talegaonkar S, Ali J, Baboota S, Ahuja A, et al. Formulation development and optimisation using nanoemulsion technique: A technical note. AAPS PharmSciTech. 2007;8(Article 28) doi: 10.1208/pt0802028. IF – 0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baboota S, Shakeel F, Ahuja A, Ali J, Shafiq S. Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm. 2007;57:315–32. doi: 10.2478/v10007-007-0025-5. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Chang X, Weng T, Zhao X, Gao Z, Yang Y, et al. A study of micro emulsion systems for transdermal delivery of triptolide. J Control Release. 2004;98:427–36. doi: 10.1016/j.jconrel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Pan J, Chen Q, Yu Q, Chen H, Xu H, et al. In vivo evaluation of the safety of triptolide-loaded hydrogel-thickened microemulsion. Food Chem Toxicol. 2008;46:3792–9. doi: 10.1016/j.fct.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 11.Winter CA. Antiinflammatory testing methods: Comparative evaluation of indomethacin and other agents. In: Garattini S, Dukes MN, editors. Nonsteroidal anti-inflammatory drugs. Vol. 82. Excerpta-Medica, Amsterdam: 1965. pp. 190–220. [Google Scholar]
- 12.Rhee YS, Choi JG, Park ES, Chi SC. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 2001;228:161–70. doi: 10.1016/s0378-5173(01)00827-4. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Agarwal SP, Ahuja A, Ali J, Choudhry R, Baboota S. Preparation, characterization, and in vitro antimicrobial assessment of nanocarrier based formulation of nadifloxacin for acne treatment. Pharmazie. 2011;66:111–4. [PubMed] [Google Scholar]
- 14.Wennerstrom H, Olsson U. Microemulsion as model systems. CR Chimie. 2009;12:4–17. [Google Scholar]


