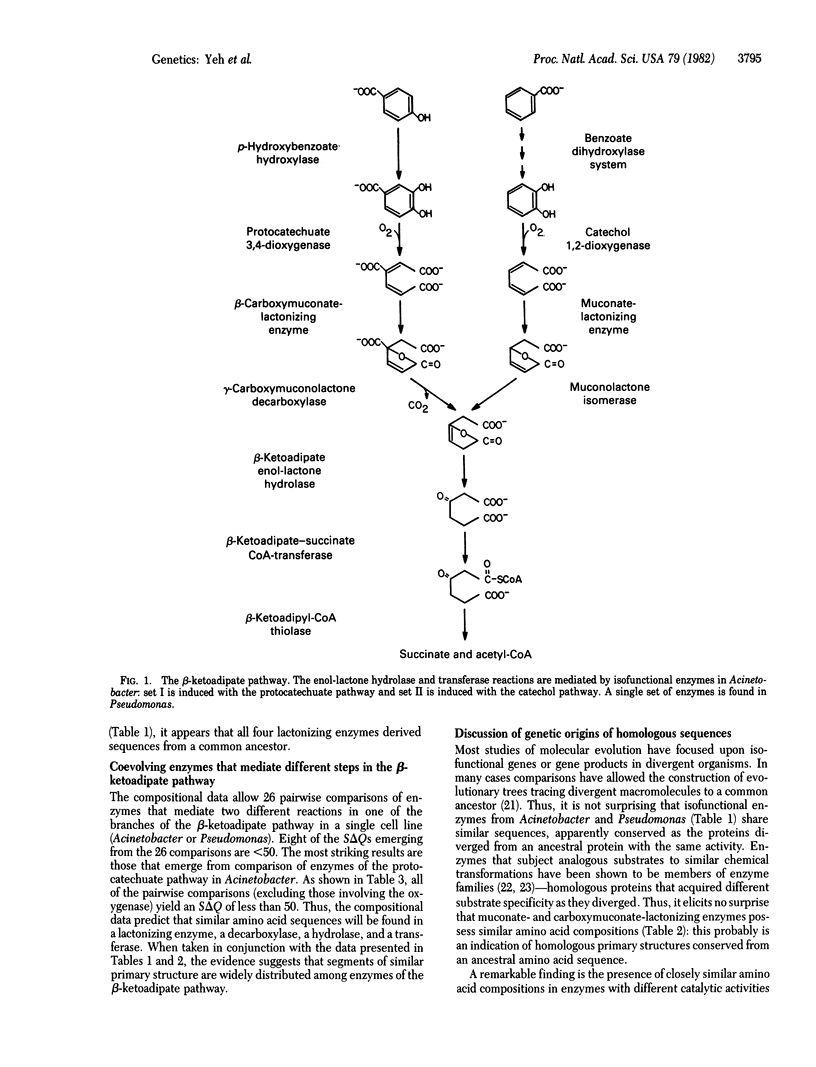
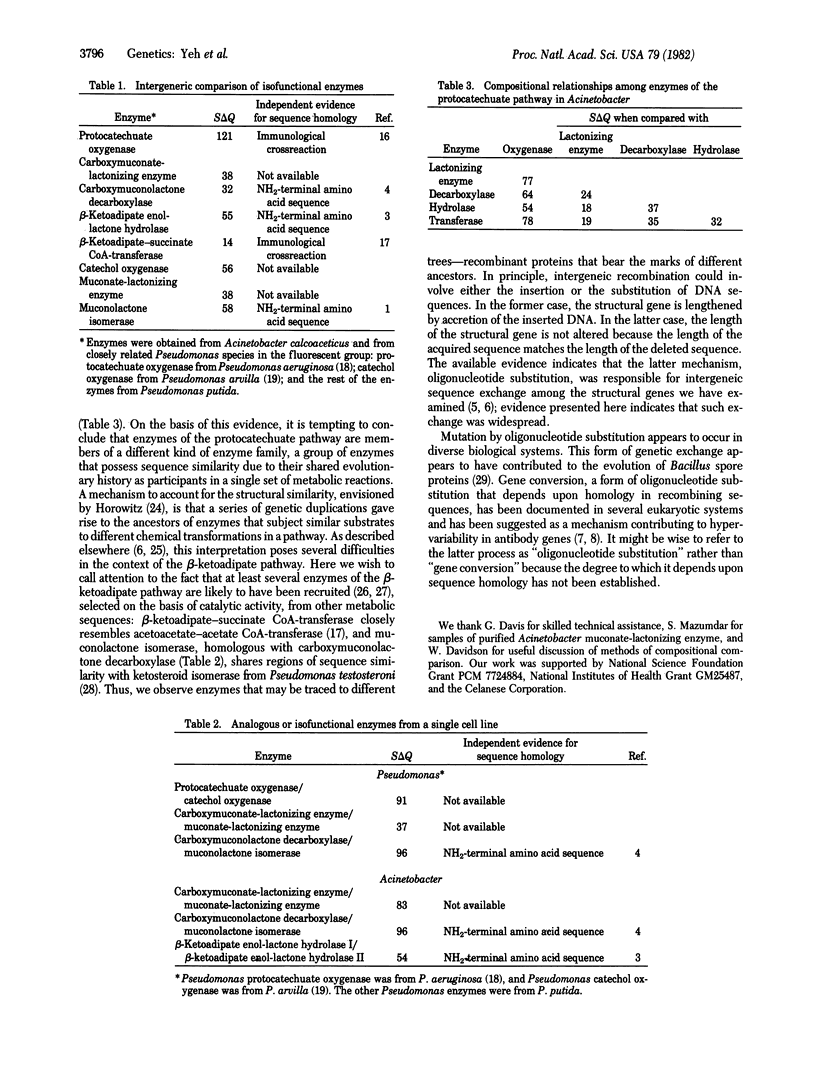
Abstract
Comparison of the amino acid compositions of purified proteins indicates the presence of overlapping evolutionary affinities among enzymes of the beta-ketoadipate pathway. Isofunctional enzymes from different bacterial genera share a common evolutionary origin. Moreover, enzymes that mediate isofunctional or chemically analogous reactions within an organism appear to be evolutionarily homologous. Most remarkably, closely similar amino acid compositions are found in enzymes that mediate the following consecutive metabolic steps: lactonization, decarboxylation, hydrolysis, and transfer of a thioester bond.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981 Jun;24(3):592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Durham D. R., Stirling L. A., Ornston L. N., Perry J. J. Intergeneric evolutionary homology revealed by the study of protocatechuate 3,4-dioxygenase from Azotobacter vinelandii. Biochemistry. 1980 Jan 8;19(1):149–155. doi: 10.1021/bi00542a023. [DOI] [PubMed] [Google Scholar]
- Egel R. Intergenic conversion and reiterated genes. Nature. 1981 Mar 19;290(5803):191–192. doi: 10.1038/290191a0. [DOI] [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg S. L. The evolution of bacterial enzyme systems. Annu Rev Microbiol. 1970;24:429–462. doi: 10.1146/annurev.mi.24.100170.002241. [DOI] [PubMed] [Google Scholar]
- Horowitz N. H. On the Evolution of Biochemical Syntheses. Proc Natl Acad Sci U S A. 1945 Jun;31(6):153–157. doi: 10.1073/pnas.31.6.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A. Enzyme recruitment in evolution of new function. Annu Rev Microbiol. 1976;30:409–425. doi: 10.1146/annurev.mi.30.100176.002205. [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Wu T. T., Bilofsky H. Variable region genes for the immunoglobulin framework are assembled from small segments of DNA--a hypothesis. Proc Natl Acad Sci U S A. 1978 May;75(5):2429–2433. doi: 10.1073/pnas.75.5.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTLOCK R. P., WOOD W. A. METABOLISM OF PENTOSES AND PENTITOLS BY AEROBACTER AEROGENES. I. DEMONSTRATION OF PENTOSE ISOMERASE, PENTULOKINASE, AND PENTITOL DEHYDROGENASE ENZYME FAMILIES. J Bacteriol. 1964 Oct;88:838–844. doi: 10.1128/jb.88.4.838-844.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorkle G. M., Yeh W. K., Fletcher P., Ornston L. N. Repetitions in the NH2-terminal amino acid sequence of beta-ketoadipate enol-lactone hydrolase from Pseudomonas putida. J Biol Chem. 1980 Jul 10;255(13):6335–6341. [PubMed] [Google Scholar]
- Metzger H., Shapiro M. B., Mosimann J. E., Vinton J. E. Assessment of compositional relatedness between proteins. Nature. 1968 Sep 14;219(5159):1166–1168. doi: 10.1038/2191166a0. [DOI] [PubMed] [Google Scholar]
- Nakai C., Kagamiyama H., Saeki Y., Nozaki M. Nonidentical subunits of pyrocatechase from Pseudomonas arvilla C-1. Arch Biochem Biophys. 1979 Jun;195(1):12–22. doi: 10.1016/0003-9861(79)90322-9. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Parke D. The evolution of induction mechanisms in bacteria: insights derived from the study of the beta-ketoadipate pathway. Curr Top Cell Regul. 1977;12:209–262. doi: 10.1016/b978-0-12-152812-6.50011-1. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N., Yeh W. K. Origins of metabolic diversity: evolutionary divergence by sequence repetition. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3996–4000. doi: 10.1073/pnas.76.8.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]
- Yeh W. K., Davis G., Fletcher P., Ornston L. N. Homologous amino acid sequences in enzymes mediating sequential metabolic reactions. J Biol Chem. 1978 Jul 25;253(14):4920–4923. [PubMed] [Google Scholar]
- Yeh W. K., Fletcher P., Ornston L. N. Evolutionary divergence of co-selected beta-ketoadipate enol-lactone hydrolases in Acinetobacter calcoaceticus. J Biol Chem. 1980 Jul 10;255(13):6342–6346. [PubMed] [Google Scholar]
- Yeh W. K., Fletcher P., Ornston N. Homologies in the NH2-terminal amino acid sequences of gamma-carboxymuconolactone decarboxylases and muconolactone isomerases. J Biol Chem. 1980 Jul 10;255(13):6347–6354. [PubMed] [Google Scholar]
- Yeh W. K., Ornston L. N. Evolutionarily homologous alpha 2 beta 2 oligomeric structures in beta-ketoadipate succinyl-CoA transferases from Acinetobacter calcoaceticus and Pseudomonas putida. J Biol Chem. 1981 Feb 25;256(4):1565–1569. [PubMed] [Google Scholar]
- Yeh W. K., Ornston L. N. Origins of metabolic diversity: substitution of homologous sequences into genes for enzymes with different catalytic activities. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5365–5369. doi: 10.1073/pnas.77.9.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Hori K., Fujiwara M., Saeki Y., Kagamiyama H. Nonidentical subunits of protocatechuate 3,4-dioxygenase. Biochemistry. 1976 Sep 7;15(18):4048–4053. doi: 10.1021/bi00663a020. [DOI] [PubMed] [Google Scholar]
- Yuan K., Johnson W. C., Tipper D. J., Setlow P. Comparison of various properties of low-molecular-weight proteins from dormant spores of several Bacillus species. J Bacteriol. 1981 Jun;146(3):965–971. doi: 10.1128/jb.146.3.965-971.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


