Abstract
Parenteral administration of pharmaceutical products is one of the most popular methods used to produce quick onset of action and also 100% bioavailability. Main problem occurs with the parenteral drug delivery is lack of convenience, affordability, accuracy, sterility, safety etc. Such drawbacks with this delivery system makes it less preferable. Hence, all the disadvantages of these systems can be easily overcome by use of prefilled syringes. The objective of this review article is to provide information regarding prefilled syringes; it's method of preparation, direction to use, advantages, its future scope, and development.
Keywords: Bioavailability, parenteral, prefilled syringes
INTRODUCTION
There are over 20 pharmaceutical companies manufacturing prefilled syringes as preferred delivery device for at least 50 injectables drugs and vaccines that have total combined annual sales of approx. US $500.[1,2] Prefilled syringes are now used across a wide array of therapeutics sectors outside of the traditional domains of anticoagulants and vaccines. The use of prefilled syringes is expected to accelerate over the coming decades. For the pharmaceutical company, the advantages of prefilled syringes are minimizing drug waste; increasing product life span and enhancing level of market share are some of driving market demand. For healthcare workers, prefilled syringes are recognized as an efficient, reliable and convenient method for drug administration. Furthermore, the ease at which patient can self-administer many types of injectables drugs make prefilled syringes of healthcare treatment out of hospital and into home.[1,2] So, in future emerging challenge in the pharmaceutical market for prefilled syringes will be there. This broad acceptance of prefilled syringes is not surprising because of the range of compelling benefits including simplicity, suitability for home use, reduction in wasted products, and greater dose precision. Prefilled are convenient and help ease the administration process.[3] Patient does not have to worry about the transfer of a drug from a vial to a syringe and therefore do not have to worry about leaving a small percentage of dose behind.
Prefilled provide greater patient safety by reducing the potential for inadvertent needle sticks and exposure to toxic products that can occur while drawing medication from vials.[4,5] Prefilled syringes, with their pre measures dosage, can reduce dosing errors and increase patient compliance. Prefilled syringes can virtually reduce the manufactures need to overfill unlike vials those are overfill by as much 20-30% to account for potential waste. This is particularly important where manufacturing and product costs are high and bulk manufacturing capacity is low. Overall, this compilation of paper is not covering energy aspects of the field of prefilled syringes, but aims to provide the reader with a better understanding of this dynamically growing field of parenteral application system.[6,7] Prefilled Syringes assures delivery of particulate free solution and accommodate volumes that typically range from 0.25 to 5.0 ml. They are therefore best suited to products administered by subcutaneous or intramuscular injection. Three categories account for the bulk of PFS: anti-thrombotics, vaccines, and biotech drugs.
ADVANTAGES
0Prefilled syringe cartridges are designed to fit into specialized syringes, which are used to administer various fluid medications. These are used in the position of standard syringes, which practitioners must fill manually before each does is administered. The first prefilled syringe cartridges were made of polypropylene. The advantages of prefilled syringe cartridges are their convenience, affordability, accuracy, sterility, and safety.[8]
Convenience
During an emergency (an allergic reaction), filling of standard syringes can be a time-consuming and complicated process, so that requires a quick injection syringes. Prefilled syringe cartridges can save time, and sequentially save lives. Prefilled cartridges allow injections to be administered more quickly specially in a busy hospital. They can diminish overcrowding in emergency rooms and other treatment areas.[9]
Affordability
Standard syringes feature cylindrical glass barrels and tightly fitting glass rods, which are much more expensive to manufacture in comparison to plastic-base prefilled cartridges and their corresponding syringes. Additionally, prefilled cartridges are much less prone to crack or break in comparison to their standard, glass counterparts.[10,11]
Accuracy
Prefilled syringe cartridges assure that patients receive accurate dosages. This is especially advantageous for patients who need to self-inject medication, but have no medical training. According to boschpackaging.com, with standard syringes it is possible to overfill (or under fill) the barrel, which could negatively impact the efficacy of the treatment.[12,13]
Sterility
Once a standard syringe is filled with a medication, it will remain optimally effective, or sterile, for approximately 12 hours. In comparison, a medication stored inside of a prefilled syringe cartridge will remain sterile for approximately two to three years (this is also referred to as a “shelf life” of two to three years).
Safety
Prefilled syringe have a high level of accuracy, which makes them safer to use. Some cartridges are designed to fit into self-aspirating syringes, which essentially pierce the skin and inject medications without having to push on a plunger. This ensures that injections reach the appropriate depth, and that dosages are administered smoothly.
Medical advantages
Healthcare professionals benefit from accurate, pre-measured doses, reduced dosing and medication errors, and reduced risk of microbial contamination. Prefills are convenient for emergency use and have potential for duplicate peel-off labels, which facilitate patient charting. Single-use prefilled syringes typically do not require preservatives. In fact, in studies conducted recently, nine out of ten healthcare professionals preferred prefills to conventional needles and syringes.[10]
Manufacturing advantages
Another key advantage is that prefills require less overfill. For example, for a 0.5 ml vial, the USP recommends 20-25% overfill. In contrast, for a 0.5 ml, required overfills is less than 2%. As a result, potentially 18-23% more doses can be produced. Simple and flexible processing formats make prefills easier to incorporate into a pharmaceutical company manufacturing line.[10]
Marketing advantages
Not only do healthcare providers prefer prefills, but healthcare products are also accepted and well known globally. Products can be found in virtually every hospital in the US and overseas. This real-time exposure gives us a unique opportunity to develop products for customers that will be well accepted by their ultimate recipients – end-users, doctors, and other healthcare professionals.[10]
REASONS FOR NOT IN USE
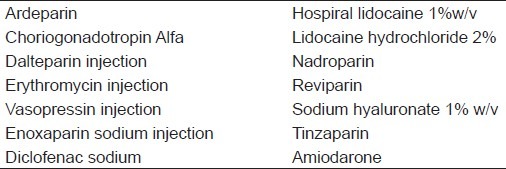
Prefilled syringes are not used widely because there are no prefilled syringes with integrated safety feature. Pharmaceutical companies marketing drugs in prefilled format have main option to comply with laws specking to protect healthcare workers from needle stick injuries.[1] There are many drugs which are used via prefilled syringes [Table 1].
Table 1.
List of drug that are used via prefilled syringes
FACTORS RESPONSIBLE FOR GROWTH OF PREFILLED SYRINGES
Ease of administration, more convenient for healthcare professional and end user, easier for home use, easier in emerging situations, reduction of medication errors, misidentification, and better dose accuracy, increase assurance of sterility, Better use of controlled drugs such as narcotics, lower injection cost by less preparation, fever material, easy storage and disposal, elimination of vials overfill for products transferred to syringes for direct diluents, removal of preservatives, i.e. thiomersal from vaccine formulation, product differentiation.[6,7]
MATERIAL USED FOR THE PREPARATION OF CONTAINER AND CLOSURE ASSEMBLY OF PREFILLED SYRINGES
Glass
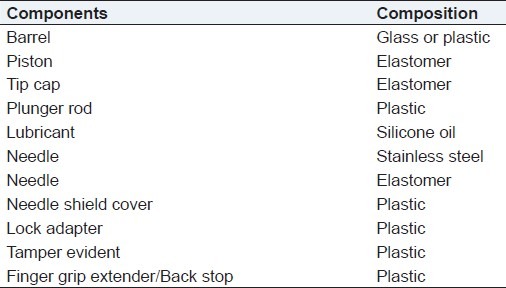
Glass is used as it is non-reactive and stable during storage. But it has main disadvantages that it is highly fragile in nature. [Table 2] Glass used in manufacturing of prefilled syringes should be made up of borosilicate glass of hydrolytic class I, which has transformation temp of 565°c.
Table 2.
Component of prefilled syringes
Marking in glass
An already established laser method was further developed within the syringe forming process and coding device into the existing manufacturing equipment. This entails the commercially available rotary indexing machine for syringe manufacturing. To optimize the laser process in terms of readability of the code, the long wave CO2 laser was programmed to 20% of laser out power.[14] This procedure also ensure about crack-free product.[15]
Siliconisation
Siliconisation seems to remain one of the processes required to allow good performance in the effective delivery of injectables. Prefilled syringes are one of the most challenging application, where silicone is a key factor in combination with glass barrel, rubbers stoppers to obtain effective and accurate drug delivery, combining packaging is mandatory requirement for all companies involved in manufacturing of primary packaging compliant to regulatory guidelines and GMP framework.[16–18]
Biocompetitable siliconisation
The demand for enhanced biocompatibility requires advanced solution to provide barrels characterized by two aspects in comfit.[17,18]
Extrusion speed at minimum pressure
Reduce overall silicone oil quantity.
Glass prefilled syringes are of two types[19]
Oil siliconised syringes
In this type of prefilled syringe system there is direct contact of rubber to glass surface leads overtime to higher break out forces and leads to chances of contamination Figure 1a. Baked on silicone syringes:-In this backed on silicone syringes provide consistent coating of the glass barrel Wales. Break out forces stay low during storage Figure 1b.
Figure 1(a-b).
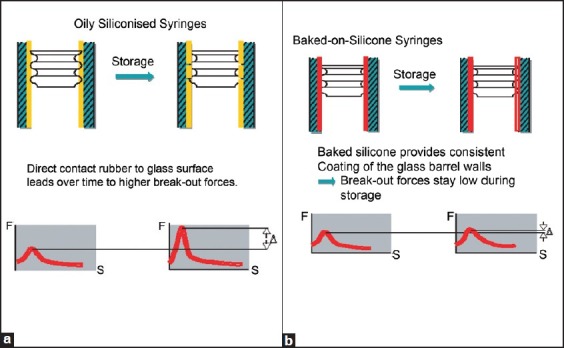
Oily siliconised syringes and baked on silicone syringe
pH shift in glass syringes
The shift in pH occurs because of use of type I glass used in prefilled syringes manufacturing is a borosilicate glass, which must be subjected to various temperature changes glass tube production process Figure 2a and 2b. Around the beginning of cooling phase at 580°c, sodium oxide is forms and remains in glass. During storage, sodium ions are released into the water for injection and increase the concentration of hydroxide ions, thus increasing alkalinity. This causes change in pH in syringes.
Figure 2a.

pH shift in glass syringes
Figure 2b.
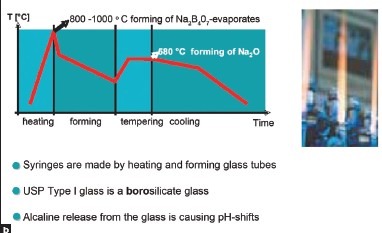
Sodium oxide released from sodium ions
PLASTIC OR POLYMER USED IN PREFILLED SYRINGES
Why plastics are selected for prefilled syringes?
Plastics syringes bring a true benefit over glass. Plastic provide improved robustness against breakability and better ergonomy (light weight),while delivering for many product an adequate stability performance level regarding water/gas permeability as well as extractible/leachable.
Types of plastics used
Plastics materials used for prefilled syringes are mainly of two types.
Cyclo olefin polymer (cop)
Cyclo olefin co polymer (coc)
Significant advantages of plastics[2,3]
Plastics makers developed a new class of thermo elastic polymers that are used as clear as glass but lighter and less prone to breakage. These resins are also more resistant that polypropylene to water transmission, which lengthen the shelf life of the drugs. They contain one of the most popular Daikyo crystal zeniths. These crystal zeniths provide an impressive array of physical and chemical properties that are attractive to drug makers:-
High heat resistance: material is autoclavable.
Excellent low temperature characteristic, including tolerance of freeze drying and liquid nitrogen exposure.
Excellent drain ability: Crystal Zenith offers a non wettable surface with low surface energy and a contact angle of 80, compared to 7 for glass.
High break resistance.
High transparency.
Low extractable: there are virtually no metal extractable from Crystal Zenith.
Solvent resistance;
Wide range of pH, from 2 to 12.
Easy, safe, environmentally friendly disposal: the syringe can be incinerated with virtually no residual ash.
Cycloolefin-copolymers (COCs) are clear amorphous copolymers based on cyclic and linear olefins. These materials form a family of engineering resins that exhibit a unique combination of properties, including high transparency, low density, excellent moisture barrier capabilities, and resistance to aqueous and polar organic media. Topas® COC is being used for pre-filled syringes, needleless injectors and other drug delivery systems.[23]
FILLING PROCESS IN PREFILLED SYRINGES
Traditional filling method includes filling of syringe with solution and then closing and then final sterilization of that prefilled syringes. But this method has disadvantages that there are chances of bubble in the prefilled syringes. Newer technique developed by HCM's (Hyaluron Contract manufacturing) patented method of syringe filling involves online vacuum filling coupled with online vacuum stoppering, Known as bubble-free filling. It eliminates the air bubble inside the syringe, (known as “head space”), that results from traditionally filling methods. Furthermore, totally removing the gas bubble improves the stability of oxygen sensitive compounds. Another advantage is that bubble-free filling is compatible with coated stoppers. This benefit arises from the nature of the filling process rather than anything to do with the gas bubble itself. Conventional filling process use a rod to push the stopper into place, but this can damage stopper coatings. In bubble-free filling, a vacuum (or, more accurately, differential pressure) is used to place the stopper.[2]
STERILIZATION OF PREFILLED SYRINGES
Sterilization of prefilled syringe is mainly done by autoclaving or by ionizing radiation. Autoclave is not suitable of glass prefilled syringes and normal plastics, as there occurs a pH shift in glass syringes during autoclave sterilization process. Mainly used method of sterilization is ionizing radiations. Gamma sterilization has proved to be an efficient means of sterilizing prefilled syringes. Ionizing radiation has the advantage of sterilization the syringe plungers while they remain in their packaging. Possible risks of contamination are therefore limited to handling when transferring the components to the sterilizing zone where the products are packed. Radio sterilization takes place without moisture. Therefore, the stoppers do not have to be dried before use. Gamma rays are highly penetrating and can be used for treating whole syringe. The exposure dose is well controlled and can be easily recorded.[20–22]
VALIDATION PROTOCOL FOR PREFILLED SYRINGES
Validation of washing process
Washing rubber parts effectively requires equipment specially designed for this purpose. Stelmi has therefore developed its washing technology and uses machines of its own design. The cleanliness of elastomeric components is particularly important for pre-filled syringe components, especially plungers where there is a large contact surface between the drug and the plunger and where contact is prolonged. The cleanliness of elastomer sealing systems principally concerns the parameters of visible and subvisible particles, bioburden and endotoxins. Regulatory requirements mainly concern the quality of the fluids used, the environment and the validation of the procedure. The quality of water to be used is clearly defined in the Guidelines issued by the FDA and the EMEA.[20,21] In both cases, it must comply with the “Purified Water”. USP Monograph for the washing and first rinsing operations and the “Water for Injectable Product” Monograph for the final rinsing. The environment should not allow any recontamination after washing. The sensitive area is packaging, which will take place in an ISO 5 standard classified zone. In order to meet these requirements, Stelmi uses the Ultra Clean 6 evolution washing process for plungers, which allows the highest particulate and microbiological cleanliness to be obtained.
Washing and first rinsing operations are carried out using Highly Purified Water, EP.
The final rinsing is in WFI, USP.
Validation is divided into three successive qualification phases, each with a specific aim.
Installation Qualification (IQ) consists of checking that all the equipment is installed in accordance with the manufacturer's designs and specifications.
Operational Qualification (OQ) consists of demonstrating that the equipment functions as anticipated under normal and extreme conditions of use.
Performance Qualification (PQ) is carried out under real production conditions. Its purpose is to show that the product obtained clearly conforms to previously established specifications.
PQ mainly covers the following parameters:
Minimum 3 log reduction of bacterial endotoxin contamination (Worst Case Challenge).
Reduction of particulate contamination.
Reduction of microbiological contamination.
Absence of residual detergent.
Homogeneity of siliconisation.
Qualitative and quantitative knowledge of the microbiological contamination is essential for plungers as contamination not controlled may call into question the validation of the sterilization process. In fact, testing particulate and microbiological cleanliness does not just rely on the washing procedure, and all manufacturing processes related to plungers must be optimized to fully limit risks of contamination. The optimization allows contamination below 0.1 CFU/cm2 before sterilization to be absolutely guaranteed. Moreover, Ultra Clean 6 evolution is part of a general programme of maximum endotoxin elimination based on the implementation of effective preventive and elimination methods, making it possible to guarantee exceptional levels: endotoxins <0.03 EU/ml. Stelmi has taken the option of regrouping all the information about pharmaceutical closure finishing in a Type-V DMF. The pharmaceutical laboratories may therefore refer to this DMF and simplify the registration of their own file with the FDA and it ensures that Stelmi's production meets all the reference requirements in force. Stelmi has prepared a unique DMF for its production sites, ensuring that the same quality is provided regardless of the production site and also allowing the availability of a second source.
Validation of sterilization process
Validation of the sterilization process with gamma irradiation has four steps:[21]
Determining the maximum dose tolerated by the product
Determining the permitted maximum irradiation dose concerns the product itself and its packaging. The most relevant tests are those described in the European Pharmacopoeia and applicable to rubber closures. They reveal the effect of gamma sterilization on the chemical and functional properties of the product. These tests may be augmented by measuring the mechanical properties in standardized test samples. The results obtained from chlorobutyl rubber closures show great stability of all the properties studied after exposure to 25 and 50 kGy. With respect to the packaging materials, the measurement of mechanical properties gives interesting information about the behavior of the chosen material. Integrity is checked at the time of determining the expiry date. The elastomer-based formulations offered by Stelmi for sterile plungers are designed to be compatible with radio sterilization. Exposing the stoppers to 25 and 50 kGy either does not affect the investigated properties or has a negligible influence on these properties. Even when irradiated and aged, these formulations do not approach the limits described in the principal norms and pharmacopoeias. The influence of ageing was also studied. The tests were performed after one and three years. No significant change in the chemical profile of the elastomer formulation was observed.
Determining the sterilizing dose
To select the sterilizing dose, Stelmi has chosen to use ISO regulation 11,137 relating to the sterilization of medical devices and more specifically to use Method 1, which involves working from information on bioburden. The advantage of this method is accurate determination of the sterilizing dose and therefore not exposing the product more than is necessary. In practice, an estimate of the average bioburden is made from three different batches of syringe plungers and a table provides the dose at which SAL (Sterility Assurance Level) is 10 for this bioburden. This value is then used as the verification dose. A sample of 100 syringe plungers is then exposed to this verification dose and the sterility of each product is tested individually. If there are no more than two positive tests out of 100, the sterilizing dose where SAL is 10 is determined using the same table. Once the dose is determined, a periodic audit must be carried out to confirm the validity of this sterilizing dose.
Determining dose mapping (irradiation dose received in each point of the batch to be sterilized)
Gamma sterilization permits products on entire pallets to be treated, which simplifies handling considerably. Depending on the density of the product, this may lead to significant dose differences in different points within the load on the pallet. The first step therefore consists of determining the configuration which allows the most homogenous irradiation dose possible to be obtained between the different points within the load being treated. This is done by choosing the service provider and its plant, as well as by optimizing the pallet-loading arrangement. Dose-mapping is then validated from three different loads. Validation involves distributing the dosimeters to different points within each load and therefore determining the position of the “cold point” (maximum dose) and the position of the “hot point” (maximum dose) so that exposure relating to these points can be compared with a routine or reference point. Afterwards, a single dosimeter will be necessary for each load and will be placed at the “routine point”. From the irradiation dose measured at the routine point, we can deduce the doses received at the cold and hot points and therefore ensure that each point within the load has at least received the sterilizing dose without exceeding the set maximum dose.
Determining the expiry date
The expiry date is determined following an aging study conducted on the finished product. This aging study allows us to check packaging integrity and the behaviour of the irradiated product. Packaging integrity is an essential factor in terms of preserving sterility. This may be demonstrated by periodically checking the vacuum level in the bags and this examination may be completed by performing sterility tests on the product itself. A review of the physico-chemical properties of the product may be conducted at the same time to check conformity over time.
VARIOUS STEPS TO USE PREFILLED SYRINGES[9]
Step 1: Verify the label on prefilled syringe as it may be serious if wrongly injected.
Step 2: Take out the syringe cap and needle cap without touching the needle tip to prevent the contamination of the syringe.
Step 3: Insert the needle. Manually inserting a needle into skin can be the most challenging element of self injection. This is to be expected because our survival has depended on avoiding injury, so our natural instinct is to avoid actions that would result in a self-inflicted wound. Again, the current generation of auto injector typically features auto insertion of the syringe needle to overcome this challenging step.
Step 4: Once injection is completed, the patient must dispose the used syringe.
CASE STUDY
Interferon beta-1a prefilled syringe - injection (Avonex) is used to treat multiple sclerosis (MS). Interferon is not a cure for MS, but it may help to decrease the number of attacks of weakness and slow the progression of the disease. This medicine is injected usually once a week into a muscle (IM-intramuscularly) or as directed by doctor.[24]
For the treatment of rheumatoid arthritis, a new anti-tumor necrosis factor alpha (TNF-α) inhibitor with a novel mechanism of action has entered phase 3 trials. Certolizumab pegol (Cimzia®) is a humanized Fab antibody fragment against TNF-α with a polyethylene glycol tail that prevents complement-dependent and antibody-dependent cell-mediated cytotoxicity or apoptosis. Prefilled Certolizumab pegol syringes for self-use are used for the treatment of rheumatoid arthritis.[25]
CONCLUSION
From this review article it can be concluded that there is vast scope for development of prefilled syringe market. As there are many advantages of prefilled syringes like convenience, affordability, accuracy, sterility, safety, marketing advantages, manufacturing advantages, and marketing advantages. Thus, prefilled syringes will eventually replace the conventional type.
Footnotes
Source of Support: By industry person
Conflict of Interest: None declared.
REFERENCES
- 1.Stephen A. Unilife – Developing prefilled products of choice. Cedar cottage, Newtimber Place Lane, West Sussex, BN6 9BU United Kingdom: ON drug delivery Ltd; 2010. [Google Scholar]
- 2.Danielle L. Advanced innovations on a new generation of plastic prefilled syringes. Cedar cottage, Newtimber Place Lane, West Sussex, BN6 9BU United Kingdom: ON drug delivery Ltd; 2010. [Google Scholar]
- 3.Bernie L. Prefilled syringes: Device suppliers meeting pharmaceutical standards. East Sussex, United Kingdom: ON Drug Delivery Ltd; 2007. The next generation of prefillable syringes: Speciaised plastics lead the way. [Google Scholar]
- 4.Shawn DK. Cedar cottage, Newtimber Place Lane, West Sussex: United Kingdom: ON drug delivery Ltd; 2007. [Last Cited on 2007 Oct 10]. A better fill for prefilled syringes: Applications and advantages of bubble free filling for today's parenteral products; pp. 17–22. Available from: http://www.ondrugdelivery.com . [Google Scholar]
- 5.Karras L, Wright L, Cox L, Kouns T, Abram, Akers MJ. Current Issues in Manufacturing and Control of Sterile Prefilled Syringes. Pharm Tech. 2000;24:188. [Google Scholar]
- 6.Palin JB. Pharm med packaging News. Los Angeles, United States: Canon Communications LLC; 2003. The ins and outs of prefilled syringes. [Google Scholar]
- 7.Karras L, Wringt L, Lox L, Kouns T, Abram D, Aker MJ. Current issue in manufacturing and control of sterile prefilled syringes. Pharm Tech. 2000;24:118. [Google Scholar]
- 8.Pagay SN, Bachorik RJ, II, Richard TL. Pre-filled syringe and prefilled cartridge having actuating cylinder/plunger rod combination for reducing syringing force. Patent Storm: US Patent 5411489 - Pre-filled syringe and pre-filled cartridge. 1995 [Google Scholar]
- 9.Glenn AT. Prefillable syringes: Trends and growth strategies. Cedar cottage, Newtimber Place Lane, West Sussex, BN6 9BU, United Kingdom: ON drug delivery Ltd; 2006. Registered in England: No 05314696. [Google Scholar]
- 10.EMEA Note for Guidance on Quality of Water for Pharmaceutical Use. 2002 [Google Scholar]
- 11.Tibor H, Karim A, Ryan MA. Reducing quality risks to drug products and meeting needs of patients with enhanced components for prefilled syringe systems. Vol. 30. 48, Albany Villas, Hove, East Sussex, BN3 2RW United Kingdom: Frederick Furness Publishing; 2012. [Last accessed on 2005]. Smart Infusion Pumps. Available from: http://www.ondrugdelivery.com . [Google Scholar]
- 12.Beele Denise. Increasing Popularity of Prefilled Syringes. Packazine. 2007;1:4–5. [Google Scholar]
- 13.Michael NE. The American Pharmaceutical Review. Cedar cottage, Newtimber Place Lane, West Sussex, BN6 9BU, United Kingdom: ON drug delivery Ltd; 2008. Offering a new choice in glass prefillable syringes; pp. 8–12. [Google Scholar]
- 14.Witzman A, Trinks U. Encoding and reading of codes on glass containers for pharmaceutical and diagnostic products. Pharm Ind. 2009;71:1945–8. [Google Scholar]
- 15.Lin DC, Dimitriadis EK, Horkay F. Elasticity of rubber-like materials measured by AFM nanoindentation. eXPRESS Polym Lett. 2007;1:576–84. [Google Scholar]
- 16.Studer K, Decker C, Beckb E, Schwalm R. Overcoming oxygen inhibition in UV-curing of acrylate coatings by carbon dioxide inerting, Part I. Prog Org Coat. 2003;48:92–100. [Google Scholar]
- 17.Simone V, Robert H, Sabine L, Andra W, Ulla T, Oliver B, et al. Encoding and reading of codes on glass containers for pharmaceutical and diagnostic products. Pharm Ind. 2009;17:1770–4. [Google Scholar]
- 18.Andrea S, Nuova O. Fine tuning of process parameters for improving biocompatibility of prefillable syringes. 48 Albany Villas. East Sussex, United Kingdom: Frederick Furness Publishing; 2010. [Google Scholar]
- 19.Thomas S, Mathias R. Prefilled syringes: why new developments are important in injectable delivery today. Government Buildings, Hebron Road, Kilkenny, Ireland: Irish Patents Office; 2010. pp. 26–32. [Google Scholar]
- 20.Mudhar P. Asian Hospital and Healthcare Management. Vol. 23. Secunderabad, Andhra Pradesh, India: Ochre Media Pvt Ltd; 2011. Are Pre-Filled Syringes the Future? [Google Scholar]
- 21.Danielle L. 48 Albany Villas, Hove, East Sussex, United Kingdom: Frederick Furness Publishing; 2007. [Last Cited on 2007 October 10]. Advanced innovations on a new generation of plastic prefilled syringes; pp. 8–13. Available from: http://www.ondrugdelivery.com . [Google Scholar]
- 22.Rubber Closures of Containers for Aqueous Parental Preparations, for Powder and for Freeze-Dried Powders. Eupropean Pharmacopeia. (6th ed) 2005:316–7. [Google Scholar]
- 23.Michael NE. West Prefillable Syringe Solutions Glass and plastic based system. [Last accessed on 2011 Jul 14];Am Pharm Rev. 2012 4:12–6. Available from: http://www.westpfssolutions.com/en/prefsys . [Google Scholar]
- 24. [Last accesssed on 2011 Jul 14]. Available from: http://www.medicinenet.com/interferon_beta-1aprefilledinj syringe/page3.htm .
- 25.Rosa J, Sabelli M, Soriano ER. Prefilled certolizumab pegol (Cimzia®) syringes for self-use in the treatment of rheumatoid arthritis. Med Devices: Evid Res. 2010;3:25–31. doi: 10.2147/mder.s7504. [DOI] [PMC free article] [PubMed] [Google Scholar]


