Abstract
Background
The aim of the present paper was to investigate the relationship between behavioral symptoms and attentional and executive functions and hematological variables related to iron deficiency and anemia, ferritin, hemoglobin, mean corpuscular volume (MCV), and red cell distribution width (RDW) in children and adolescents with attention deficit–hyperactivity disorder (ADHD).
Methods
The sample consisted of 52 ADHD children (42 boys, 10 girls; age 7–13 years; mean ± SD, 9.9±2.1 years). Conners Parent and Teacher Rating Scales were obtained. The neuropsychological test battery included Wisconsin Card-Sorting Test (WCST), Stroop, Continuous Performance Test, Digit Symbol and Digit Span subtests of the Wechsler Intelligence Scale for Children Revised (WISC-R), and Trail Making Test A and B, which taps abstraction –flexilibity (WCST), sustained attention (CPT), mental tracking and complex attention (WISC-R Digit Span, Digit Symbol, Trail Making A and B) and interference control (Stroop). Multiple linear regression was used to evaluate the relation of ferritin, hemoglobin, MCV, RDW, age, gender, and presence of comorbidity.
Results
While seven children had iron deficiency, none of them was anemic. Lower ferritin levels were associated with higher hyperactivity scores in parental ratings. While performance increased with age for most of the neuropsychological tests utilized, ferritin, hemoglobin, MCV and RDW and gender were not significantly related with cognitive performance in this sample.
Conclusions
At least for the present clinical sample, ferritin levels might be related with behavioral but not cognitive measures in ADHD cases.
Keywords: attention deficit hyperactivity disorder, ferritin, iron deficiency, neuropsychological assessment
Attention deficit – hyperactivity disorder (ADHD) is one of the most common neuropsychiatric disorders of childhood. ADHD consists of two symptom domains, hyperactivity/impulsivity and inattentiveness.1 Although numerous studies conducted with children with ADHD have been published each year, the exact pathophysiology of the symptoms is unclear at the moment. However, many studies using different methodologies have indicated that dopamine is a key element of ADHD pathophysiology. Rats with lesions involving the ventral tegmental area have hyperactivity and problems in focusing on specific stimulus. These animals also have difficulties in shifting behavior. 2 Similarly, cortical DA deficiency causes hyperactivity, problems in inhibition, spatial orientation and temporal organization. The changes in prefrontal cortical areas also lead to reactive changes of the subcortical dopaminergic system.3 These studies indicate that dopamine may have an important role in all of the main symptom domains of ADHD. Some authors suggested that the lack of efficient dopaminergic control in the cortical and limbic striatal areas might result in selective attention and behavioral inhibition. 4,5
Iron is a coenzyme of tyrosine hydroxylase, which is critical in dopamine synthesis. 6 Iron is also related with monoamine oxidase, which is critical for the degradation of dopamine. Iron is colocalized with dopaminergic neurons in the brain, 6 and D2 and D4 receptor and dopamine transporter densities decrease with decreased brain iron levels.7,8 All these results suggest that iron metabolism may have important role in ADHD pathophysiology. However, the relationship of ADHD and iron metabolism has not been thoroughly studied. One study showed that children with ADHD had lower mean ferritin levels when compared with normal controls and that low serum ferritin levels were related to more severe symptoms, as indicated by higher Conners Parent Rating Scale scores. 9 Other studies have focused on the utility of iron supplementation in ADHD, with conflicting results. 10 The published studies have not investigated the possible relationship between iron levels and cognitive functioning of children with ADHD. This is surprising in the light of the previous studies that children with ADHD perform relatively worse than controls in vigilance and sustained attention tasks, executive function tests such as planning and organization, complex problem solving, set shifting, verbal learning, memory tests, motor inhibition and response monitoring. 11–20 Iron deficiency without anemia can cause cognitive problems because brain iron levels decrease before anemia becomes evident.21 Indeed, children with iron deficiency with or without anemia have been shown to have significant cognitive problems including lower IQ, motor problems, spatial memory, and selective attention,22,23 which can last for years. 24
The aim of the present study was to investigate the relationship between ferritin, hemoglobin, mean corpuscular volume (MCV) and red cell distribution width (RDW) and neuropsychological measures obtained by a broad test battery in children and adolescents with ADHD. We selected these variables because iron deficiency is usually defined by low serum ferritin levels, or low MCV and high RDW. Anemia is clearly defined as having low serum hemoglobin values. The neuropsychological test battery was selected to cover executive functions and attentional processes.
Methods
Population and sampling
Subjects were 52 ADHD children (42 boys, 10 girls; age 7 –13 years; mean±SD, 9.9±2.1 years). All of the children were Caucasian. All the subjects, who fulfilled the inclusion criteria, were recruited from the general outpatient clinic of a general hospital during February –June 2005. Diagnosis was based on DSM-IV criteria and made by the first author (OO), an experienced child psychiatrist using Schedule for Affective Disorders and Schizophrenia for School-Age Children —Present and Lifetime Version (K-SADS-PL) semi-structured interview. Forty-seven of the ADHD children met the criteria for combined, four for inattentive and one for hyperactive–impulsive subtype. Informed consent was verbal, as is customary given the literacy level of the parents. Nineteen children had various comorbid conditions: nine had comorbid oppositional defiant disorder, one had conduct disorder, six had anxiety disorder, one had depression, and two had enuresis nocturna.
All ADHD children had unremarkable medical history other than ADHD and were clinically screened for psychosis, eating disorders, substance use disorders, pervasive developmental disorders, and mental retardation. All patients were diagnosed for the first time and had never been evaluated for psychiatric disorders or treated with psychopharmacological medicine. None of the children was taking iron supplements or multivitamins with iron at the time of the study The parents could choose to opt out of the study but none of the parents refused to participate.
Symptom severity was evaluated with Conners Parent and Teacher Rating Scales.
Behavioral measures
Conners Parent Rating Scale
The Conners Parent Rating Scale (CPRS) includes 48 items and evaluates behavior of children assessed by their parents.25 The scale includes oppositional behavior, inattentiveness, hyperactivity, psychosomatic and irritability domains. Turkish translation has good validity and reliability.26
Conners Teacher Rating Scale
The Conners Teacher Rating Scale (CTRS) includes 28 items and rates classroom behavior of children as assessed by teachers.27 There are three subscales of the form: eight items refer to inattentiveness, seven items refer to hyperactivity and eight items refer to conduct problems. The CTRS has been translated into Turkish and the Turkish form showed adequate validity and reliability (Cronbach’s α = 0.95).28
Cognitive measures
The neuropsychological test battery consisted of Wisconsin Card-Sorting Test (WCST), Stroop, Continuous Performance Test (CPT), Digit Symbol and Digit Span subtests of the Wechsler Intelligence Scale for Children Revised (WISC-R), and Trail Making Test A and B.
Stroop Color–Word Test
This measures the ability to shift perceptual set with the changing demands, to inhibit a habitual behavior pattern, and to behave in an unusual way. Defects in these abilities result in perseveration, stereotypic behavior, and difficulty in controlling behavior. These functions are mainly frontal lobe functions. Higher interference score indicates worse performance. Stroop Test also assesses information processing rate, and parallel processing of attended and non-attended stimuli, and attention. 29
Wisconsin Card-Sorting Test
The WCST was revised by Heaton et al., and it measures abstract reasoning, building and canceling strategies, problem solving, maintaining attention, and mental flexibility abilities. 30 These abilities are deficient in patients with frontal lobe syndrome, and the deficiency in frontal lobe functions lead to perseveration.
Wechsler Intelligence Scale for Children–Revised
The WISC-R consists of 10 subtests that assess verbal and performance abilities. Verbal subtests are Information, Similarities, Vocabulary, Comprehension and Digit Span. Performance subtests are Picture Completion, Picture Arrangement, Block Design, Object Assembly, and Digit Symbol. Verbal and Performance IQ scores are obtained from the test. Reliability and validity studies of the Turkish form have been conducted. 31 Although the WISC-R is a relatively outdated form of the test, it is widely used, and we do not have updated forms translated into Turkish.
Continuous Performance Test
The CPT is a frequently used test to measure sustained attention and impulsivity. Errors of omission are recorded when a patient does not respond in the presence of a target stimulus, indicating problems in sustained attention. Errors of commission occur when a subject responds to a non-target stimulus, indicating impulsivity. CPT has also been one of the most frequently used measures to investigate sustained attention and vigilance.
The subjects were given verbal instructions, and they had the chance to practice the test until they understood the test instructions. The test takes approximately 10 min. In the CPT a series of 450 continuous letters were presented. The stimuli were single letters 3.5 cm in height, and were displayed in the center of a standard computer monitor for 1000 ms. When the subjects saw the letter X after the letter A, they pushed the button. These targets occurred randomly, for a total of 108 times.
Trail-Making Test A–B
The basic task is connecting a series of stimuli (numbers expressed as numerals) in a specified order as quickly as possible. The score is the number of seconds required to complete the task. In Trail B the patients had to alternate between numbers and letters in a specified order, which taps mental tracking ability. Trail-making test performance is heavily influenced by attention. The test takes 5–12 min. Higher scores in these two tests indicate worse performance. We used the Comprehensive Trail-Making Test (CTMT, PAR, FL, USA).
All tests were administered by a trained psychologist blind to subjects’ diagnostic status and laboratory results. The total battery took almost 1 h. Parents were not present during testing. The tests were given in a fixed order: WISC-R Digit Span, Digit Symbol, Trail-Making Test A and B, Stroop, WCST, and CPT. Serum ferritin, hemoglobin, MCV and RDW were measured in the morning with fasting blood. Laboratory tests were obtained in the same week of the neuropsychological testing. Iron deficiency was defined as ferritin<12 ng/mL or MCV<70 fL and RDW>14.5. Anemia was defined as serum hemoglobin<11.0 g/dL. All patients with iron deficiency or anemia were referred to pediatricians for treatment.
Analysis
Multiple regression was used in order to evaluate the effects of age, gender, ferritin and hemoglobin levels, MCV and RDW, and presence of comorbid conditions on the CPRS –CTRS Inattentiveness and Hyperactivity scores, WCST Percent of Correct Responses and Categories Completed variables, WISC-R Digit Span and Digit Symbol scores, Trail Making A and B scores, CPT Total Correct Responses and Total Errors, and Stroop Interference scores. Ferritin, hemoglobin, MCV and RDW were chosen because they were the factors evaluated in anemia and iron deficiency criteria. Model fit in the regression analysis was evaluated using Durbin –Watson test. Two-tailed significance tests (P < 0.05) are reported throughout. SPSS 10.0 was used for analysis (SPSS, Chicago, IL, USA).
Results
Iron measures
The mean ± SD of various measures related to iron metabolism and hematological variables are summarized in Table 1.
Table 1.
Ferritin and hematological measures
| Range | Mean | SD | |
|---|---|---|---|
| Ferritin (ng/mL) | 7.9–73.0 | 30.6 | 15.4 |
| Hemoglobin (g/dL) | 11.9–15.0 | 13.5 | .70 |
| MCV (fL) | 63.5–90.6 | 81.8 | 4.3 |
| RDW (%) | 11.7–30.7 | 13.7 | 2.6 |
MCV, mean corpuscular volume; RDW, red cell distribution width.
Behavioral measures
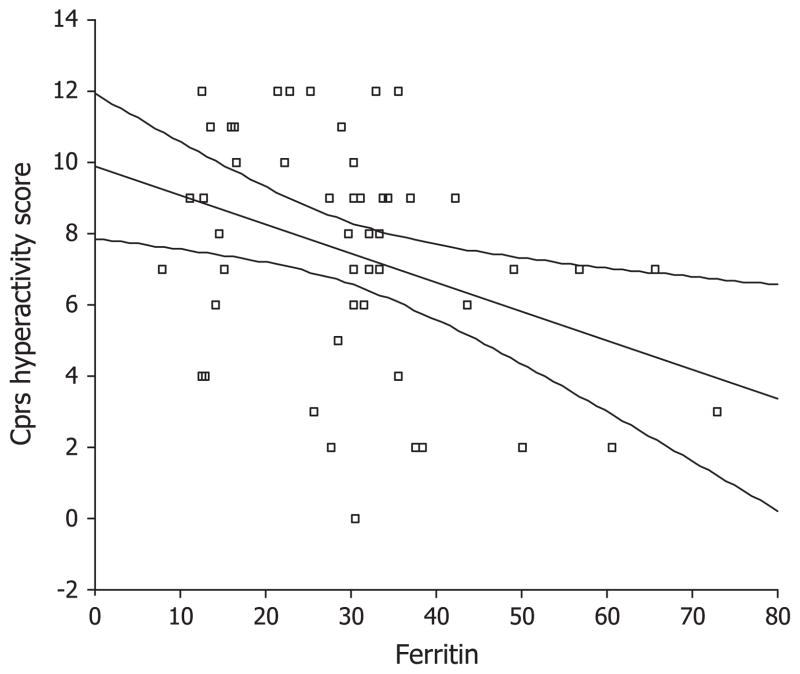
The CPRS Hyperactivity scores were significantly related with serum ferritin level; ADHD children with lower ferritin levels had higher scores, indicating more severe problems (B = −0.41, t = −2.9, P = 0.006). CTRS scores were not associated with the independent variables. Boys had higher CTRS Hyperactivity scores (B = 0.31, t = −2.2, P = 0.034).
Cognitive measures
None of the neuropsychological test scores was significantly related with ferritin, hemoglobin, MCV, or RDW. Gender was also not significantly related with cognitive performance in this sample. WISC-R Digit Symbol (B = 0.32, t = 2.1, P = 0.041), Digit Span (B = 0.43, t = 2.8, P = 0.008), Trail Making A (B = −0.61, t = −4.5, P = 0.001) and B (B = −0.56, t = −4.2, P = 0.001), Stroop (B = −0.58, t = −4.1, P = 0.001), and CPT Total Correct responses (B = 0.63, t = 4.4, P = 0.001) and Total Errors (B = −0.65, t = −4.7, P = 0.001) improved significantly with age. Presence of comorbid conditions predicted lower WISC-R Digit Symbol scores (B = −0.37, t = −2.7, P = 0.006).
Seven ADHD children had iron deficiency and none had anemia. We could not directly compare the subjects with and without iron deficiency due to the small number of subjects with iron deficiency.
Discussion
To the authors’ knowledge this is the first study investigating the relationship between ferritin and hemoglobin levels and MCV and RDW and cognitive variables in ADHD children. We are also aware of one former study that investigated the relationship between ferritin levels and parent ADHD ratings. Consistent with that previous study, we found that lower ferritin levels were associated with higher hyperactivity scores on the CPRS. 9 These authors investigated the correlation of serum ferritin levels with behavioral ratings. We believe that we have extended and elaborated their findings because we used regression analysis and controlled the effects of other variables such as gender, age, and comorbidity, which might influence the ratings. These results suggest that low iron stores, even if not associated with strictly defined iron deficiency or anemia, may contribute to the ADHD symptoms.
In contrast we did not find any significant relationship among ferritin or hemoglobin levels and MCV and RDW with cognitive variables, as well as CPRS and CTRS Inattentiveness score. We used a broad neuropsychological test battery that taps abstraction –flexilibity (WCST), sustained attention (CPT), mental tracking and complex attention (WISC-R Digit Span, Digit Symbol, Trail Making A and B) and interference control (Stroop). The present results show that, as expected, attentional and executive functions improve with age, and age was the only significant predictor of neuropsychological performance in this sample. Lack of significant associations with Inattentiveness scores might also support this result. However, lack of significant association between laboratory and cognitive measures does not necessarily mean that iron deficient ADHD children would not have worse cognitive performance, or that iron deficiency is not related to cognitive variables. Several studies have shown that iron deficiency, even without anemia, is clearly related to cognitive impairment.22,23 Iron metabolism is closely related to brain monoamine metabolism, which makes the association of iron deficiency and cognitive problems biologically plausible. 32 However, the relationship of cognitive variables with iron deficiency had been studied mainly in infant samples. 22 Studies showed that older children and adolescents with iron deficiency had impaired verbal learning, math ability and memory. 23,33,34 In the present sample only seven ADHD children had iron deficiency and none had anemia. The frequency of iron deficiency was reported to be considerably higher in the Konofal et al. study.9 They reported that >80% of their ADHD sample had iron deficiency. However, the present definition of iron deficiency was more stringent then theirs(ferritin < 12 ng/mL or MCV < 70 fL and RDW > 14.5 vs ferritin < 30 ng/mL). If we had used their criteria, 44% of the present subjects would have had iron deficiency, which is still lower than their figure. In contrast, when we reanalyzed our data using these iron deficiency criteria, the results remained essentially the same; ADHD children with iron deficiency had higher CPRS Hyperactivity and Inattentiveness scores and there were no significant differences in other variables (data not shown).
Fig 1.
Conners Parent Rating Scale (CPRS) Hyperactivity scores and ferritin levels.
We did not find any significant relationship between teacher ratings and ferritin levels. This raised the possibility that the significant association of parent ratings and ferritin levels might be due to chance. However, the association was significant and was consistent with the previous study.9 Studies with bigger sample sizes are needed to confirm this.
The present study implies that iron stores might be important in hyperactivity symptoms in children and adolescents with ADHD. However, ferritin level was not related to cognitive variables and inattentiveness symptoms. Thus, it can be speculated that the effects of iron replacement therapy might be differential in these domains. This issue must be evaluated in future randomized controlled trials with larger sample sizes.
Acknowledgments
Ozgur Oner, MD, was supported by NIMH Fogarty International Mental Health and Developmental Disabilities Research Training Program (D43TW05807) at Children’s Hospital Boston.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, IV. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 2.Nieoullon A. Dopamine and regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 3.Oades RD, Taghzouti K, Rivet JM, Simon H, Le Moal M. Locomotor activity in relation to dopamine and noradrenaline in the nucleus accumbens, septal and frontal areas: A 6-hydroxydopamine study. Neuropsychobiology. 1986;16:37–42. doi: 10.1159/000118294. [DOI] [PubMed] [Google Scholar]
- 4.Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate-putamen of an animal model of attention-deficit hyperactivity disorder: The spontaneously hypertensive rat. Brain Res. 1995;676:343–51. doi: 10.1016/0006-8993(95)00135-d. [DOI] [PubMed] [Google Scholar]
- 5.Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–9. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 6.Wigglesworth JM, Baum H. Iron dependent enzymes in the brain. In: Yuodim MBH, editor. Brain Iron: Neurochemical and Behavioral Aspects. Taylor and Francis; New York: 1998. pp. 25–66. [Google Scholar]
- 7.Erikson K, Pinero D, Connor J, Beard J. Iron status and distribution on iron in the brains of the developing rats. J Nutr. 1997;127:2030–38. doi: 10.1093/jn/127.10.2030. [DOI] [PubMed] [Google Scholar]
- 8.Ashkenazi R, Ben-Shachar D, Youdim MBH. Nutritional iron deficiency and dopamine binding sites in the rat brain. Pharmacol Biochem Behav. 1982;17:43–7. doi: 10.1016/0091-3057(82)90509-3. [DOI] [PubMed] [Google Scholar]
- 9.Konofal E, Lecendreux M, Arnulf I, Mouren MC. Iron deficiency in children with attention deficit hyperactivity disorder. Arch Pediatr Adolesc Med. 2004;158:1113–15. doi: 10.1001/archpedi.158.12.1113. [DOI] [PubMed] [Google Scholar]
- 10.Sever Y, Ashkenazi A, Tyrano S, Weizman A. Iron treatment in children with attention deficit hyperactivity disorder. Neuropsychobiology. 1997;35:178–80. doi: 10.1159/000119341. [DOI] [PubMed] [Google Scholar]
- 11.Barkley RA, Grodzinsky G, DuPaul GJ. Frontal lobe functions in attention deficit disorder with and without hyperactivity: A review and research report. J Abnorm Child Psychol. 1992;20:163–88. doi: 10.1007/BF00916547. [DOI] [PubMed] [Google Scholar]
- 12.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 13.Grodzinsky GM, Diamond R. Frontal lobe functioning in boys with attention deficit hyperactivity disorder. Dev Neuropsychol. 1992;8:427–45. [Google Scholar]
- 14.Seidman LJ, Benedict KB, Biederman J, et al. Performance of children with ADHD on the Rey-Osterrieth complex figure: A pilot neuropsychological study. J Child Psychol Psychiatry. 1995;36:1459–73. doi: 10.1111/j.1469-7610.1995.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 15.Seidman LJ, Biederman J, Faraone SV, Weber W, Ouellette C. Toward defining a neuropsychology of attention deficit-hyperactivity disorder: Performance of children and adolescents from a large clinically referred sample. J Consult Clin Psychol. 1997;65:150–60. doi: 10.1037/0022-006X.65.1.150. [DOI] [PubMed] [Google Scholar]
- 16.Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 17.Shallice T, Marzocchi GM, Coser S, Del Savio M, Meuter RF, Rumiati RI. Executive function profile of children with attention-deficit hyperactivity disorder. Dev Neuropsychol. 2002;21:43–71. doi: 10.1207/S15326942DN2101_3. [DOI] [PubMed] [Google Scholar]
- 18.Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuro psychological executive functions and DSM-IV ADHD subtypes. J Am Acad Child Adolesc Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Kempton S, Vance A, Maruff P, Luk E, Costin J, Pantelis C. Executive function and attention deficit hyperactivity disorder: Stimulant medication and better executive function performance in children. Psychol Med. 1999;29:527–38. doi: 10.1017/s0033291799008338. [DOI] [PubMed] [Google Scholar]
- 20.Houghton S, Douglas G, West J, et al. Differential patterns of executive function in children with attention-deficit hyperactivity disorder according to gender and subtype. J Child Neurol. 1999;14 :801–5. doi: 10.1177/088307389901401206. [DOI] [PubMed] [Google Scholar]
- 21.Yehuda S, Youdim MBH. Brain iron: A lesson for animal models. Am J Clin Nutr. 1989;50:618–29. doi: 10.1093/ajcn/50.3.618. [DOI] [PubMed] [Google Scholar]
- 22.Grantham-McGregor S, Ani C. A review of studies on the effects of iron deficiency on cognitive development in children. J Nutr. 2001;131:649S–68S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 23.Halterman JS, Kaczorowski JM, Aligne A, Auinger P, Szilagyi PG. Iron deficiency and cognitive achievement among school-aged children and adolescents in the United States. Pediatrics. 2001;107:1381–6. doi: 10.1542/peds.107.6.1381. [DOI] [PubMed] [Google Scholar]
- 24.Lozoff B, Jimenez E, Hagen E, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency anemia. Pediatrics. 2000;105:51–62. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 25.Conners CK. Conners’ Rating Scales–Revised. Multi-Health Systems Publishing; North Tonawada, NY: 1997. [Google Scholar]
- 26.Dereboy C, Senol S, Sener S. Adaptation of Conners’ parent rating scale in Turkish). Proceedings of the 10th National Congress of Psychology; Ankara, Turkey. 1998. p. 42. [Google Scholar]
- 27.Goyette CH, Conners CK, Ulrich RF. Normative data on revised Conners’ parent and teacher rating scales. J Abnorm Child Psychol. 1978;6:221–36. doi: 10.1007/BF00919127. [DOI] [PubMed] [Google Scholar]
- 28.Sener S, Dereboy C, Dereboy IF, Sertcan Y. Conners’ Teacher Rating Scale Turkish version-I. Turk J Child Adolesc Ment Health. 1995;2:131–41. [Google Scholar]
- 29.MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychol Bull. 1991;109:162–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 30.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test (Manual, Revised and Expanded) Psychological Assessment Resources; Odessa, FL: 1993. [Google Scholar]
- 31.Savaşir I, Şahin N. WISC uyarlama çalişmalariön rapor -I. Turk J Psychol. 1978;1:33–7. [Google Scholar]
- 32.Beard J. Iron deficiency alters brain development and functioning. J Nutr. 2003;133:1468S–72S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- 33.Bruner AB, Joffe A, Duggan AK, Casella JF, Brandt J. Randomized study of cognitive effects of iron supplementation in non-anaemic iron deficient adolescent girls. Lancet. 1996;348:992–6. doi: 10.1016/S0140-6736(96)02341-0. [DOI] [PubMed] [Google Scholar]
- 34.Webb TE, Oski FA. Iron deficiency and scholastic achievement in young adolescents. J Pediatr. 1973;82:827–30. doi: 10.1016/s0022-3476(73)80074-5. [DOI] [PubMed] [Google Scholar]