Abstract
The energetics of a fusion pathway is considered, starting from the contact site where two apposed membranes each locally protrude (as “nipples”) toward each other. The equilibrium distance between the tips of the two nipples is determined by a balance of physical forces: repulsion caused by hydration and attraction generated by fusion proteins. The energy to create the initial stalk, caused by bending of cis monolayer leaflets, is much less when the stalk forms between nipples rather than parallel flat membranes. The stalk cannot, however, expand by bending deformations alone, because this would necessitate the creation of a hydrophobic void of prohibitively high energy. But small movements of the lipids out of the plane of their monolayers allow transformation of the stalk into a modified stalk. This intermediate, not previously considered, is a low-energy structure that can reconfigure into a fusion pore via an additional intermediate, the prepore. The lipids of this latter structure are oriented as in a fusion pore, but the bilayer is locally compressed. All membrane rearrangements occur in a discrete local region without creation of an extended hemifusion diaphragm. Importantly, all steps of the proposed pathway are energetically feasible.
In biological membrane fusion, the lipid bilayers of two distinct membranes become one, and formerly separated aqueous compartments become continuous. Even though biological fusion is mediated by proteins, the lipids must transiently leave their lamellar orientations for lipid merger to occur. It is generally thought that the lipid rearrangements of biological fusion proceed in two stages. In the first, the contacting monolayer (referred to as “cis”) leaflets have merged, but the distal (denoted “trans”) leaflets have remained intact, a stage known as hemifusion. In the second, the trans leaflets merge and, in so doing, complete fusion pore formation (1). Although in diagrams pores are often depicted as forming within an extended hemifusion diaphragm, electron microscopy indicates that pores, in fact, form at discrete loci, termed “contact sites” (2).
The transient disruptions of cis monolayers during hemifusion will expose hydrophobic portions of the lipids to water. Because of the high interfacial energy between a hydrophobic phase and water, the area of hydrophobic regions exposed to water must be kept small and would favor a minimum number of disrupted lipids. On the basis of such reasoning, more than 20 years ago a structure, termed a “stalk,” was proposed as the structure of local merger (3). A stalk is created when the cis monolayers bend and merge; the trans monolayers have not yet come into contact. The energy of bending monolayers into a stalk was calculated for two parallel membranes and found to be rather large for monolayers of zero spontaneous curvature (4, 5). Because the two trans monolayers must contact each other after stalk formation for the pore to form, the bending energy necessary for the trans monolayers to come into contact was calculated (6). This latter bending energy was found to be inordinately large, more than 100 kT. These unrealistically large predicted energies mean that a theoretical understanding of membrane deformation has not yet been achieved. In addition, the physics responsible for pore formation from the point of stalk formation were not accounted for in the prior quantitative theoretical treatments. In this paper, we present a model that quantitatively answers three questions that have not yet been addressed by current theory: (i) How can a stalk form and trans monolayers come into contact with the expenditure of realistic energies? (ii) How can a pore form without the creation of an extended hemifusion diaphragm? (iii) What reconfigurations of lipids from the point of stalk formation could lead to the formation of a fusion pore?
We base our model on the fact that fusion pores do not form between parallel membranes. In exocytosis, electron microscopy shows that the plasma membrane bends inward toward the granule membrane to establish local and intimate membrane contact. Viewed from the outside of the cell, the projection of membrane appears as a “dimple” (7, 8). When cells expressing the fusion protein of influenza virus, hemagglutinin, are fused to red blood cells, both morphological (2) and functional (9) evidence indicates that the membranes bend toward each other to establish local contact. Because here each projection is outward, we refer to them as “nipples.” Possible means by which fusion proteins could create nipples have been quantitatively considered (10).
In the present study, we consider membrane deformations after nipples have formed—and the fact that membranes deform not only by bending, but also by tilting of lipids (11, 12) and compression of monolayer thickness (13)—and propose an energetically viable pathway by which stalks may expand and convert to pores.
Qualitative Description of the Model.
The energy required to bend a membrane into a nipple (Fig. 1, N) is considerable (10); nipples cannot, in practice, form spontaneously. The fusion proteins undoubtedly provide this energy. When energy has been garnered into nipple formation, however, the bending energies needed for stalk and pore formation have largely been provided. After nipple formation, transient displacements of polar headgroups from each other yield small hydrophobic patches (14) at the tip of a nipple. Because hydrophobic surfaces attract each other, cis leaflets can merge to create the stalk without significant further bending (Fig. 1, S). As a result of stalk formation, a void exists in the enclosure formed by the monolayers, yielding hydrocarbon–vacuum interfaces (6).
Figure 1.
Illustrations of the intermediate structures of membrane fusion: nipples (N), stalk (S), modified stalk (m-S), and prepore (p-P). The three-dimensional structures are obtained by rotating the illustrated cross sections around the symmetry axis of the system (i.e., the vertical dashed line). The bold solid lines are drawn along the polar head groups of the lipid molecules. The thin solid lines are the neutral surfaces of the bilayer. The dividing surface of m-S, used to calculate the tilt energy, is through the head group region and just on the bilayer side of the thick bold lines. The gray ellipses illustrate the lipid cross-section area along the dividing surface, at, and perpendicular to the molecule axis, a0. The dotted lines are the neutral surfaces of the monolayers. Variables are described in the text.
The trans and cis monolayers should come into greater contact to minimize this hydrophobic void energy. We propose that the trans leaflets deform not simply by bending but also by tilting their lipids with respect to the surface of the monolayer. This titling significantly lowers the free energy of stalk expansion. When contact is made, the central molecules are oriented parallel to the stalk's axis of symmetry (dashed line, Fig. 1, m-S), with the flanking lipids fanning out smoothly to the unaltered region of the nipple. Such a structure has not previously been quantitatively considered. We refer to it as a “modified stalk.” A modified stalk has been pictorially proposed by others (15).
We show that at an energy of about 40 kT above that of the initial stalk, the lipids of the modified stalk can reconfigure to a fusion pore-like structure. In this latter structure, the trans monolayers have merged, and the narrowest aqueous pathway between the two compartments consistent with the repulsive hydration forces has been created. Because the pore radius has not yet expanded beyond that of the modified stalk, the acyl chains of both monolayers are compressed, predominantly in the equatorial region. We refer to this structure as a “prepore” (Fig. 1, p-P). The prepore spontaneously expands to a true fusion pore: its free energy decreases with increased radius because of decompression. We now consider this model in quantitative detail. More explicit derivations are available at the internet web site http://zimmerberg.nichd.nih.gov.
The Fusion Pathway
Nipple Apposition.
Consider two parallel planar membranes of thickness 2h (i.e., each monolayer has thickness h) separated by an aqueous space of distance 2Hm. Fusion occurs where the two identical nipples abut. The nipples are modeled as portions of spheres (with Rn the radius of curvature of the neutral surface) that merge into the planar membrane (Fig. 1, N). The assumption that nipples are identical is not important qualitatively, but it does significantly simplify the calculations. We estimate Rn from the radius of the base of the nipple, rn (see Fig. 1, N), and the fact that the sum of the nipple heights should be approximately the membrane separation (i.e., 2Hm). Assuming that six hemagglutinin (HA) trimers associate tightly to buttress the two apposed nipples, we estimate rn as ≈10 nm. We take the intermembrane gap, 2Hm, to be ≈10 nm, somewhat less than the length of the HA trimer (≈13.5 nm). Straightforward geometrical reasoning yields that Rn ≈ r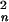 /2Hm ≈ 10 nm. The energy required to bend the spherical segments of the two nipples, Wn, is ≈200 kT for a monolayer bending modulus, B, of ≈10 kT. Unless stated otherwise, we use the energy required to bend the two membranes into nipples as the reference energy and set the spontaneous monolayer curvature equal to zero.
/2Hm ≈ 10 nm. The energy required to bend the spherical segments of the two nipples, Wn, is ≈200 kT for a monolayer bending modulus, B, of ≈10 kT. Unless stated otherwise, we use the energy required to bend the two membranes into nipples as the reference energy and set the spontaneous monolayer curvature equal to zero.
The distance between the tips of the nipples is denoted as l. When this distance is a few angstroms, a strong repulsive hydration force, Fh, prevents the nipples from coming closer together. The equilibrium distance between the nipples, l0, is determined from a balance between Fh and the force generated by the fusion proteins, Fp, that draws the membranes together. We estimate Fp as
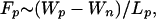 |
1 |
where Lp is a length that characterizes protein movement during conformational changes, and Wp is the energy released by these conformational changes. We assume that Fp is constant for small variations of l.
The hydration force is given by refs. 14, 16, and 17 as
 |
2 |
where, by using cylindrical coordinates, r is the radius, and z(r) is the distance between nipples as a function of r. P0 is the repulsive pressure at zero distance of separation, and ξh is the characteristic length for decay of the interaction. Assuming ξh, l << Rn, we obtain l0:
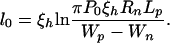 |
3 |
Because l0 varies logarithmically with Wp − Wn and Lp, its dependence on these variables is weak. For Lp ≈ 5 nm, P0 = 109 − 1010 N/m2 (17), ξh = 0.25 nm, Rn = 10 nm, and (Wp − Wn) ≈ 400 kT (10), we obtain l0 ≈ 0.8–1.4 nm.
The Creation of the Stalk.
We calculate the energy barrier, ΔW, that separates closely apposed nipples from becoming a stalk by considering the simultaneous fluctuation of the intermembrane distance l and the radius, rf, of a hydrophobic patch. The change in free energy dW(rf, l) of the system results from changes in the hydration (dWh) and the hydrophobic (dWf) energies as well as the work performed by the protein, −Fpdl. This yields (ignoring the constant of integration),
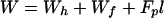 |
4 |
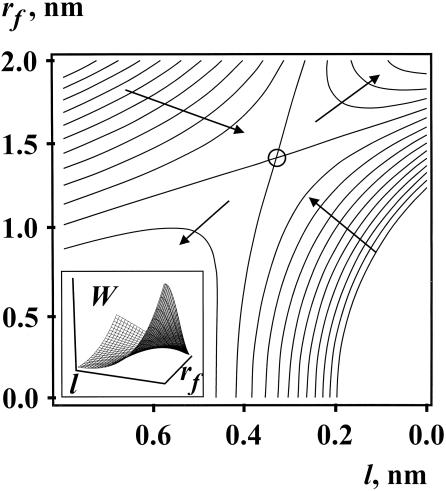
We obtain Wh by integrating Eq. 2, remembering that the hydration forces are absent for the circular hydrophobic patch of radius rf, (rf, l, ξh << Rn). We calculate the energy of interaction of two hydrophobic disks (Wf) of radius rf with an intervening water layer of thickness l (18) by using σ0 = 50 erg/cm2 as the specific energy for a surface of a hydrocarbon–water interface and ξf = 1 nm as the characteristic length for hydrophobic attraction. Fp is given by Eq. 1. The equipotentials of the surface of W(rf, l) are shown in Fig. 2. The process of merger of the cis monolayers follows from the saddle-shaped topology of this energy surface. The free energy is small when the nipples are separated by a large distance, l; the hydrophobic patches do not form (i.e., rf = 0). The free energy increases as the tips of the nipples approach each other and, as a result, hydrophobic patches then form. Eventually, l decreases to the point at which patches become large enough to cause the hydrophobic attraction to dominate and the cis monolayers merge. The unaltered portions of the two apposed nipples have not moved with respect to each other as a result of stalk formation. The height of the energy barrier separating the nipples and the stalk for the most favored pathway is ΔW = 37 kT.
Figure 2.
Equipotentials of the surface of free energy W(rf, l) of two nipples with hydrophobic patches as a function of their separation, l, and radii, rf, of the hydrophobic patches. The contours are 10 kT apart. The circle denotes a saddle-like point; the lowest energy pathway from nipples to stalks is to pass over it, yielding a total energy for transition of 37 kT. Arrows point in the direction of decreases in energy. A three-dimensional representation is shown in the Inset. The trajectory of stalk formation is from the Lower Left to Upper Right (l = 0). The parameters used for calculation are: Rn = 8 nm, P0 = 109 N/m2, ξh = 0.25 nm, Wp − Wn = 400 kT, Lp = 5 nm.
The energy barrier can be estimated from the waiting time, τ, of stalk formation and is in agreement with our model, 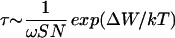 , where ω is the characteristic frequency of monolayer fluctuations, S is the area of the fusion site, and N is the number of fusion sites in the system. For τ ≈ 1 sec, we obtain ΔW ≈ 40 kT, for ω ≈ 1011 s−1nm−2 (14), S ≈ 102 nm2, and N ≈ 102 (2). Because ω, S, and N are preexponential, their precise values do not greatly affect the value of ΔW obtained from τ.
, where ω is the characteristic frequency of monolayer fluctuations, S is the area of the fusion site, and N is the number of fusion sites in the system. For τ ≈ 1 sec, we obtain ΔW ≈ 40 kT, for ω ≈ 1011 s−1nm−2 (14), S ≈ 102 nm2, and N ≈ 102 (2). Because ω, S, and N are preexponential, their precise values do not greatly affect the value of ΔW obtained from τ.
The Energetics and Structure of the Modified Stalk.
In general, both bending and compression (stretching) energies contribute to the free energy of all intermediates. We calculate each as quadratic expressions for deviations of curvature and area per lipid from the spontaneous, planar state of a monolayer. Because each equation of energy contains only the lowest-order term of a higher-order expansion, they should be valid only for small deviations. In compression, areas per lipid do not change significantly, and thus its equation of energy is, a priori, adequate. In bending, deformations can be large and thus it might be thought that higher-order terms must be considered. However, practice has shown that the bending energies calculated by the lowest-order (quadratic) term quantitatively accounts for experimentally observed large changes in curvature (16, 17, 19, 20). Thus, we can describe the elastic energies with two constants of proportionality: the bending modulus, B, and the stretching modulus, kA. The free energy is calculated at the neutral surface, defined as the surface at which bending and compression occur independently of each other. Therefore, when a monolayer bends without compression (or stretching) in the lateral direction, the area per lipid is the same in the curved and flat portions of the neutral surface. This occurs, for example, for the cis monolayer of a stalk. We smoothly connect all toroidal segments (e.g., that of a stalk) to the nipples at the neutral surface and place this surface at a fixed distance rh from the monolayer-solution interface (Fig. 1, S and p-P). Lipids are considered volumetrically incompressible.
At the point of stalk formation, the trans leaflets have not yet approached each other, but the energy caused by the void is negligible. The free energy of deformation of bending the cis monolayers within the stalk, Wc, can be determined analytically as
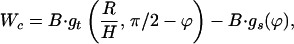 |
5 |
where
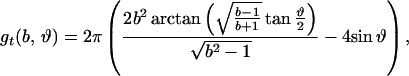 |
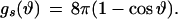 |
6 |
R is the distance from the axis of symmetry to the center of an arc of radius H whose rotation generates the neutral surface of the cis monolayer. ϕ is the angle that the line through the center of the arc and the connection between the toroidal and spherical portions of the neutral surface makes with the axis of symmetry (see Fig. 1, S). R, H, and ϕ are determined from the geometry of the system as
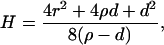 |
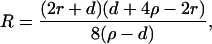 |
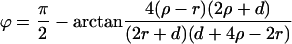 |
7 |
where r = rp − rh, ρ = Rn + h − rh, and d = l0 + 2rh; rp is the outer radius of the stalk at the narrowest point of the neck (Fig. 1, S). The first term in Eq. 5 is the bending energy of the cis monolayers of the stalk (i.e., the toroidal portion that comprises the stalk), and the second term is the bending energy of that portion of the nipple tips used to make to the stalk.
It is likely that the neutral surface of a highly curved monolayer is located near the glycerol backbone of the phospholipid molecules (19, 20). We therefore used rh = 0.6 nm. The asymmetric placement of the neutral surface combined with the volumetric incompressibility of monolayers means that the monolayer thickness, h, varies with monolayer curvature. (h would be constant if the neutral surface were symmetrically located at the midplane of a monolayer.) This dependence of thickness on curvature prevented us from deriving analytic expressions for energies of the void, the modified stalk, the prepore, and the fusion pore. We obtained these energies numerically (Figs. 3 and 4) as functions of rp, the outer radius of the stalk and pore neck (see Fig. 1, S and p-P).
Figure 3.
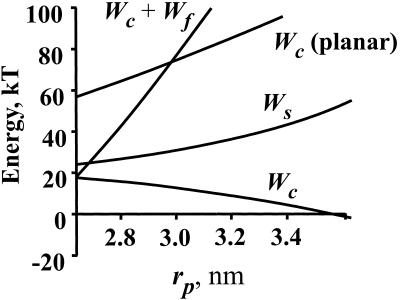
Free energy of a stalk as a function of its outer radius, rp, in the equatorial plane. Wc (planar) and Wc are the free energies of bending cis monolayers into stalks from planar membranes and nipples, respectively. Wf is the free energy of the hydrophobic void that would result if trans monolayers did not approach each other. Ws is the free energy of the modified stalk. The parameters of calculation were: Rn = 8 nm, B = 10 kT, σ0 = 27 erg/cm2, kA = 100 erg/cm2, h = 2 nm, rh = 0.6 nm, l0 = 0.8 nm.
Figure 4.
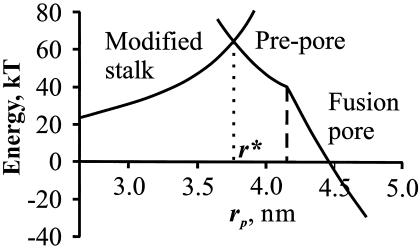
Free energy of a modified stalk, a prepore, and a fusion pore. Lipids rearrange from a modified stalk to the prepore at the intersection point, r*. The prepore spontaneously converts to a conducting fusion pore. Parameters are: Rn = 8 nm, KA = 100 erg/cm2, B = 10 kT, h = 2 nm, rh = 0.6 nm, rw = 0.4 nm, l0 = 0.8 nm.
With stalk expansion, the void becomes significant. We obtain a lower bound for the void energy, Wf, as a function of rs = rp − he for rs < h/2 by approximating the surface of the void as a cylinder of radius rs and height (2h + l0) (neglecting the area of the ends) (21); he is the monolayer thickness at the equatorial plane.
The relative contributions of the bending and void energies of the system as a function of stalk radius can be appreciated from Fig. 3. For comparison, the energy to bend the cis monolayers from planar membranes into a stalk, Wc(planar), is also shown; clearly Wc << Wc(planar). If a stalk expanded without the trans monolayers coming into contact, the void would enlarge. The required energy (Wc + Wf) becomes so high with increased radius that a large void would never form (Fig. 3). On the other hand, the trans monolayers cannot come into contact by bending, because this is energetically and sterically prohibitive—smooth bending would lead to hydrophobic voids. Clearly, for the trans monolayer to come into contact and to merge, an alternate route must exist. We have located an energetically feasible route.
Lipids in planar membranes can fluctuate normal to the neutral surface (22). Hydrophobic attraction between the separated trans monolayers would facilitate these out-of-plane lipid displacements. Through such displacement of lipids, the trans monolayers can contact each other—yielding the modified stalk (Fig. 1, m-S)—without creating voids. In the modified stalk, lipids in the trans monolayers have tilted with respect to the normal to the neutral surface. In the nipple, we assume each lipid's orientation is directed toward the origin of the nipple's spherical cap (Fig. 1, m-S). For the sake of explicit calculation, this direction is maintained as the stalk expands into a modified stalk. Intuitively, with this maintained direction, there is no change in bending energy for the nipple to convert into modified stalk—the change in free energy is entirely because of tilting of the lipids.
To calculate the tilting energy, consider a curved surface dividing the water from the hydrophobic tails. This surface is located in the region of the polar headgroups, and we refer to it as a “dividing surface.” The area per lipid molecule at the dividing surface is larger for tilted lipids, at, than for lipids perpendicular, a0, to the surface. Tilting thus exposes portions of the hydrophobic tails to water. We assume that the dividing surface is parallel to the surface of the free termini of the acyl chains of the cis monolayer. The distance between these two surfaces varies with the degree of tilting. Because this variation contributes as a second order term in energy, whereas the exposure of hydrophobic tails provides the first order term, we fixed the distance between the surfaces as the monolayer thickness h. The energy per lipid (w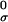 ) required for tilting may be written as
) required for tilting may be written as
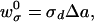 |
8 |
where Δa = at − a0, and σd is the surface tension at the dividing surface and is readily obtained from the relation KA = 3σd (13) where KA is the elastic modulus for stretching the monolayer area. KA ≈ 100 dyn/cm for bilayer membranes, independent of the length or degree of saturation of the acyl chains (13).
The specific energy, wσ = σdΔa/at, per unit area of dividing surface, is
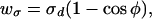 |
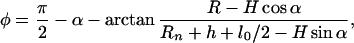 |
9 |
where φ is the angle between the axis of the tilted lipid and the normal to the dividing surface. The angles φ and α are shown in Fig. 1, m-S. H and R are given by Eq. 7, but expressions for r, d, and ρ are different and given by r = rp − he, d = l0 + 2h and ρ = Rn. For small deviations, Eq. 9 is equivalent to the specific energy of tilting derived by others (12). The total energy to tilt the lipids within the trans monolayers, Wσ, is obtained by using Eq. 9 to integrate wσ along the dividing surface over the entire stalk up to the location of merger with the unperturbed nipples. The explicit expression for Wσ(rp) cannot be obtained analytically, and it was therefore computed numerically. Adding Wc and Wσ, we obtain the free energy of the modified stalk, Ws, as a function of rp (Fig. 3). Ws is significantly lower than the free energy of an expanded stalk with a hydrophobic void (Wc + Wf); Ws increases ≈25 kT as the stalk expands from its initial radius to rp ≈ 3.5 nm (Fig. 3). In other words, the energy of this pathway is small enough to permit the formation and expansion of the modified stalk.
The Energetics and Structure of the Prepore.
The free energy of an extended hemifusion diaphragm is extremely large [for a line tension of 10 kT/nm (6), it is about 150 kT] and relatively independent of radius (calculations not shown). The free energy of a fusion pore is much lower, and therefore the evolution of a stalk into a pore should proceed without the creation of an extended diaphragm. To understand how a modified stalk converts to a pore, it is useful to view the process in the reverse direction. Obviously, a pore's radius is greater than that of a stalk. The pore radius, rp, decreases by shrinking the radius, rw, of the water-filled lumen (Fig. 1, p-P). However, a point is reached in which a pore cannot shrink any further by eliminating additional water from the lumen. For example, experiments show that the radius of water-filled tubes within phospholipid HII phases cannot decrease to less than ≈0.4–0.6 nm (23). Because the geometry of a fusion pore at the equatorial region is similar to that of the cylindrical tube of an HII phase, we assume that a pore does not shrink beyond rw = 0.4 nm. Further reduction of rp could only occur by compressing the monolayers in the collapsed region.
The energy of compression per lipid molecule, w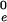 , for each monolayer is
, for each monolayer is
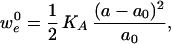 |
10 |
where the areas refer to the neutral surface. The area a varies with the monolayer thickness, which in turn depends on the curvature. rp decreases through compression of each monolayer, consistent with the overall bilayer compression. The toroidal neutral surface of the trans monolayer remains fixed because rw does not vary. Instead, both H and R (see Eq. 7 and Fig. 1, S), which define the neutral surface of the cis monolayer, change with compression. To determine the amount each monolayer compresses as rp is decreased, we calculated the minimum compression energy. We did this by integrating Eq. 10 over the neutral surfaces of both monolayers and determining the position of the interface between the two monolayers that minimized the compression energy. The bending energy (24) of the prepore (which, as always, is independent of how much each monolayer was compressed) was calculated over the neutral surface by using Eqs. 5–7.
The numerically calculated free energies of the modified stalk, the prepore, and the fusion pore are shown in Fig. 4. At rp = r* ≈ 3.7 nm, the free energy of the prepore matches that of the modified stalk. At this intersection point, a reorientation of lipids within the trans monolayer is thermodynamically permitted. Therefore, the modified stalk can transform into a prepore that will then spontaneously expand to an uncompressed pore. The height of the energy barrier at the transition radius r* is ≈40 kT for a nipple radius of Rn ≈ 7–10 nm. In other words, the energy profiles of the modified stalk and the prepore converge at an achievable energy value. Thus, we can account for the conversion of a stalk to a pore in an energetically viable manner through deformations of monolayers that are known to occur.
The Required Monolayer Deformations Are Feasible.
Because our assumed pathway yields modest energy barriers, we have demonstrated that it is energetically favorable for fusion to occur at the discrete site of the stalk, as probably occurs experimentally (2). In our model, the initial stalk resides in a local energy minimum and thus is metastable. In contrast, the two new proposed intermediate structures—the modified stalk and the prepore—do not reside at energy minima and thus are transient structures.
All of the deformations in our model fusion pathway have been considered in other contexts and are reasonably well understood. Fluctuations of lipids normal to the surface of planar bilayers have been experimentally identified (22) and theoretically considered in both mean-field approximations (25) and computer simulations (26). Lipid tilting has been considered in theoretical detail and successfully applied to lipid phase transitions (11, 12). Compression of monolayer thickness has been extensively measured, and the theory describes it well (13).
The energy surface for the fusion reaction is multidimensional, and thus if fusion does not proceed through one of our proposed intermediates, it is because a pathway even energetically more favorable exists. We have shown that our model is energetically realistic, and therefore a pore should form at a discrete site. We have delineated reconfigurations of lipids that would allow the initial stalk to expand and a fusion pore to form.
Acknowledgments
We thank Michael Kozlov for useful suggestions and providing a preprint of his work on lipid tilting, Grigory Melikyan for fruitful discussions, Adrian Parsegian and Vadim Frolov for a critical reading of the manuscript, and Sergey Akimov for assistance with the numerical calculations. This work was supported in part by Fogarty International Research Collaboration Award R03 TW00715, National Institutes of Health Grant GM 27367, and the Russian Foundation for Basic Research (Grants nos. 99-04-48426, 99-04-48427, and 00-15-97849).
References
- 1.Chernomordik L V, Melikyan G B, Chizmadzhev Yu A. Biochem Biophys Acta. 1987;906:309–352. doi: 10.1016/0304-4157(87)90016-5. [DOI] [PubMed] [Google Scholar]
- 2.Frolov V A, Cho M-S, Bronk P, Reese T S, Zimmerberg J. Traffic. 2000;1:622–630. doi: 10.1034/j.1600-0854.2000.010806.x. [DOI] [PubMed] [Google Scholar]
- 3.Gingell D, Ginsberg L. In: Membrane Fusion. Poste G, Nicholson G L, editors. Amsterdam: Elsevier; 1978. pp. 791–833. [Google Scholar]
- 4.Kozlov M M, Markin V S. Biofizika. 1983;28:255–261. [PubMed] [Google Scholar]
- 5.Markin V S, Kozlov M M, Borovjagin V L. Gen Physiol Biophys. 1984;5:361–377. [PubMed] [Google Scholar]
- 6.Siegel D P. Biophys J. 1993;65:2124–2140. doi: 10.1016/S0006-3495(93)81256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandler D E, Heuser J E. J Cell Biol. 1980;86:666–674. doi: 10.1083/jcb.86.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ornberg R L, Reese T S. J Cell Biol. 1981;90:40–50. doi: 10.1083/jcb.90.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markosyan R M, Melikyan G B, Cohen F S. Biophys J. 1999;77:943–952. doi: 10.1016/S0006-3495(99)76945-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozlov M M, Chernomordik L V. Biophys J. 1988;75:1384–1396. doi: 10.1016/S0006-3495(98)74056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamm M, Kozlov M M. Eur Phys J B. 1998;6:519–528. [Google Scholar]
- 12.Hamm M, Kozlov M M. Eur Phys J E. 2000;3:323–335. [Google Scholar]
- 13.Rawicz W, Olbrich K C, McIntosh T, Needham D, Evans E. Biophys J. 2000;79:328–339. doi: 10.1016/S0006-3495(00)76295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leikin S L, Kozlov M M, Chernomordik L V, Markin V S, Chizmadzhev Yu A. J Theor Biol. 1987;129:411–425. doi: 10.1016/s0022-5193(87)80021-8. [DOI] [PubMed] [Google Scholar]
- 15.Hui S W, Stewart T P, Boni L T, Yeagle P L. Science. 1981;212:921–923. doi: 10.1126/science.7233185. [DOI] [PubMed] [Google Scholar]
- 16.Rand R P, Parsegian V A. Biochim Biophys Acta. 1989;988:351–376. [Google Scholar]
- 17.Parsegian V A, Rand R P. In: Handbook of Biological Physics. Lipowsky R, Sackmann E, editors. Vol. 1. Amsterdam: Elsevier; 1995. pp. 643–690. [Google Scholar]
- 18.Israelachvili J N, Pashley R M. J Coloid Interface Sci. 1984;98:500–504. [Google Scholar]
- 19.Rand R P, Fuller N L. Biophys J. 1994;66:2127–2138. doi: 10.1016/S0006-3495(94)81008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leikin S L, Kozlov M M, Fuller N L, Rand R P. Biophys J. 1996;71:2623–2632. doi: 10.1016/S0006-3495(96)79454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaser R W, Leikin S L, Chernomordik L V, Pastushenko V F, Sokirko A I. Biochim Biophys Acta. 1988;940:275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- 22.Pfeiffer W, Henkel Th, Sackmann E, Knoll W, Richter D. Europhys Lett. 1989;8:201–206. [Google Scholar]
- 23.Gruner S M, Parsegian V A, Rand R P. Faraday Discuss Chem Soc. 1986;81:29–37. doi: 10.1039/dc9868100029. [DOI] [PubMed] [Google Scholar]
- 24.Helfrich W. Z Naturforsch. 1973;28c:693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 25.Leermakers F A M, Scheutjens J M H M. J Chem Phys. 1988;89:6912–6924. [Google Scholar]
- 26.Feller S E, Pastor R W. J Chem Phys. 1999;111:1281–1287. [Google Scholar]