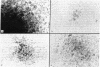
Abstract
Mouse marrow and spleen cells formed colonies consisting of 40-1,000 blast cells after 16 days of incubation in methylcellulose culture in the presence of medium conditioned by pokeweed mitogen-stimulated mouse spleen cells. These colonies could be distinguished from other hemopoietic colonies in situ by the complete absence of signs of terminal differentiation. Replating of these colonies (tentatively named stem cell colonies) revealed their self-renewal capacity and the extensive ability to generate secondary colonies, many of which were multipotential hemopoietic colonies. Some of the colonies revealed 100% replating efficiencies. Analyses of individual stem cells colonies revealed concurrent and high incidences of spleen colony-forming units and the macroscopic granulocyte-erythrocyte-macrophage-megakaryocyte colony-forming units (CFU-GEMM) in culture. Replating comparison between the stem cell colonies and GEMM colonies strongly indicated that the progenitors for the stem cell colonies are higher in the hierarchy of stem cell differentiation than are CFU-GEMM. Quantitation of stem cell colonies provides an assay for the class of primitive hemopoietic progenitors described here.
Full text
PDF
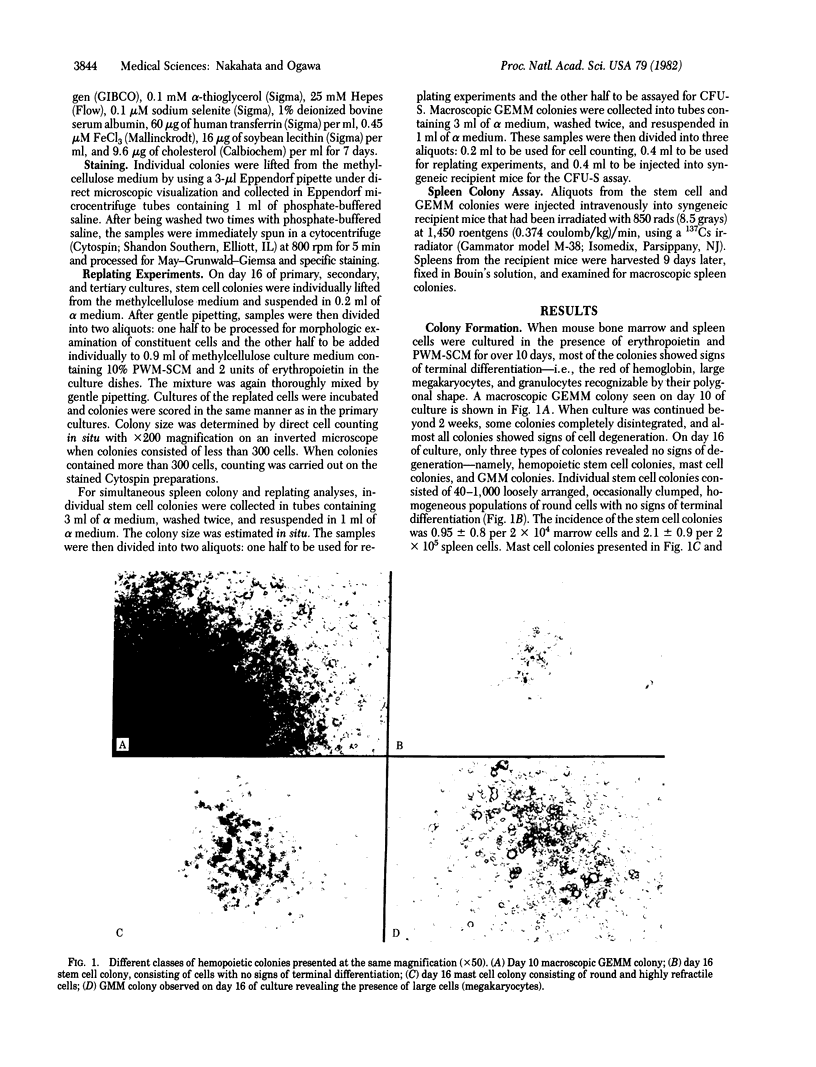
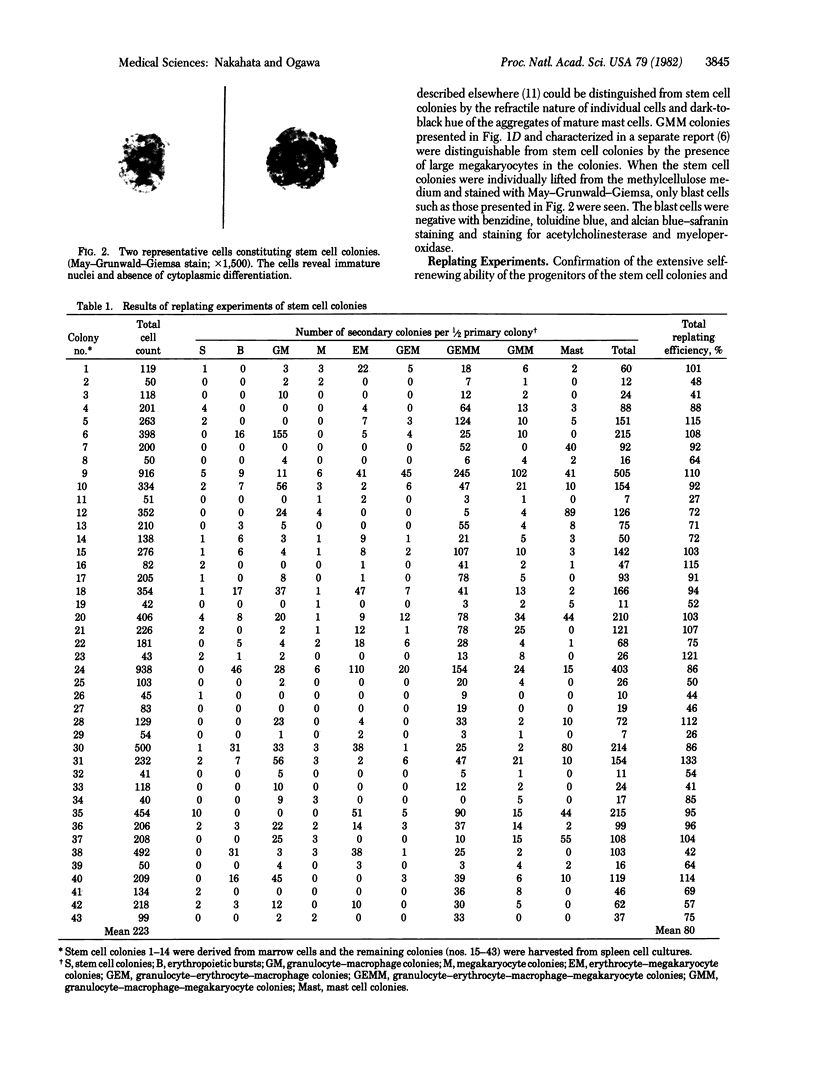
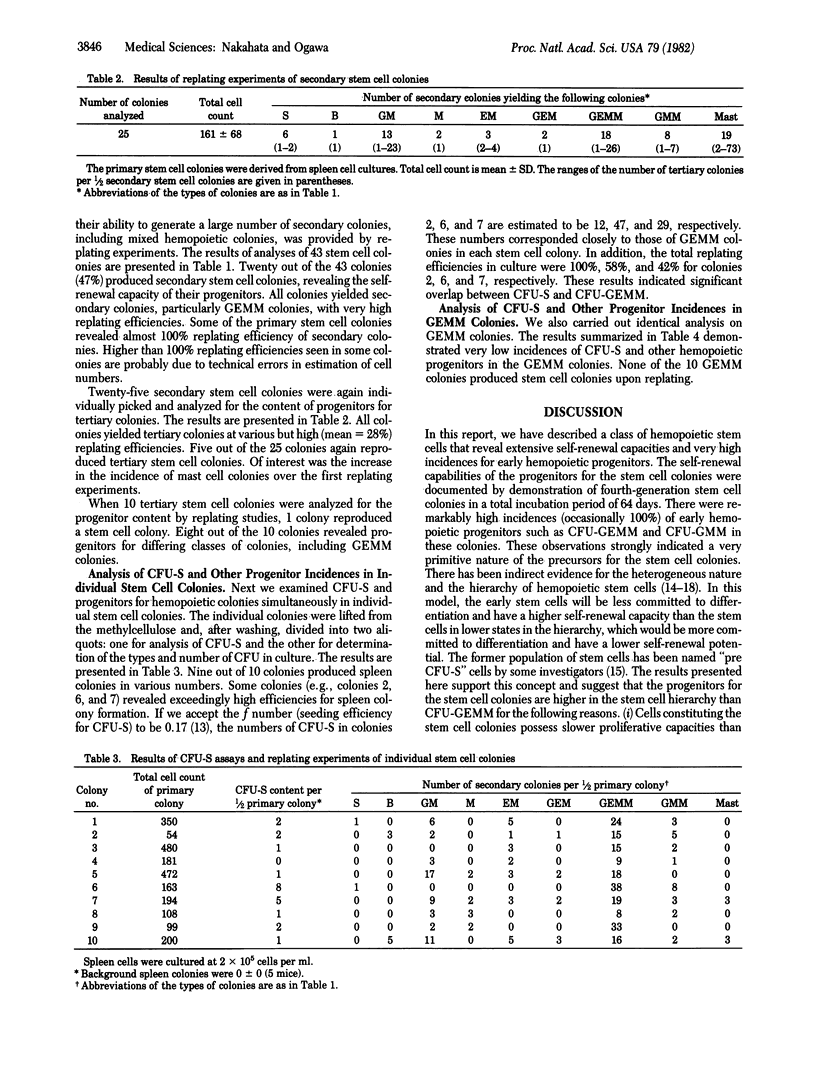

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botnick L. E., Hannon E. C., Hellman S. Nature of the hemopoietic stem cell compartment and its proliferative potential. Blood Cells. 1979 Jun 15;5(2):195–210. [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Proliferative state of human pluripotent hemopoietic progenitors (CFU-GEMM) in normal individuals and under regenerative conditions after bone marrow transplantation. Blood. 1979 Nov;54(5):1197–1200. [PubMed] [Google Scholar]
- Guilbert L. J., Iscove N. N. Partial replacement of serum by selenite, transferrin, albumin and lecithin in haemopoietic cell cultures. Nature. 1976 Oct 14;263(5578):594–595. doi: 10.1038/263594a0. [DOI] [PubMed] [Google Scholar]
- Hara H., Ogawa M. Murine hemopoietic colonies in culture containing normoblasts, macrophages, and megakaryocytes. Am J Hematol. 1978;4(1):23–34. doi: 10.1002/ajh.2830040105. [DOI] [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979 Oct 4;281(5730):381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- Humphries R. K., Eaves A. C., Eaves C. J. Self-renewal of hemopoietic stem cells during mixed colony formation in vitro. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3629–3633. doi: 10.1073/pnas.78.6.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa Y., Pluznik D. H., Sachs L. In vitro control of the development of macrophage and granulocyte colonies. Proc Natl Acad Sci U S A. 1966 Aug;56(2):488–495. doi: 10.1073/pnas.56.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F. Erythroid progenitors in mouse bone marrow detected by macroscopic colony formation in culture. Exp Hematol. 1975 Jan;3(1):32–43. [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Pure and mixed erythroid colony formation in vitro stimulated by spleen conditioned medium with no detectable erythropoietin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3879–3882. doi: 10.1073/pnas.74.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch P., Greenberger J. S., Botnick L., Hannon E., Hellman S. Evidence for structured variation in self-renewal capacity within long-term bone marrow cultures. Proc Natl Acad Sci U S A. 1980 May;77(5):2927–2930. doi: 10.1073/pnas.77.5.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R., Mandel T. E. Colony formation in agar by multipotential hemopoietic cells. J Cell Physiol. 1979 Feb;98(2):401–420. doi: 10.1002/jcp.1040980216. [DOI] [PubMed] [Google Scholar]
- Metcalf D., MacDonald H. R., Odartchenko N., Sordat B. Growth of mouse megakaryocyte colonies in vitro. Proc Natl Acad Sci U S A. 1975 May;72(5):1744–1748. doi: 10.1073/pnas.72.5.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., von Melchner H., Burgess A. W. Isolation of murine fetal hemopoietic progenitor cells and selective fractionation of various erythroid precursors. Blood. 1981 Aug;58(2):376–386. [PubMed] [Google Scholar]
- SIMINOVITCH L., MCCULLOCH E. A., TILL J. E. THE DISTRIBUTION OF COLONY-FORMING CELLS AMONG SPLEEN COLONIES. J Cell Physiol. 1963 Dec;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- Schofield R., Lord B. I., Kyffin S., Gilbert C. W. Self-maintenance capacity of CFU-S. J Cell Physiol. 1980 May;103(2):355–362. doi: 10.1002/jcp.1041030221. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]