Abstract
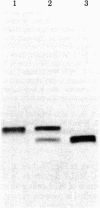
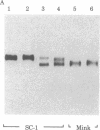
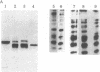
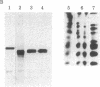
Gross passage A murine leukemia virus (MuLV) derived from extracts of C3Hf/Bi mouse leukemias has been shown to be a virus complex consisting of ecotropic, xenotropic, and recombinant, dualtropic MuLV components. The three virus components were distinguished biochemically by differences in the molecular weights and peptide maps of their primary env gene products synthesized in infected cells in vivo and in vitro. Virus expression was studied in primary leukemias induced in C3Hf/Bi mice by Gross passage A virus extracts and by the individual ecotropic and recombinant MuLV components that were isolated in vitro. Our findings suggest that expression of the recombinant MuLV component of the Gross passage A virus complex is necessary and sufficient for the induction of leukemias in C3Hf/Bi mice. In contrast, induction of leukemias by the ecotropic virus component appears to involve generation of a second virus with characteristics of recombinant, dualtropic MuLV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchhagen D. L., Pedersen F. S., Crowther R. L., Haseltine W. A. Most sequence differences between the genomes of the Akv virus and a leukemogenic Gross A virus passaged in vitro are located near the 3' terminus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4359–4363. doi: 10.1073/pnas.77.7.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhagen D. L., Pincus T., Stutman O., Fleissner E. Leukemogenic activity of murine type C viruses after long-term passage in vitro. Int J Cancer. 1976 Dec 15;18(6):835–842. doi: 10.1002/ijc.2910180616. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Gupta S., Rands E., Lowy D. R. Origin of mink cytopathic focus-forming (MCF) viruses:comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology. 1981 Sep;113(2):465–483. doi: 10.1016/0042-6822(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Verma I. M., Shih T. Y., Scolnick E. M., Davidson N. Heteroduplex analysis of the sequence relations between the RNAs of mink cell focus-inducing and murine leukemia viruses. J Virol. 1978 Oct;28(1):352–360. doi: 10.1128/jvi.28.1.352-360.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Rapp U. R., Todaro G. J., Stephenson J. R. Acquisition of oncogenicity by endogenous mouse type C viruses: effects of variations in env and gag genes. J Virol. 1978 Nov;28(2):457–465. doi: 10.1128/jvi.28.2.457-465.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulari N. G., English K. J. Env gene products of AKR dual-tropic viruses: examination of peptide maps and cell surface expression. J Virol. 1981 Dec;40(3):971–976. doi: 10.1128/jvi.40.3.971-976.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulari N. G., Jelalian K. Cell surface expression of the env gene polyprotein of dual-tropic mink cell focus-forming murine leukemia virus. J Virol. 1979 Jun;30(3):720–728. doi: 10.1128/jvi.30.3.720-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulari N. G., Tung J. S., O'Donnell P. V., Fleissner E. Murine leukemia virus env-gene expression in preleukemic thymocytes and leukemia cells of AKR strain mice. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1281–1287. doi: 10.1101/sqb.1980.044.01.140. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Dunlop N. M. Genomic masking of nondefective recombinant murine leukemia virus in Moloney virus stocks. Science. 1978 Aug 4;201(4354):457–459. doi: 10.1126/science.663667. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Frankel A. E., Elder J. H., Lerner R. A., Ihle J. N., Bolognesi D. P. Biological, immunological, and biochemical evidence that HIX virus is a recombinant between Moloney leukemia virus and a murine xenotropic C type virus. Virology. 1978 Oct 15;90(2):241–254. doi: 10.1016/0042-6822(78)90308-2. [DOI] [PubMed] [Google Scholar]
- GROSS L. Development and serial cellfree passage of a highly potent strain of mouse leukemia virus. Proc Soc Exp Biol Med. 1957 Apr;94(4):767–771. doi: 10.3181/00379727-94-23080. [DOI] [PubMed] [Google Scholar]
- Green N., Hiai H., Elder J. H., Schwartz R. S., Khiroya R. H., Thomas C. Y., Tsichlis P. N., Coffin J. M. Expression of leukemogenic recombinant viruses associated with a recessive gene in HRS/J mice. J Exp Med. 1980 Aug 1;152(2):249–264. doi: 10.1084/jem.152.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L., Dreyfuss Y. Relative loss of oncogenic potency of mouse leukemia virus (Gross) after prolonged propagation in tissue culture. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3989–3992. doi: 10.1073/pnas.75.8.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Patch V. Cell-surface antigens associated with dualtropic and thymotropic murine leukemia viruses inducing thymic and nonthymic lymphomas. J Exp Med. 1980 Jun 1;151(6):1321–1333. doi: 10.1084/jem.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Patch V. Genomic masking and rescue of dual-tropic murine leukemia viruses: role of pseudotype virions in viral lymphomagenesis. J Virol. 1980 Sep;35(3):583–591. doi: 10.1128/jvi.35.3.583-591.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K., Yanagihara K., Kamiya K., Seyama T., Yokoro K. Leukemogenicity and cell transformation mechanisms in vitro by Gross murine leukemia virus: analysis of virus subpopulations. J Virol. 1981 Apr;38(1):327–335. doi: 10.1128/jvi.38.1.327-335.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J. S-tropic murine type-C viruses: frequency of isolation from continuous cell lines, leukemia virus preparations and normal spleens. Int J Cancer. 1974 May 15;13(5):587–598. doi: 10.1002/ijc.2910130503. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Hays E. F. Oncogenicity of AKR endogenous leukemia viruses. J Virol. 1978 Jul;27(1):13–18. doi: 10.1128/jvi.27.1.13-18.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. V., Stockert E., Obata Y., Old L. J. Leukemogenic properties of AKR dualtropic (MCF) viruses: amplification of murine leukemia virus-related antigens on thymocytes and acceleration of leukemia development in AKR mice. Virology. 1981 Jul 30;112(2):548–563. doi: 10.1016/0042-6822(81)90301-9. [DOI] [PubMed] [Google Scholar]
- Oliff A., Ruscetti S., Douglass E. C., Scolnick E. Isolation of transplantable erythroleukemia cells from mice infected with helper-independent Friend murine leukemia virus. Blood. 1981 Aug;58(2):244–254. [PubMed] [Google Scholar]
- Pedersen F. S., Crowther R. L., Tenney D. Y., Reimold A. M., Haseltine W. A. Novel leukaemogenic retroviruses isolated from cell line derived from spontaneous AKR tumour. Nature. 1981 Jul 9;292(5819):167–170. doi: 10.1038/292167a0. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Davis L., Feild J., Oliff A. Friend murine leukemia virus-induced leukemia is associated with the formation of mink cell focus-inducing viruses and is blocked in mice expressing endogenous mink cell focus-inducing xenotropic viral envelope genes. J Exp Med. 1981 Sep 1;154(3):907–920. doi: 10.1084/jem.154.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E., O'Donnell P. V., Obata Y., Old L. J. Inhibition of AKR leukemogenesis by SMX-1, a dualtropic murine leukemia virus. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3720–3724. doi: 10.1073/pnas.77.6.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M. Properties of "mink cell focus-inducing" (MCF) virus isolated from spontaneous lymphoma lines of BALB/c mice carrying Moloney leukemia virus as an endogenous virus. Virology. 1979 Feb;93(1):226–236. doi: 10.1016/0042-6822(79)90290-3. [DOI] [PubMed] [Google Scholar]
- van Griensven L. J., Vogt M. Rauscher "mink cell focus-inducing" (MCF) virus causes erythroleukemia in mice: its isolation and properties. Virology. 1980 Mar;101(2):376–388. doi: 10.1016/0042-6822(80)90451-1. [DOI] [PubMed] [Google Scholar]