Abstract
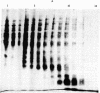
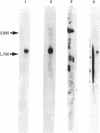
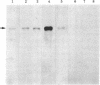
Five recombinant DNA plasmids have been constructed that contain structural gene sequences for rat tyrosine hydroxylase [TyrOHase; tyrosine 3-monooxygenase; L-tyrosine, tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2]. Rat pheochromocytoma PC 12 cell line, which contains relatively high levels of catecholamine-synthesizing enzymes, was used to purify RNA. TyrOHase cDNA clones were identified by screening 350 cDNA clones constructed from partially purified TyrOHase mRNA. A rapid and powerful screening of the recombinant clones by differential colony hybridization was possible because TyrOHase is a tissue-specific protein. The final selection relied on the ability of cDNA inserts to hybridize specifically to TyrOHase mRNA as judged by cell-free translation and immunoprecipitation. Blot hybridization analysis of polyadenylylated RNA from PC 12 cells indicated a major mRNA species of 1.9 kilobases. A species of the same size was identified from a human pheochromocytoma tumor, indicating a crossreactivity between rat TyrOHase cDNA and human TyrOHase mRNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetge E. E., Kaplan B. B., Reis D. J., Joh T. H. Translation of tyrosine hydroxylase from poly(A)-mRNA in pheochromocytoma cells is enhanced by dexamethasone. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1269–1273. doi: 10.1073/pnas.78.2.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berod A., Hartman B. K., Pujol J. F. Importance of fixation in immunohistochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem. 1981 Jul;29(7):844–850. doi: 10.1177/29.7.6167611. [DOI] [PubMed] [Google Scholar]
- Black I. B., Patterson P. H. Developmental regulation of neurotransmitter phenotype. Curr Top Dev Biol. 1980;15(Pt 1):27–40. doi: 10.1016/s0070-2153(08)60115-5. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Edgar D. H., Thoenen H. Selective enzyme induction in a nerve growth factor-responsive pheochromocytoma cell line (PC 12). Brain Res. 1978 Oct 6;154(1):186–190. doi: 10.1016/0006-8993(78)91070-3. [DOI] [PubMed] [Google Scholar]
- Gergen J. P., Stern R. H., Wensink P. C. Filter replicas and permanent collections of recombinant DNA plasmids. Nucleic Acids Res. 1979 Dec 20;7(8):2115–2136. doi: 10.1093/nar/7.8.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R., Herschman H. R. Nerve growth factor-mediated induction of tyrosine hydroxylase in a clonal pheochromocytoma cell line. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4587–4590. doi: 10.1073/pnas.75.9.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh T. H., Geghman C., Reis D. Immunochemical demonstration of increased accumulation of tyrosine hydroxylase protein in sympathetic ganglia and adrenal medulla elicited by reserpine. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2767–2771. doi: 10.1073/pnas.70.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh T. H., Park D. H., Reis D. J. Direct phosphorylation of brain tyrosine hydroxylase by cyclic AMP-dependent protein kinase: mechanism of enzyme activation. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4744–4748. doi: 10.1073/pnas.75.10.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M. The ontogeny of the neural crest in avian embryo chimaeras. Nature. 1980 Aug 14;286(5774):663–669. doi: 10.1038/286663a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Aloe L., Mugnaini E., Oesch F., Thoenen H. Nerve growth factor induces volume increase and enhances tyrosine hydroxylase synthesis in chemically axotomized sympathetic ganglia of newborn rats. Proc Natl Acad Sci U S A. 1975 Feb;72(2):595–599. doi: 10.1073/pnas.72.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomedico P. T., Saunders G. F. Preparation of pancreatic mRNA: cell-free translation of an insulin-immunoreactive polypeptide. Nucleic Acids Res. 1976 Feb;3(2):381–391. doi: 10.1093/nar/3.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet J., Privat A., Samolyk D., Bérod A., Keller A., Pujol J. F. Synthèse in vitro de la tyrosine hydroxylase. C R Seances Acad Sci III. 1981 Feb 9;292(6):455–460. [PubMed] [Google Scholar]
- Markey K. A., Kondo H., Shenkman L., Goldstein M. Purification and characterization of tyrosine hydroxylase from a clonal pheochromocytoma cell line. Mol Pharmacol. 1980 Jan;17(1):79–85. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T., Brutlag D. Addition of homopolymers to the 3'-ends of duplex DNA with terminal transferase. Methods Enzymol. 1979;68:41–50. doi: 10.1016/0076-6879(79)68005-9. [DOI] [PubMed] [Google Scholar]
- Otten U., Thoenen H. Effect of glucocorticoids on nerve growth factor-mediated enzyme induction in organ cultures of rat sympathetic ganglia: enchanced response and reduced time requirement to initiate enzyme induction. J Neurochem. 1977 Jul;29(1):69–75. doi: 10.1111/j.1471-4159.1977.tb03925.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Molecular cloning of a human histocompatibility antigen cDNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6081–6085. doi: 10.1073/pnas.77.10.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts T. M., Lauer G. D. Maximizing gene expression on a plasmid using recombination in vitro. Methods Enzymol. 1979;68:473–482. doi: 10.1016/0076-6879(79)68036-9. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Trans-synaptic enzyme induction. Life Sci. 1974 Jan 16;14(2):223–235. doi: 10.1016/0024-3205(74)90052-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef M., Berod A., Sotelo C. Dopaminergic dendrites in the pars reticulata of the rat substantia nigra and their striatal input. Combined immunocytochemical localization of tyrosine hydroxylase and anterograde degeneration. Neuroscience. 1981;6(11):2125–2139. doi: 10.1016/0306-4522(81)90003-8. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]