Abstract
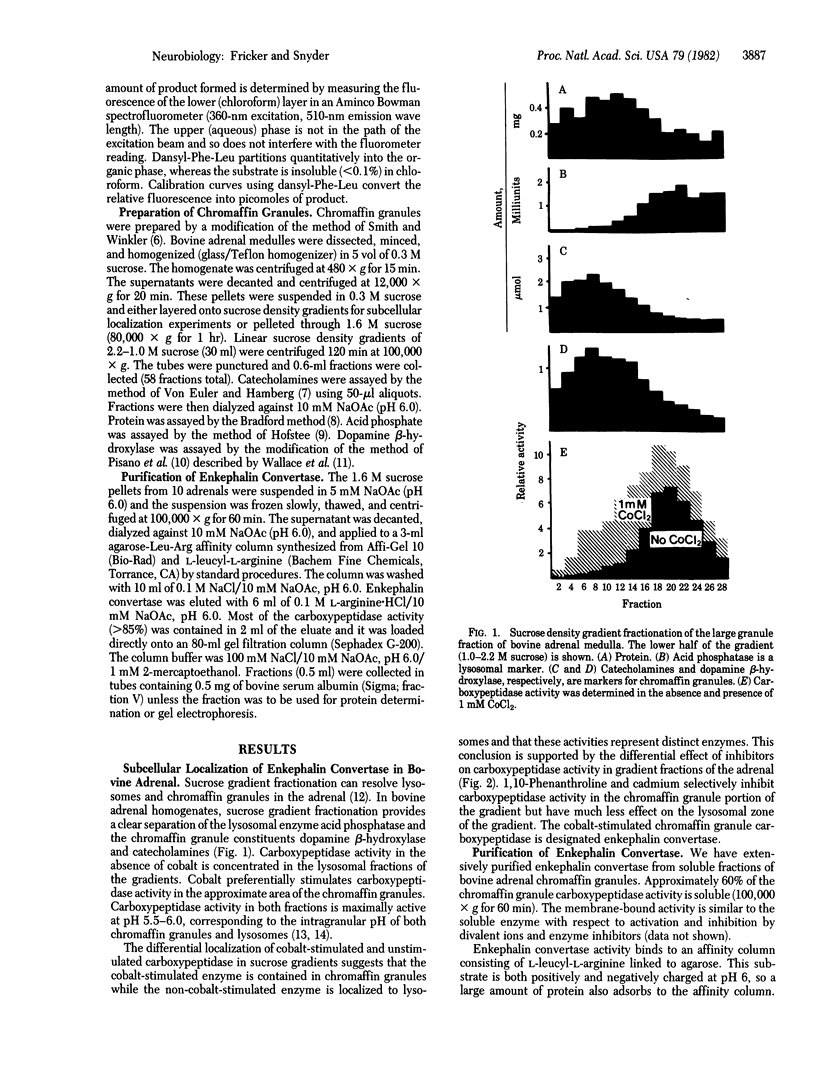
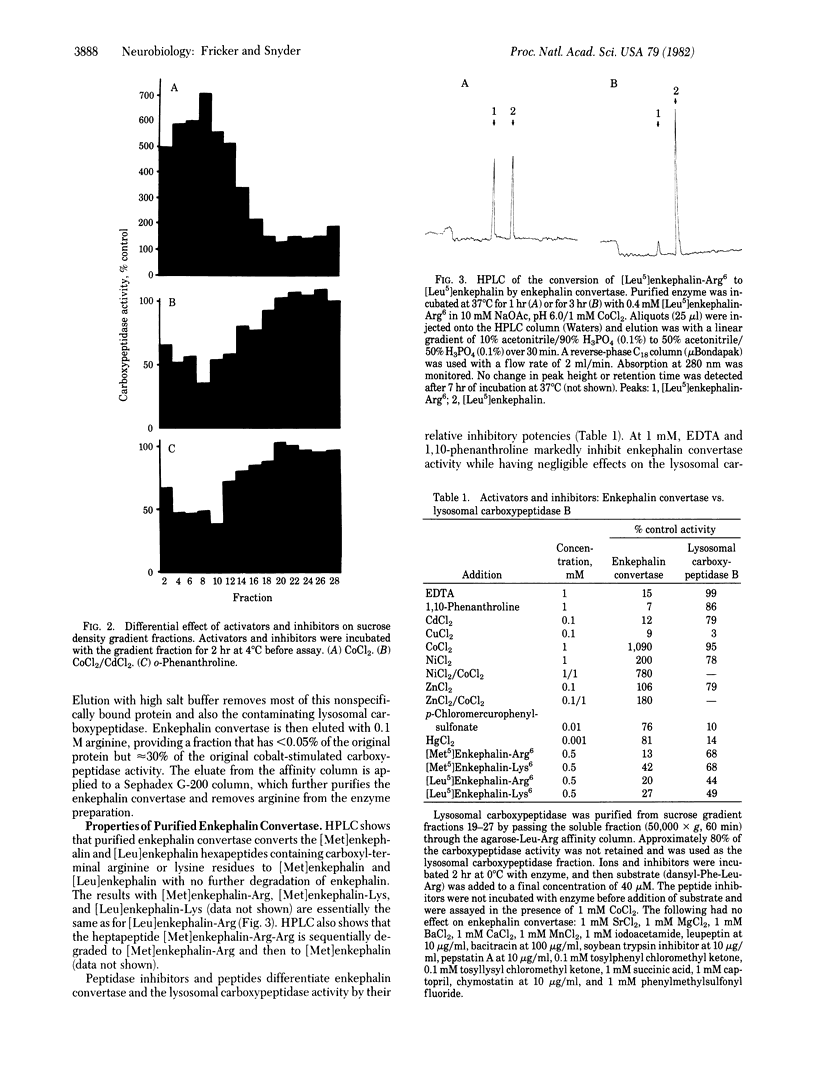
A specific carboxypeptidase that converts enkephalin precursors into enkephalin in adrenal chromaffin granules has been purified and characterized. In the adrenal this enzyme, designated enkephalin convertase, is uniquely localized to the chromaffin granules, which contain enkephalin and precursor peptides. Enkephalin convertase is markedly stimulated by CoCl2 and inhibited by EDTA or 1,10-phenanthroline, unlike the lysosomal carboxypeptidase. The purified enzyme has a high affinity for the hexapeptides [Met5]- and [Leu5]enkephalin-Arg6 (51 and 83 microM, respectively) and a somewhat lower affinity for the hexapeptides [Met5]- and [Leu5]enkephalin-Lys6 (195 and 174 microM). Brain enkephalin convertase shows 10-fold regional variations, unlike other carboxypeptidases, which are uniformly distributed. Enkephalin convertase appears to be associated selectively and physiologically with biosynthesis of the enkephalins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett S. F., Smith A. D. Adrenal chromaffin granules: isolation and disassembly. Methods Enzymol. 1974;31:379–389. doi: 10.1016/0076-6879(74)31042-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Gubler U., Seeburg P., Hoffman B. J., Gage L. P., Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982 Jan 21;295(5846):206–208. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. Direct and continuous spectrophotometric assay of phosphomonoesterases. Arch Biochem Biophys. 1954 Jul;51(1):139–146. doi: 10.1016/0003-9861(54)90461-0. [DOI] [PubMed] [Google Scholar]
- Hook V. Y., Eiden L. E., Brownstein M. J. A carboxypeptidase processing enzyme for enkephalin precursors. Nature. 1982 Jan 28;295(5847):341–342. doi: 10.1038/295341a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Internal pH of isolated chromaffin vesicles. J Biol Chem. 1976 Apr 10;251(7):2189–2191. [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Hirose T., Inayama S., Nakanishi S., Numa S. Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature. 1982 Jan 21;295(5846):202–206. doi: 10.1038/295202a0. [DOI] [PubMed] [Google Scholar]
- Riejngoud D. J., Tager J. M. Measurement of intralysosomal pH. Biochim Biophys Acta. 1973 Jan 24;297(1):174–178. doi: 10.1016/0304-4165(73)90061-5. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. The localization of lysosomal enzymes in chromaffin tissue. J Physiol. 1966 Mar;183(1):179–188. doi: 10.1113/jphysiol.1966.sp007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. S., Lewis R. V., Kimura S., Rossier J., Stein S., Udenfriend S. Opioid hexapeptides and heptapeptides in adrenal medulla and brain possible implications on the biosynthesis of enkephalins. Arch Biochem Biophys. 1980 Dec;205(2):606–613. doi: 10.1016/0003-9861(80)90144-7. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Diliberto E. J., Jr, Hazum E., Chang K. J. Opiate-like materials in the adrenal medulla: evidence for storage and secretion with catecholamines. Mol Pharmacol. 1979 Nov;16(3):1101–1108. [PubMed] [Google Scholar]
- Wallace E. F., Krantz M. J., Lovenberg W. Dopamine-beta-hydroxylase: a tetrameric glycoprotein. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2253–2255. doi: 10.1073/pnas.70.8.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience. 1976;1(2):65–80. doi: 10.1016/0306-4522(76)90001-4. [DOI] [PubMed] [Google Scholar]



