Abstract
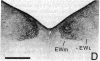
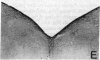
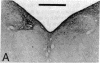
Retrograde and anterograde pathway tracing techniques were used in the pigeon to study afferent visual input from the suprachiasmatic nucleus (SCN) of the hypothalamus to the nucleus of Edinger-Westphal (EW), the parasympathetic visceral efferent component of the oculomotor complex. Horseradish peroxidase injected into the EW retrogradely labeled numerous neurons in the contralateral SCN, a retinorecipient hypothalamic nucleus, as well as a few neurons in the ipsilateral SCN. Autoradiographic orthograde pathway tracing experiments confirmed that the SCN projects heavily upon the medial subdivision of the contralateral EW and lightly upon the medial subdivision of the ipsilateral EW. Some neurons of the SCN contain substance P and a substance P-positive plexus of fibers was seen in precisely that medial portion of the EW to which the SCN was found to project in the autoradiographic experiments. This substance P-positive fiber plexus in the medial EW was eliminated by bilateral electrolytic lesions of the SCN. These results show that the avian suprachiasmatic nucleus has a heavy contralateral and a much lighter ipsilateral projection to the medial subdivision of the EW and that this projection may be largely substance P positive. Previous studies have suggested that neurons of the medial EW specifically project to the neurons of the ciliary ganglion that control the choriocapillary blood flow of the eye. Since the SCN has been implicated in the control of circadian rhythms in vertebrates, the projection of the SCN to the EW may represent a pathway by which a circadian rhythmicity is imposed on choriocapillary blood flow. Alternatively or in addition, the SCN-EW pathway described in this paper may provide the central neural substrate for a homeostatic regulatory mechanism by which choriocapillary blood flow is controlled by the intensity of retinal illumination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam E. A., Banas J., Criley J. M., Loeb H. S., Mueller H., Willerson J. T., Mason D. T. Clinical assessment of external pressure circulatory assistance in acute myocardial infarction. Report of a cooperative clinical trial. Am J Cardiol. 1980 Feb;45(2):349–356. doi: 10.1016/0002-9149(80)90658-x. [DOI] [PubMed] [Google Scholar]
- Benevento L. A., Rezak M., Santos-Anderson An autoradiographic study of the projections of the pretectum in the rhesus monkey (Macaca mulatta): evidence for sensorimotor links to the thalamus and oculomotor nuclei. Brain Res. 1977 May 27;127(2):197–218. doi: 10.1016/0006-8993(77)90536-4. [DOI] [PubMed] [Google Scholar]
- Berk M. L., Finkelstein J. A. An autoradiographic determination of the efferent projections of the suprachiasmatic nucleus of the hypothalamus. Brain Res. 1981 Dec 7;226(1-2):1–13. doi: 10.1016/0006-8993(81)91079-9. [DOI] [PubMed] [Google Scholar]
- Cowan W. M., Gottlieb D. I., Hendrickson A. E., Price J. L., Woolsey T. A. The autoradiographic demonstration of axonal connections in the central nervous system. Brain Res. 1972 Feb 11;37(1):21–51. doi: 10.1016/0006-8993(72)90344-7. [DOI] [PubMed] [Google Scholar]
- Cuello A. C., Galfre G., Milstein C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3532–3536. doi: 10.1073/pnas.76.7.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbesson S. O. On the organization of central visual pathways in vertebrates. Brain Behav Evol. 1970;3(1):178–194. doi: 10.1159/000125470. [DOI] [PubMed] [Google Scholar]
- Erichsen J. T., Reiner A., Karten H. J. Co-occurrence of substance P-like and Leu-enkephalin-like immunoreactivities in neurones and fibres of avian nervous system. Nature. 1982 Feb 4;295(5848):407–410. doi: 10.1038/295407a0. [DOI] [PubMed] [Google Scholar]
- GAY A. J., GOLDOR H., SMITH M. CHORIORETINAL VASCULAR OCCLUSIONS WITH LATEX SPHERES. Invest Ophthalmol. 1964 Dec;3:647–656. [PubMed] [Google Scholar]
- Goldor H., Gay A. J. Chorioretinal vascular occlusions with latex microspheres (a long-term study). II. Invest Ophthalmol. 1967 Feb;6(1):51–58. [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Hartwig H. G. Electron microscopic evidence for a retinohypothalamic projection to the suprachiasmatic nucleus of Passer domesticus. Cell Tissue Res. 1974;153(1):89–99. doi: 10.1007/BF00225448. [DOI] [PubMed] [Google Scholar]
- Hess A. Developmental changes in the structure of the synapse on the myelinated cell bodies of the chicken ciliary ganglion. J Cell Biol. 1965 Jun;25(3 Suppl):1–19. doi: 10.1083/jcb.25.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A., Pilar G., Weakly J. N. Correlation between transmission and structure in avian ciliary ganglion synapses. J Physiol. 1969 Jun;202(2):339–354. doi: 10.1113/jphysiol.1969.sp008815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten H. J., Brecha N. Localisation of substance P immunoreactivity in amacrine cells of the retina. Nature. 1980 Jan 3;283(5742):87–88. doi: 10.1038/283087a0. [DOI] [PubMed] [Google Scholar]
- Korte G. E., Reiner A., Karten H. J. Substance P-like immunoreactivity in cerebellar mossy fibers and terminals in the red-eared turtle, Chrysemys scripta elegans. Neuroscience. 1980;5(5):903–914. doi: 10.1016/0306-4522(80)90159-1. [DOI] [PubMed] [Google Scholar]
- Kucera P., Favrod P. Suprachiasmatic nucleus projection to mesencephalic central grey in the woodmouse (Apodemus sylvaticus L.). Neuroscience. 1979;4(11):1705–1716. doi: 10.1016/0306-4522(79)90029-0. [DOI] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Selective reinnervation of two cell populations in the adult pigeon ciliary ganglion. J Physiol. 1970 Nov;211(1):203–216. doi: 10.1113/jphysiol.1970.sp009275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanum J. The damaging effects of light on the retina. Empirical findings, theoretical and practical implications. Surv Ophthalmol. 1978 Jan-Feb;22(4):221–249. doi: 10.1016/0039-6257(78)90070-x. [DOI] [PubMed] [Google Scholar]
- Ljungdahl A., Hökfelt T., Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat--I. Cell bodies and nerve terminals. Neuroscience. 1978;3(10):861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- Lorén I., Emson P. C., Fahrenkrug J., Björklund A., Alumets J., Håkanson R., Sundler F. Distribution of vasoactive intestinal polypeptide in the rat and mouse brain. Neuroscience. 1979;4(12):1953–1976. doi: 10.1016/0306-4522(79)90068-x. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwitt R., Pilar G., Weakly J. N. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 1971 Jan 22;25(2):317–334. doi: 10.1016/0006-8993(71)90441-0. [DOI] [PubMed] [Google Scholar]
- Moore R. Y. Retinohypothalamic projection in mammals: a comparative study. Brain Res. 1973 Jan 30;49(2):403–409. doi: 10.1016/0006-8993(73)90431-9. [DOI] [PubMed] [Google Scholar]
- Narayanan C. H., Narayanan Y. An experimental inquiry into the central source of preganglionic fibers to the chick ciliary ganglion. J Comp Neurol. 1976 Mar 1;166(1):101–109. doi: 10.1002/cne.901660107. [DOI] [PubMed] [Google Scholar]
- Nishino H., Koizumi K. Responses of neurons in the suprachiasmatic nuclei of the hypothalamus to putative transmitters. Brain Res. 1977 Jan 14;120(1):167–172. doi: 10.1016/0006-8993(77)90509-1. [DOI] [PubMed] [Google Scholar]
- Parver L. M., Auker C., Carpenter D. O. Choroidal blood flow as a heat dissipating mechanism in the macula. Am J Ophthalmol. 1980 May;89(5):641–646. doi: 10.1016/0002-9394(80)90280-9. [DOI] [PubMed] [Google Scholar]
- Reiner A., Gamlin P. On noncarcinogenic chromogens for horseradish peroxidase histochemistry. J Histochem Cytochem. 1980 Feb;28(2):187–191. doi: 10.1177/28.2.7354215. [DOI] [PubMed] [Google Scholar]
- Repérant J. Nouvelles donnèes sur less projections visuelles chez le pigeon (Columba livia. J Hirnforsch. 1973;14(1):151–187. [PubMed] [Google Scholar]
- Rusak B. Neural mechanisms for entrainment and generation of mammalian circadian rhythms. Fed Proc. 1979 Nov;38(12):2589–2595. [PubMed] [Google Scholar]
- Ruskell G. L. Facial parasympathetic innervation of the choroidal blood vessels in monkeys. Exp Eye Res. 1971 Sep;12(2):166–172. doi: 10.1016/0014-4835(71)90085-6. [DOI] [PubMed] [Google Scholar]
- Steiger H. J., Büttner-Ennever J. A. Oculomotor nucleus afferents in the monkey demonstrated with horseradish peroxidase. Brain Res. 1979 Jan 5;160(1):1–15. doi: 10.1016/0006-8993(79)90596-1. [DOI] [PubMed] [Google Scholar]
- Stephan F. K., Berkley K. J., Moss R. L. Efferent connections of the rat suprachiasmatic nucleus. Neuroscience. 1981;6(12):2625–2641. doi: 10.1016/0306-4522(81)90108-1. [DOI] [PubMed] [Google Scholar]
- Stjernschantz J., Alm A., Bill A. Effects of intracranial oculomotor nerve stimulation on ocular blood flow in rabbits: modification by indomethacin. Exp Eye Res. 1976 Oct;23(4):461–469. doi: 10.1016/0014-4835(76)90175-5. [DOI] [PubMed] [Google Scholar]
- Streit P., Stella M., Cuénod M. Transneuronal labeling in the pigeon visual system. Neuroscience. 1980;5(4):763–777. doi: 10.1016/0306-4522(80)90169-4. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Cowan W. M. The efferent connections of the suprachiasmatic nucleus of the hypothalamus. J Comp Neurol. 1975 Mar 1;160(1):1–12. doi: 10.1002/cne.901600102. [DOI] [PubMed] [Google Scholar]
- Takahashi J. S., Menaker M. Physiology of avian circadian pacemakers. Fed Proc. 1979 Nov;38(12):2583–2588. [PubMed] [Google Scholar]
- Turek F. W. Are the suprachiasmatic nuclei the location of the biological clock in mammals? Nature. 1981 Jul 23;292(5821):289–290. doi: 10.1038/292289a0. [DOI] [PubMed] [Google Scholar]
- Weber J. T., Harting J. K. The efferent projections of the pretectal complex: an autoradiographic and horseradish peroxidase analysis. Brain Res. 1980 Jul 21;194(1):1–28. doi: 10.1016/0006-8993(80)91315-3. [DOI] [PubMed] [Google Scholar]