Abstract
Impulse control disorders such as pathological gambling (PG) are a serious and common adverse effect of dopamine (DA) replacement medication in Parkinson’s disease (PD). Patients with PG have increased impulsivity and abnormalities in striatal DA, in common with behavioural and substance addictions in the non-PD population. To date, no studies have investigated the role of extrastriatal dopaminergic abnormalities in PD patients with PG. We used the PET radiotracer, [11C] FLB-457, with high-affinity for extrastriatal DA D2/3 receptors. 14 PD patients on DA agonists were imaged while they performed a gambling task involving real monetary reward and a control task. Trait impulsivity was measured with the Barratt Impulsivity Scale (BIS). Seven of the patients had a history of PG that developed subsequent to DA agonist medication. Change in [11C] FLB-457 binding potential (BP) during gambling was reduced in PD with PG patients in the midbrain, where D2/D3 receptors are dominated by autoreceptors. The degree of change in [11C] FLB-457 binding in this region correlated with impulsivity. In the cortex, [11C] FLB-457 BP was significantly greater in the anterior cingulate cortex (ACC) in PD patients with PG during the control task, and binding in this region was also correlated with impulsivity. Our findings provide the first evidence that PD patients with PG have dysfunctional activation of DA autoreceptors in the midbrain and low DA tone in the ACC. Thus, altered striatal and cortical DA homeostasis may incur vulnerability for the development of PG in PD, linked with the impulsive personality trait.
Keywords: Parkinson’s disease, Dopamine agonists, Pathological gambling, Impulsivity
Introduction
Symptomatic relief of motor symptoms in Parkinson’s disease (PD) is achieved by increasing endogenous dopamine (DA) levels using levodopa, or by synthetic activation of DA receptors using DA agonists. Dopamine agonists in particular may contribute to the development of impulse control disorders (ICDs) in about 13% of PD patients (Voon et al., 2006; Weintraub et al., 2010). These include pathological gambling (PG), hypersexuality, compulsive shopping and compulsive eating, and are considered as behavioural addictions with epidemiology and phenomenology similar to substance addiction (Potenza, 2008; Ray and Strafella, 2010; Voon et al., 2011a,c). Neurobiological commonalities in DA and 5HT neurotransmitter systems have also been noted (Leeman and Potenza, 2012; Zack and Poulos, 2009).
Proposed mechanisms for the development of an ICD in PD include excessive dopaminergic stimulation of the mesolimbic pathway (Cools et al., 2003), and interference with reward based learning by tonically occupying post-synaptic DA receptors (van Eimeren et al., 2009, 2010; Voon et al., 2010). The later is associated with decreased blood oxygen level dependent (BOLD) activity in the orbitofrontal (OFC) and anterior cingulate cortex (ACC) (Voon et al., 2011b). These areas are also hypoactive in substance addiction, associated with decreased striatal D2 receptor availability measured with [11C] Raclopride and PET (Goldstein and Volkow, 2011; Volkow et al., 2011, 2012).
The impulsive personality trait represents a significant risk factor for the development of an ICD in PD (Voon and Dalley, 2011). In the non-PD population, impulsive individuals have greater amphetamine-induced release of DA in the striatum (Buckholtz et al., 2010), an abnormality also seen in PD patients with PG in response to monetary reward (Steeves et al., 2009). Buckholtz et al. (2010), using the PET ligand [18F] Fallypride, also found that trait impulsivity was negatively associated with binding to DA D2/3 receptors in the midbrain. DA receptors in this region are dominated by autoreceptors (Khan et al., 1998), which function to limit striatal DA release following reward. This suggests that midbrain autoreceptors influence individuals’ propensity for impulsivity, and opens the possibility that excessive striatal DA release following gambling rewards in PD patients with PG, shown in Steeves et al. (2009), stems from reduced control over striatal DA by midbrain autoreceptors.
DA function in the cortex may also contribute to the impulsive personality trait. Genetic studies suggest that polymorphisms of the catechol-O-methyltransferase (COMT) gene, associated with increased rate of synaptic dopamine catabolism in the prefrontal cortex (PFC), and therefore reduced basal DA levels in these areas, are more frequent in more impulsive people (Paloyelis et al., 2010). To the best of our knowledge, no studies have investigated extrastriatal abnormalities in PD patients with PG.
Here were report the results of our PET and [11C] FLB-457 study, a radiotracer with high affinity for DA D2/3 receptors, and therefore sensitivity to extrastriatal, but not striatal D2/3 receptors (Olsson et al., 1999), showing midbrain and PFC dopaminergic differences in PD patients with and without PG.
Methods
Participants and experimental design
14 PD patients meeting UK Brain Bank criteria for the diagnosis of idiopathic PD were recruited from the movement disorders clinic at the Toronto Western Hospital. Patients were screened for cognitive deficits using the Montreal Cognitive Assessment (MOCA) (Nasreddine et al., 2005). Seven of the patients had developed PG subsequent to taking dopamine agonists, confirmed by psychiatric assessment. All seven had recovered from pathological gambling after reduction of DA agonist intake, but reported some residual, controllable, gambling urges. All completed the gambling symptom assessment scale (G-SAS) (Kim et al., 2009). This scale gives a severity score during patients worst week of gambling activity (past gambling), and a severity score for current gambling activity. Patients also completed a Barratt Impulsivity Scale (Patton et al., 1995), which is a self report 30 item questionnaire of impulsivity traits. Total scores were used for further analysis, with greater scores equating to more impulsive personality. Disease severity was measured while on medication with the Unified Parkinson’s Disease Rating Scale (UPDRS). One patient in the PD with PG group had a deep brain stimulator implanted in the bilateral subthalamic nucleus. The presence of the implant prevented us from acquiring an MRI for this subject. Thus, normalisation to standard space (see below) was achieved by matching the raw PET scans to an MNI normalised [11C] FLB-457 template image created from an average of the normalised scans from the remaining patients, using SPM8. We do not expect the inclusion of this patient has greatly influenced our results since the scans were performed while DBS was switched off.
The PD with and without PG groups were matched for age, sex, disease severity disease duration and total levodopa equivalent doses (LEDD) (Tomlinson et al., 2010) (see Table 1). However it must be noted that due to heterogeneity of PD itself, and differing responses to dopamine restoration, it was not possible to match patients on every aspect of their treatment. For example, two patients with PG were no longer using DA agonists, and the remainder had DA agonists reduced, with concomitant increases in levodopa (Table 2). Thus, we considered it more important to match patients for total levodopa equivalent doses rather than either levodopa or DA agonists, since both may have an impact on DA receptor expression (Chernoloz et al., 2009; Kaasinen et al., 2000; Pitts et al., 1995; Subramaniam et al., 1992; Thobois et al., 2004).
Table 1.
Demographic characteristics of the PD patients with and without pathological gambling.
| PD without PG
|
PD with PG
|
Significance levels | |||
|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | ||
| Age | 60.57 | 9.73 | 59.71 | 10.98 | NS |
| Disease duration | 8.14 | 4.53 | 10.43 | 5.53 | NS |
| UPDRS III | 17.14 | 6.44 | 21.00 | 8.04 | NS |
| Total LEDD (mg) | 644.43 | 337.73 | 888.29 | 479.96 | NS |
| G-SAS (past) | 1.29 | 3.40 | 35.71 | 7.59 | ** |
| G-SAS (current) | 1.29 | 3.40 | 14.00 | 11.30 | * |
| BIS | 57.00 | 4.43 | 65.00 | 7.0 | * |
Legend: UPDRS=Unified Parkinson’s disease rating scale (section III), Total LEDD= total levodopa equivalent dose (calculated as L-dopa dose+pramipexole (mg)×100, ropinirole (mg)×20, amantadine×1, rasagiline×100) (Tomlinson et al., 2010). G-SAS (past/present)=Gambling symptom assessment scale (during the worst week of problem gambling in memory/at the time of filling in the assessment). NS=not significant,
indicates significance at P<0.05 and
indicates significance at P<0.01.
Table 2.
Medication list of PD patients with and without pathological gambling.
| Medications | |
|---|---|
| Patient (without PG) | |
| 1 | Pramipexole, Levodopa/Carbidopa |
| 2 | Rasagiline, Ropinirole, Levodopa/Carbidopa |
| 3 | Pramipexole, Levodopa/Carbidopa |
| 4 | Pramipexole, Levodopa/Carbidopa |
| 5 | Amantadine, Pramipexole, Levodopa/Carbidopa |
| 6 | Pramipexole |
| 7 | Selegiline, Pramipexole, Levodopa/Carbidopa |
| Patient (with PG) | |
| 8 | Levodopa/Carbidopa, Pramipexole |
| 9 | Levodopa/Carbidopa |
| 10 | Levodopa/Carbidopa, Pramipexole |
| 11 | Levodopa/Carbidopa, Amantadine |
| 12 | Levodopa/Carbidopa, Pramipexole, Amantadine |
| 13 | Levodopa/Carbidopa, Pramipexole, Amantadine |
| 14 | Levodopa/Carbidopa, Pramipexole, Rasagiline |
Behavioural tasks
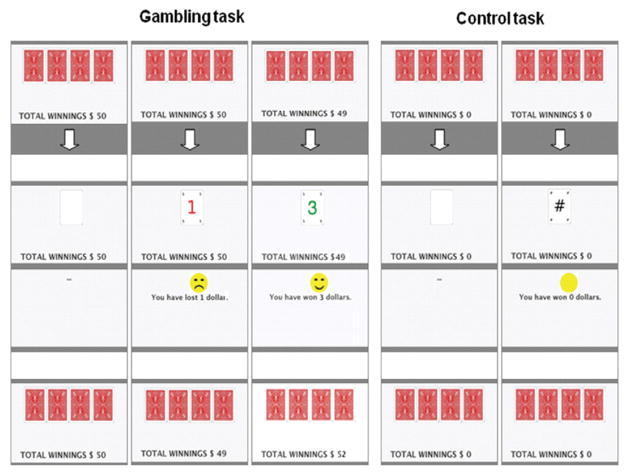
During the scans patients performed, in a counterbalanced order, a control task or a gambling task involving real monetary reward (see Fig. 1). The tasks were displayed via video eyewear (VR920; Vuzix Corporation, New York, USA). For the gambling task, subjects selected with their right hand one of four cards displayed on the video screen. On reward trials subjects were presented with a green reward amount ($1, $3 or $5 CA), a smiling face and the sound of a cash register. On penalty trials subjects were presented with a red number ($1, $3 or $5) and a frowning face. A running total of the subjects’ current earnings was constantly displayed, starting at $30. The card sequence was determined by a computerised random sequence generator providing a ratio of 3:1 reward vs penalty, ensuring final winnings of $70. Subjects were not aware that their choices did not influence winnings, which they were aware they would keep. This simplified version of a gambling situation ensured that differences between gamblers and controls in terms of cognitive involvement and skill were minimised. Both tasks included blocks of 150 trials each with 2 min of rest in between. In the control task, subjects selected cards as above but received neither a reward nor penalty. Cards revealed green and red nonsense symbols, and a green symbol was accompanied by a clicking sound. Thus the tasks were matched for visual, auditory and motor aspects. Both tasks were started 5 min before the injection of the radiotracer and continued until end of the scan.
Fig. 1.
The gambling task is shown on the left and the control/baseline task on the right.
Scanning procedures
Patients withheld antiparkinsonian medications for 12 hrs prior to the PET scans, and were given 1 mg of pramipexole 1 hr prior to the scan. We reasoned that differences between patients with and without PG might be elevated when on pramipexole, since a single dose of 1 mg pramipexole has previously been shown to alter behaviour even in drug naïve individuals (Campbell-Meiklejohn et al., 2011). PET images were acquired using [11C] FLB-457, a high affinity marker used for assaying extrastriatal D2/D3 receptors (Farde et al., 1997), and which has been shown to be sensitive to endogenous DA levels in the PFC and midbrain (Montgomery et al., 2007; Narendran et al., 2009; Vandehey et al., 2010). PET scans were obtained with a high resolution PET CT, Siemens-Biograph HiRez XVI (Siemens Molecular Imaging, Knoxville, TN, U.S.A.) operating in 3D mode with an in-plane resolution of approximately 4.6 mm full width at half-maximum. Head movements were restricted using a custom-made thermoplastic facemask, used for both scans, and a head fixation system (Tru-Scan Imaging, Annapolis). Before each emission scan, following the acquisition of a scout view for accurate positioning of the subject, a low dose (0.2 mSv) CT scan was acquired and used for attenuation correction. [11C] FLB-457 was injected into the left antecubital vein over 60s and emission data were then acquired over a period of 90 min in fifteen 1-min frames and fifteen 5-min frames. The injected mass was not different between conditions (t=0.7, P=0.47), or groups (t= 0.12, P=0.38).
High-resolution MRIs (GE Signa 1.5 T, a T1-weighted image and a proton density weighted image, 1 mm slice thickness) of each subject’s brain were acquired and transformed into standardised stereotaxic space using nonlinear automated feature-matching to the MNI template (Collins et al., 1994).
PET frames were summed, registered to the corresponding MRI (Woods et al., 1993) and transformed into standardised stereotaxic space using the transformation parameters of the individual structural MRIs (Collins et al., 1994). Voxelwise [11C] FLB-457 binding potential (BP) was calculated using a simplified reference tissue (cerebellum) method (Gunn et al., 1997; Lammertsma and Hume, 1996; Sudo et al., 2001).
Volume of interest analysis
We used proton density weighted images, in which the substantia nigra (SN) is clearly visible, to define a midbrain volume of interest (VOI). Images were normalised to an MNI template using the transformation parameters of the T1-weighted MRIs, and averaged across all patients. A 3 mm radius spherical VOI was placed medial to the SN on the transverse slice inferior to the slice containing the most prominent view of the SN. Given the spatial resolution of PET, this midbrain region will include signal predominately from the VTA and also the SN (Kessler et al., 1984). Both the VTA and SN contain, in different degrees, mesostriatal and mesolimbic projection fibres, and both respond to reward and are implicated in addiction (Domesick, 1988; Fallon, 1988; Wise, 2009). DA receptors in these regions are predominantly autoreceptors (Jang et al., 2011, Khan et al., 1998; Morelli et al., 1987). In this VOI, we measured changes in mean [11C] FLB-457 BP due to gambling to compare between groups (using a between-groups t-test, and post-hoc within-subjects t-tests for simple effects analysis) and to correlate with the BIS (Spearman’s correlation test).
Voxel-based parametric analysis
The MNI normalised BP images collected during the tasks for all patients were subjected to a within subjects t-test in SPM8 to assess the main effect of task, with the whole brain included. Images were thresholded at P<0.005 and only voxel clusters with P values<0.05 (FWE corrected) were considered significant. This contrast revealed a large frontally located cluster, detailed in the results section. Further analyses were limited to this cluster, allowing us to limit our search to those voxels showing DAergic modulation during gambling. This procedure avoids the risk of making a type II error due to the inclusion, and subsequent correction for, voxels not expected to show changes in DA due to gambling at the level of DA D2/3 receptors. Thus, an explicit mask retaining voxels within the cluster was applied to two between-groups t-tests that tested a) the main effect of group [(PG/gambling+PG/control) − (controls/gambling+ controls/control)] and b) the interaction [(PG/gambling − PG/control) − (controls/gambling − controls/control)].
Results
Table 1 presents a summary of the patients’ clinical and demographic characteristics. Patients with and without PG did not differ in age (t (12)=0.16, P=0.88), disease severity (t (12)=0.99, P= 0.34) or disease duration (t (12)=0.84, P=0.41) or LEDD (t= −0.538, P=0.663). PD with PG patients were currently using less DA agonists than those without PG (t=9.98, P<0.0001).
PD patients with PG scored significantly higher on the BIS (t (12)=2.41, P0.05). Present gambling was reduced compared with past gambling in PG (t (6)=4.20, P<0.01), but was still greater than in control patients (t (12)=2.85, P<0.05).
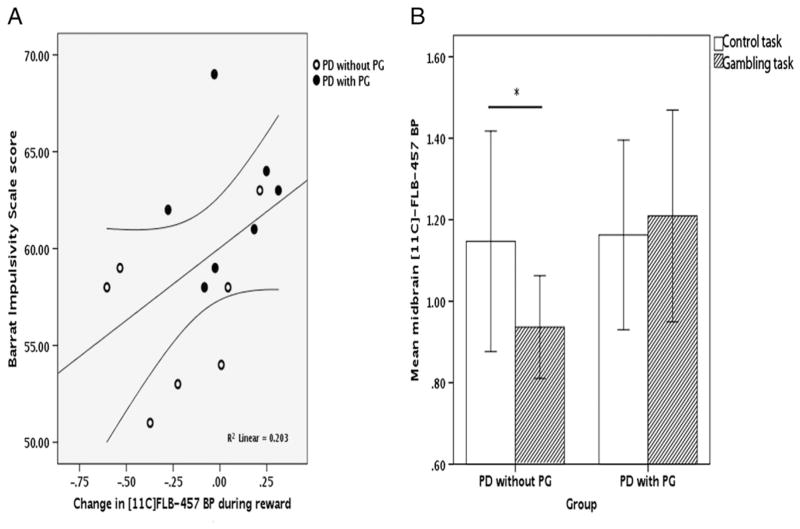
In the midbrain VOI, BIS scores were positively correlated with the change in BP in the midbrain region due to gambling (r=0.45, P<0.05) (Fig 2A), and the gambling task induced a significantly greater reduction in BP in PD patients without PG (t (12)=1.85, P<0.05). Simple effects revealed that BP decreased during gambling in the PD without PG group (t (6)=2.01, P<0.05), while it did not significantly change in the PD with PG group (t (6)=0.26, P=0.4) (Fig 2B).
Fig. 2.
A displays the relationship between the change in [11C] FLB-457 BP during reward in the midbrain volume in the PD patients with (closed circles) and without (open circles) PG and scores on the BIS. 95% confidence intervals for the regression line are also indicated. In B, the baseline task is represented by the unpatterned bars and the reward task is represented by the corrugated bars. The single asterisk denotes a significant difference between BP during the two scans only in the PD patients without PG at the P<0.05 level. Whiskers represent 2 standard errors.
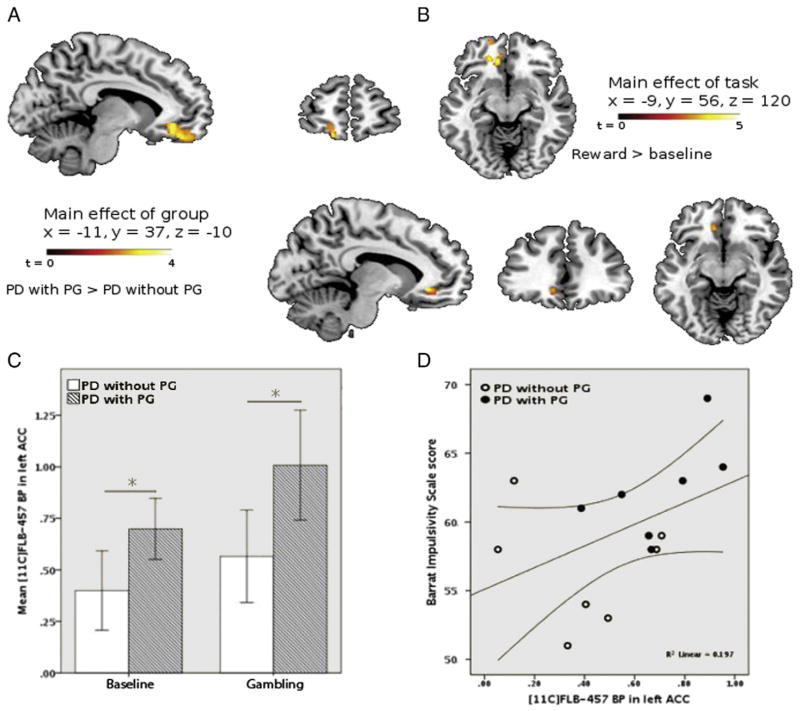
In the exploratory voxel-based analysis that included the whole brain we found a main effect of task at the level of the left medial PFC encompassing the OFC (BA 11) and extending into the left ACC (BA 32) (Fig 3A) for the contrast gambling>baseline (k (no of voxels)=217). Within this area, we found a significant main effect of group in the left ACC, revealing increased BP in the PD with PG group (P<0.05, FWE corrected). Mean BP in this region (shown in Fig 3B) was greater in the PD with PG group during both control and gambling scans (Fig 3C) possibly reflecting reduced basal DA levels and/or increased expression of D2/3 receptors in the PD with PG group. Finally, as predicted, baseline BP in this ACC region positively correlated with the BIS (r=0.55, P<0.05).
Fig. 3.
A shows the cluster revealed during the whole brain voxel-based analysis by the contrast reward>baseline, showing therefore increased BP due to gambling across all patients, superimposed on the MNI normalised brain. The cluster (k=217) peaked in the left OFC (Brodmann’s area 11) and in the left ACC (Brodmann’s area 32). B shows the main effect of group (PD with PG>PD without PG). C shows mean [11C] FLB-457 BP during the baseline and reward tasks in the PD patients with (crossed bars) and without (plain bars) PG, extracted from the whole area. Whiskers are 2 standard errors. D shows the relationship between BP in this area during the baseline scan and scores on the BIS. PD with PG is represented by closed circles and PD without PG by open circles. 95% confident limits are indicated.
Exploratory analyses including the whole brain testing for the main effect of group and the interaction revealed no significant increase or decrease of BP.
Discussion
[11C] FLB-457 BP in the midbrain
We found that changes in [11C] FLB-457 BP in the midbrain due to gambling were associated with impulsivity and were reduced in PD patients with PG compared to controls. DA D2/3 receptors in the midbrain are dominated by autoreceptors; which exert negative feedback control over striatal DA release (Santiago and Westerink, 1991). A previous study using [18F] Fallypride in healthy controls found that low levels of midbrain autoreceptors were associated with increased amphetamine-induced release of DA in the striatum (Buckholtz et al., 2010). These findings may link our current finding of impaired D2 receptor activation during gambling with our previous observations of elevated striatal DA responses to gambling in PD patients with DA agonist-induced PG (Steeves et al., 2009).
Natural variation in DA autoreceptor function may account for a proportion of the difference we observed between the groups of patients. However, pre-clinical studies have found that chronic administration of DA agonists cause decreased midbrain autoreceptor sensitivity (Chernoloz et al., 2009; Pitts et al., 1995; Subramaniam et al., 1992); potentially a compensatory mechanism for the temporary reductions in striatal DA release after acute doses. Thus, diminished occupation of D2 autoreceptors during gambling could represent DA agonist-induced lowered sensitivity of midbrain auto-receptors to endogenous DA release.
These presynaptic abnormalities may contribute to findings of both increased learning from positive feedback ( Voon et al., 2010) and decreased learning from negative feedback in PD patients with ICDs (van Eimeren et al., 2009). In this framework, greater striatal DA release following reward due to impaired autoreceptor sensitivity would augment reward based learning. At the same time, greater levels of striatal DA would blunt learning from punishment by analogy to the deficits observed on probabilistic learning tasks after levodopa (Frank et al., 2007).
Homoeostatic control of striatal DA is also mediated by dopamine transporter (DAT) proteins located on presynaptic terminals. D2 autoreceptors have been found to associate with DAT, and support DAT trafficking (Bolan et al., 2007; Lee et al., 2007). Thus, our results are consistent with recent SPECT data showing reduced striatal DAT binding in PD patients with ICDs (Cilia et al., 2010). We suggest that impaired DA homeostasis in the midbrain, resulting in increased striatal DA release during reward, may be responsible for increased impulsivity and therefore vulnerability for addiction in these patients.
[11C]-FLB BP in the cortex
PD patients with PG had significantly greater BP in the ACC compared with control PD patients. Previous studies have found lowered BOLD activity in the ACC and OFC during risk-based decision making in PD patients with ICDs (Voon et al., 2011b). Regional cerebral blood flow measured with [15O] H2O and PET is reduced in the ACC and OFC in response to DA agonists only in PD patients with an ICD (van Eimeren et al., 2010). In the non-PD population, addiction is associated with reduced brain glucose metabolism in the ACC and OFC, which is proposed to result from lowered DAergic modulation of these areas (see Volkow et al., 2011). Our data provide the first evidence that impaired function in the ACC in PD patients with an ICD may have a dopaminergic basis; specifically, low basal synaptic DA and/or increased D2/3 receptor expression. Given the involvement of the ACC in impulse control, low synaptic DA in this region may result in an impaired ability to control motivational urges to engage in rewarding behaviours.
Evidence that low frontal DA may be a characteristic of addiction, regardless of DA agonist use, comes from genetic studies. Individuals with polymorphisms of the COMT gene associated with high levels of DA catabolism in the frontal cortex, and therefore reduced basal DA levels, experience less pleasure from similar rewards than those with higher basal DA levels (Wichers et al., 2008). These observations are consistent with the reward deficiency syndrome account of addiction (Blum et al., 2007).
In the present study, BP in the ACC during the control task was correlated with impulsivity. Thus, if increased BP indicates lower basal DA levels, this implies that low DA modulation of the ACC contributes to the impulsive personality trait, perhaps by impairing inhibitory control. Indeed, a previous fMRI study found that oral methlyphenidate, which increases DA levels, reduced behavioural impulsivity in cocaine addicted and control subjects, and was associated with normalised ACC activity in the cocaine addicted group (Goldstein et al., 2010). Low DA tone in this area has also been associated with apathy (Thobois et al., 2010), which would seem incongruous with our suggestion that it is associated with impulsivity. However, both apathy and impulsivity are caused by changes within a large network that also relies on activity within other neurotransmitter systems. It is likely that low DA tone in the ACC represents only one aspect of this complexity.
We observed an increase in binding, meaning there is a greater availability of D2 receptors in the OFC and ACC during gambling as compared to the baseline task across all PD patients. The role of DA in the frontal cortex is extremely complex, with opposing actions being exerted via different receptor types (D1- and D2-class receptors) (Lapish et al., 2007; Seamans and Yang, 2004), and the functional implications of this are not yet understood. For example, it has been shown that D1 versus D2 receptors in the PFC are activated at different levels of DA tone, with preferential D1 receptor activation occurring at high DA levels and preferential D2 receptor activation occurring at low DA levels (Trantham-Davidson et al., 2004). Thus, increased D2 binding across all PD patients during the gambling task may reflect this complex relationship between D1 versus D2 activation.
While interactions exist between the mesocortical and mesostriatal DA systems (Jenner, 2002), we do not consider there to be a direct relationship between activation of midbrain autoreceptors and DA levels in the cortex since there is a lack of somatodendritic autoreceptors on mesocortical projection fibres (Chiodo et al., 1984). Rather, differences in autoreceptor sensitivity in the midbrain impact only on mesostriatal and mesolimbic DA.
Patients were on 1 mg pramipexole during both scans. Post synaptic occupation of DA receptors in the PFC is not likely to account for our findings as this should be equivalent across groups. Interestingly however, our findings in the midbrain may depend on the presence of a DA agonist via altered feedback control of striatal DA due to tonic occupation of autoreceptors. Our results would then suggest that DA agonists differently alter autoreceptor function, and, in turn, risk for ICDs, depending on trait impulsivity.
Among possible limitations we should emphasise that, as with all studies in medicated PD patients, but in particular in PD patients with PG who have had atypical adjustments applied to their medication regimes, it is necessary to interpret our results with caution. The PD with PG group was taking lower chronic DA agonist doses, along with compensatory increases in levodopa doses, in order to retain benefit to PD symptoms. Since both DA agonists and levodopa effect DA receptor expression (Chernoloz et al., 2009; Kaasinen et al., 2000; Pitts et al., 1995; Subramaniam et al., 1992; Thobois et al., 2004) we chose to match groups on total LEDD, but it must be acknowledged that these differences may have influenced our results.
Conclusions
Natural variation in DA homeostasis in the midbrain and cortex can impact an individuals’ propensity for impulsivity and, as such, modulate risk for ICDs in PD. DA agonists may exaggerate these dopaminergic influences over behaviour, turning a previous tendency to engage in rewarding activities into a pathological inability to abstain from them.
Acknowledgments
This work was supported by Parkinson Disease Foundation, Canadian Institutes of Health Research (MOP 110962). We thank Mr Keith O. Dorricott. A.P.S. is also supported Canada Research Chair program. N.J.R is supported by the Parkinson Society Canada.
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
References
- Blum K, Chen TJH, Meshkin B, et al. Manipulation of catechol-O-methyl-transferase (COMT) activity to influence the attenuation of substance seeking behavior, a subtype of Reward Deficiency Syndrome (RDS), is dependent upon gene polymorphisms: a hypothesis. Med Hypotheses. 2007;69 (5):1054–1060. doi: 10.1016/j.mehy.2006.12.062. [DOI] [PubMed] [Google Scholar]
- Bolan EA, Kivell B, Jaligam V, et al. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol. 2007;71 (5):1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329 (5991):532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Meiklejohn D, Wakeley J, Herbert V, Cook J, Scollo P, Ray MK, Selvaraj S, Passingham RE, Cowen P, Rogers RD. Serotonin and dopamine play complementary roles in gambling to recover losses. Neuropsychopharmacology. 2011 Jan;36(2):402–410. doi: 10.1038/npp.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoloz O, El Mansari M, Blier P. Sustained administration of pramipexole modifies the spontaneous firing of dopamine, norepinephrine, and serotonin neurons in the rat brain. Neuropsychopharmacology. 2009;34 (3):651–661. doi: 10.1038/npp.2008.114. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bannon MJ, Grace AA, Roth RH, Bunney BS. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience. 1984;12 (1):1–16. doi: 10.1016/0306-4522(84)90133-7. [DOI] [PubMed] [Google Scholar]
- Cilia R, Ko JH, Cho SS, et al. Reduced dopamine transporter density in the ventral striatum of patients with Parkinson’s disease and pathological gambling. Neurobiol Dis. 2010;39 (1):98–104. doi: 10.1016/j.nbd.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18 (2):192–205. [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41 (11):1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Domesick VB. Neuroanatomical organization of dopamine neurons in the ventral tegmental area. Ann N Y Acad Sci. 1988;537:10–26. doi: 10.1111/j.1749-6632.1988.tb42094.x. [DOI] [PubMed] [Google Scholar]
- Fallon JH. Topographic organization of ascending dopaminergic projections. Ann N Y Acad Sci. 1988;537:1–9. doi: 10.1111/j.1749-6632.1988.tb42093.x. [DOI] [PubMed] [Google Scholar]
- Farde L, Suhara T, Nyberg S, et al. A PET-study of [11C] FLB 457 binding to extrastriatal D2-dopamine receptors in healthy subjects and antipsychotic drug-treated patients. Psychopharmacology (Berl) 1997;133 (4):396–404. doi: 10.1007/s002130050420. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318 (5854):1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12 (11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Maloney T, et al. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Natl Acad Sci U S A. 2010;107 (38):16667–16672. doi: 10.1073/pnas.1011455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. NeuroImage. 1997;6 (4):279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Jang JY, Jang M, Kim SH, Um KB, Kang YK, Kim HJ, Chung S, Park MK. Regulation of dopaminergic neuron firing by heterogeneous dopamine autoreceptors in the substantia nigra pars compacta. J Neurochem. 2011 Mar;116(6):966–974. doi: 10.1111/j.1471-4159.2010.07107.x. [DOI] [PubMed] [Google Scholar]
- Jenner P. Pharmacology of dopamine agonists in the treatment of Parkinson’s disease. Neurology. 2002;58 (4 Suppl 1):S1–S8. doi: 10.1212/wnl.58.suppl_1.s1. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Någren K, Hietala J, Oikonen V, Vilkman H, Farde L, Halldin C, Rinne JO. Extrastriatal dopamine D2 and D3 receptors in early and advanced Parkinson’s disease. Neurology. 2000 Apr 11;54(7):1482–1487. doi: 10.1212/wnl.54.7.1482. [DOI] [PubMed] [Google Scholar]
- Kessler RM, Ellis JR, Jr, Eden M. Analysis of emission tomographic scan data: limitations imposed by resolution and background. J Comput Assist Tomogr. 1984 Jun;8(3):514–522. doi: 10.1097/00004728-198406000-00028. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Godman-Rakic PS. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95 (13):7731–7736. doi: 10.1073/pnas.95.13.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Grant JE, Potenza MN, Blanco C, Hollander E. The Gambling Symptom Assessment Scale (G-SAS): a reliability and validity study. Psychiatry Res. 2009;166 (1):76–84. doi: 10.1016/j.psychres.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4 (3 Pt 1):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Lapish CC, Kroener S, Durstewitz D, Lavin A, Seamans JK. The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology (Berl) 2007;191 (3):609–625. doi: 10.1007/s00213-006-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FJS, Pei L, Moszczynska A, et al. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 2007;26 (8):2127–2136. doi: 10.1038/sj.emboj.7601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Potenza MN. Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacology (Berl) 2012;219 (2):469–490. doi: 10.1007/s00213-011-2550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery AJ, Asselin MC, Farde L, Grasby PM. Measurement of methylphenidate-induced change in extrastriatal dopamine concentration using [11C] FLB 457 PET. J Cereb Blood Flow Metab. 2007;27 (2):369–377. doi: 10.1038/sj.jcbfm.9600339. [DOI] [PubMed] [Google Scholar]
- Morelli M, Carboni E, Devoto S, Di Chiara G. 6-Hydroxydopamine lesions reduce specific [3H] sulpiride binding in the rat substantia nigra: direct evidence for the existence of nigral D-2 autoreceptors. Eur J Pharmacol. 1987;140 (1):99–104. doi: 10.1016/0014-2999(87)90639-x. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Mason NS, et al. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: a comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C] FLB 457 and [11C] fallypride. Synapse. 2009;63 (6):447–461. doi: 10.1002/syn.20628. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53 (4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C] FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab. 1999;19:1164–1173. doi: 10.1097/00004647-199910000-00013. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35 (12):2414–2426. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51 (6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pitts DK, Wang L, Kelland MD, Freeman AS, Chiodo LA. Repeated stimulation of dopamine D2-like receptors: reduced responsiveness of nigrostriatal and mesoaccumbens dopamine neurons to quinpirole. J Pharmacol Exp Ther. 1995;275 (1):412–421. [PubMed] [Google Scholar]
- Potenza MN. Review. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos Trans R Soc Lond B Biol Sci. 2008;363 (1507):3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray N, Strafella AP. Dopamine, reward, and frontostriatal circuitry in impulse control disorders in Parkinson’s disease: insights from functional imaging. Clin EEG Neurosci. 2010;41 (2):87–93. doi: 10.1177/155005941004100208. [DOI] [PubMed] [Google Scholar]
- Santiago M, Westerink BH. The regulation of dopamine release from nigrostriatal neurons in conscious rats: the role of somatodendritic autoreceptors. Eur J Pharmacol. 1991;204 (1):79–85. doi: 10.1016/0014-2999(91)90838-h. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74 (1):1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Steeves TDL, Miyasaki J, Zurowski M, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132 (Pt 5):1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Lucki I, McGonigle P. Effects of chronic treatment with selective agonists on the subtypes of dopamine receptors. Brain Res. 1992;571 (2):313–322. doi: 10.1016/0006-8993(92)90670-5. [DOI] [PubMed] [Google Scholar]
- Sudo Y, Suhara T, Inoue M, et al. Reproducibility of [11 C] FLB 457 binding in extrastriatal regions. Nucl Med Commun. 2001;22 (11):1215–1221. doi: 10.1097/00006231-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Thobois S, Vingerhoets F, Fraix V, Xie-Brustolin J, Mollion H, Costes N, Mertens P, Benabid AL, Pollak P, Broussolle E. Role of dopaminergic treatment in dopamine receptor down-regulation in advanced Parkinson disease: a positron emission tomographic study. Arch Neurol. 2004 Nov;61(11):1705–1709. doi: 10.1001/archneur.61.11.1705. [DOI] [PubMed] [Google Scholar]
- Thobois S, Ardouin C, Lhommée E, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain. 2010 Apr;133:1111–1127. doi: 10.1093/brain/awq032. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010 Nov 15;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in pre-frontal cortex. J Neurosci. 2004;24 (47):10652–10659. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eimeren T, Pellecchia G, Cilia R, et al. Drug-induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology. 2010;75 (19):1711–1716. doi: 10.1212/WNL.0b013e3181fc27fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eimeren T, Ballanger B, Pellecchia G, et al. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: a trigger for pathological gambling in Parkinson’s disease? Neuropsychopharmacology. 2009;34 (13):2758–2766. doi: 10.1038/sj.npp.npp2009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandehey NT, Moirano JM, Converse AK, et al. High-affinity dopamine D2/D3 PET radioligands 18F-fallypride and 11C-FLB457: a comparison of kinetics in extrastriatal regions using a multiple-injection protocol. J Cereb Blood Flow Metab. 2010;30 (5):994–1007. doi: 10.1038/jcbfm.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain (*) Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108 (37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67 (7):1254–1257. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- Voon V, Dalley JW. Parkinson disease: impulsive choice-Parkinson disease and dopaminergic therapy. Nat Rev Neurol. 2011;7 (10):541–542. doi: 10.1038/nrneurol.2011.139. [DOI] [PubMed] [Google Scholar]
- Voon V, Gao J, Brezing C, et al. Dopamine agonists and risk: impulse control disorders in Parkinson’s disease. Brain. 2011a;134 (Pt 5):1438–1446. doi: 10.1093/brain/awr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Mehta AR, Hallett M. Impulse control disorders in Parkinson’s disease: recent advances. Curr Opin Neurol. 2011c;24 (4):324–330. doi: 10.1097/WCO.0b013e3283489687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Pessiglione M, Brezing C, et al. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron. 2010;65 (1):135–142. doi: 10.1016/j.neuron.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Sohr M, Lang AE, et al. Impulse control disorders in Parkinson disease: a multicenter case–control study. Ann Neurol. 2011b;69 (6):986–996. doi: 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67 (5):589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- Wichers M, Aguilera M, Kenis G, et al. The catechol-O-methyl transferase Val158Met polymorphism and experience of reward in the flow of daily life. Neuropsychopharmacology. 2008;33 (13):3030–3036. doi: 10.1038/sj.npp.1301520. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci. 2009;32 (10):517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17 (4):536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Zack M, Poulos CX. Parallel roles for dopamine in pathological gambling and psychostimulant addiction. Curr Drug Abuse Rev. 2009;2 (1):11–25. doi: 10.2174/1874473710902010011. [DOI] [PubMed] [Google Scholar]