SUMMARY
Understanding the mechanisms regulating pluripotency in embryonic and induced pluripotent stem cells is required to ensure their safe use in clinical applications. Glycogen synthase kinase-3 (GSK-3) has emerged as an important regulator of pluripotency, based primarily on studies with small-molecule GSK-3 inhibitors. Here, we use mouse embryonic stem cells (ESCs) lacking GSK-3 to demonstrate that a single GSK-3 substrate, β-catenin, controls the ability of ESCs to exit the pluripotent state and to differentiate into neurectoderm. Unexpectedly, the effects of β-catenin on pluripotency do not appear to be dependent on TCF-mediated signaling, based on experiments utilizing a β-catenin C-terminal truncation mutant or highly efficient dominant-negative TCF strategies. Alternatively, we find that stabilized β-catenin forms a complex with and enhances the activity of Oct-4, a core component of the transcriptional network regulating pluripotency. Collectively, our data suggest previously underappreciated, divergent TCF-dependent and TCF-independent roles for β-catenin in ESCs.
INTRODUCTION
The advent of induced pluripotent stem cell technology has resulted in renewed optimism with respect to using pluripotent or multipotent stem cells for applications in regenerative medicine. Our understanding of the signaling mechanisms that regulate the acquisition and retention of the pluripotent state remains limited and must be improved if pluripotent stem cells are to be used safely for clinical purposes. Recently, glycogen synthase kinase-3 (GSK-3) has emerged as an important regulator of pluripotency, based largely on studies with small-molecule GSK-3 inhibitors (Bone et al., 2009; Buehr et al., 2008; Li et al., 2009; Sato et al., 2004; Ying et al., 2008).
GSK-3 regulates numerous cellular substrates, which include transcription factors, signaling molecules, and structural proteins (Doble and Woodgett, 2003). Because of its involvement in numerous signaling pathways and metabolic processes, the mechanisms underlying the effects of GSK-3 inhibition on stem cell pluripotency are unclear. Several mechanisms have been proposed, including GSK-3’s regulation of c-Myc, Wnt/β-catenin, and PI-3K signaling (Bechard and Dalton, 2009; Bone et al., 2009; Sato et al., 2004; Storm et al., 2007).
A key GSK-3 substrate is β-catenin, the effector molecule of the Wnt/β-catenin signaling pathway, which acts as a transactivator of target genes through its interactions with TCF/LEF transcription factors (reviewed in Arce et al., 2006; MacDonald et al., 2009). GSK-3-mediated phosphorylation of β-catenin results in its ubiquitination and proteasomal degradation. We have shown previously that mouse embryonic stem cells (mESCs) devoid of GSK-3 display extremely high levels of signaling-competent β-catenin and have a profound block in their capacity to differentiate into cell types of the three germ lineages (Doble et al., 2007). Most notably, teratomas and embryoid bodies derived from GSK-3α/β double-knockout (DKO) mESCs exhibited a complete lack of detectable neurectodermal differentiation.
A correlation between high levels of β-catenin signaling and blocked neuronal differentiation of mESCs has been observed in other models where dominant-active β-catenin was overexpressed (Haegele et al., 2003) or the β-catenin destruction complex was disrupted because of mutations in the tumor-suppressor adenomatous polyposis coli (APC) (Kielman et al., 2002). Conversely, antagonism of Wnt signaling has been implicated as a requirement for neural differentiation of mESCs (Aubert et al., 2002; Cajánek et al., 2009).
Activation of Wnt/β-catenin signaling has been linked to the maintenance of the pluripotent state in human and mouse ESCs (Sato et al., 2004), although these results are somewhat controversial because self-renewal of the ESCs in Wnt-3a-supplemented medium was not examined over multiple passages (Bakre et al., 2007; Dravid et al., 2005). Wnt/β-catenin signaling has also been shown to enhance the induction of pluripotency in somatic cells, obtained through viral transduction or cell fusion (Lluis et al., 2008; Marson et al., 2008).
We hypothesized that hyperactivated β-catenin/TCF-mediated transcriptional activation was responsible for the phenotype of the DKO mESCs. To address this possibility, we examined the consequences of blocking β-catenin activity at the level of TCF-mediated transcription in GSK-3α/β DKO cells through the stable expression of dominant-negative TCF1 or TCF4 (encoded in humans by the genes TCF7 and TCF7L2, respectively). Surprisingly, although expression of these dominant-negative TCFs efficiently attenuated β-catenin/TCF gene transactivation, these cells were not rescued with respect to their ability to differentiate into neurectoderm and were capable of self-renewal in the absence of exogenous factors. However, we demonstrate that suppression of β-catenin expression, through shRNA-mediated knockdown, permits the multilineage differentiation of DKO mESCs. We provide evidence for alternative β-catenin-mediated signaling, not requiring its TCF-transactivation domain, which reinforces pluripotency and blocks efficient differentiation of mESCs. We show that β-catenin can form a complex with and modulate the activity of Oct-4 and propose that this is part of the mechanism through which β catenin exerts its TCF-independent effects on mESCs. Taken together, our data suggest that β-catenin is the primary GSK-3 substrate regulating the differentiation of mESCs, through divergent nuclear signaling mechanisms.
RESULTS
Generation of DKO Flp-in Cell Lines
We generated a GSK-3α/β DKO mESC cell line (DKO2.1) amenable to the generation of isogenic stable cell lines. Starting with GSK-3α−/− mESCs (Doble et al., 2007), we targeted both GSK-3β loci to generate DKO cells with a single FRT site for Flp-mediated site-specific recombination (Figure 1A). Transgenes were cloned into pEF5/FRT/V5-D-TOPO (“Flp-In system”; Invitrogen) and single-copy transgenic lines were generated in the DKO2.1 cell line (Figure 1B). We used Southern blotting to confirm proper targeting (Figure S1A available online).
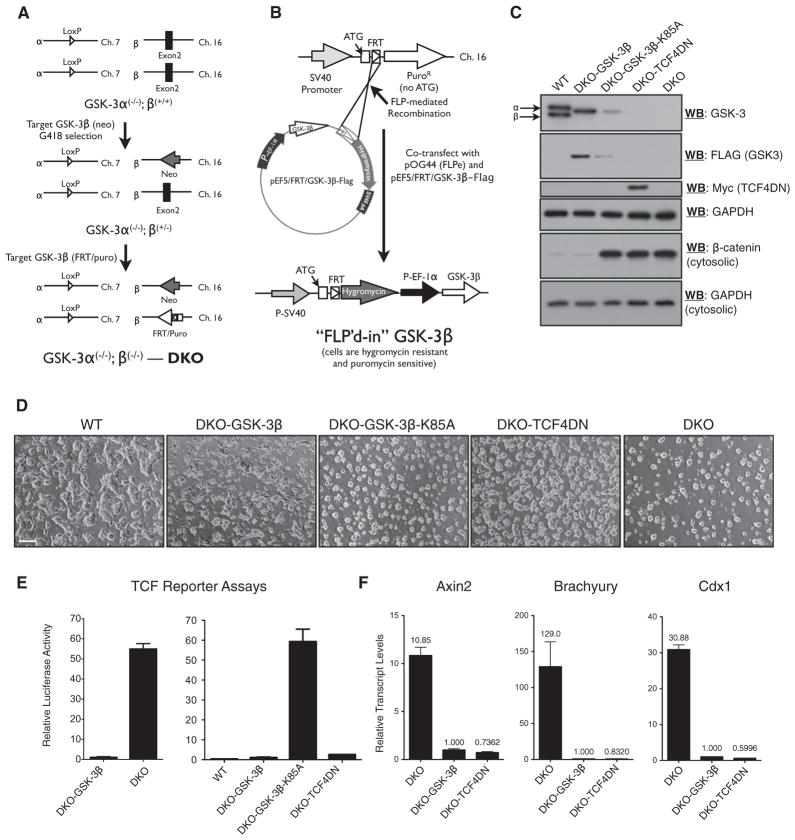
Figure 1. Generation and Validation of Isogenic DKO mESC Lines Expressing GSK-3 Variants or Dominant-Negative TCF4.
(A) Targeting steps used to create DKO mESCs, which incorporate an FRT recombination site for the Flp-in system in the GSK-3β locus.
(B) Flp-mediated site-specific integration of an EF-1α-driven transgene.
(C) Immunoblot analyses of transgene expression in mESC lines expressing TCF4DN or variants of GSK3.
(D) Morphology of WT, DKO, and DKO-Flp-in lines as indicated, via light microscopy. Scale bar represents 100 μm.
(E) TCF reporter assays demonstrate that re-expression of GSK3 in DKO mESCs restores steady-state inhibition of Wnt signaling (left) and that reporter activity is not altered by the expression of kinase-inactive GSK3 (K85A) but is efficiently attenuated by expression of TCF4DN in DKO cells (right).
(F) Quantitative RT-PCR analysis of Axin2, Brachyury, and Cdx1 transcript levels in the indicated cell lines.
Bars represent the mean of three independent experiments ± SEM. See also Figure S1.
The DKO2.1 cell line, hereafter referred to as “DKO,” was used to generate isogenic FLP-in cell lines expressing (1) wild-type (WT) GSK-3β (DKO-GSK3β), (2) dominant-negative TCF4 (DKO-TCF4DN), (3) dominant-negative TCF1 (DKO-TCF1DN), and (4) kinase-inactive GSK-3β (DKO-GSK-3β-K85A). PCR analyses with genomic DNA template isolated from each transgenic cell line and primers designed to amplify the junction of a properly recombined Flp-in event yielded bands of the expected size (Figure S1B).
The DKO mESCs lacked detectable GSK-3α and GSK-3β protein and displayed high levels of cytosolic β-catenin (Figure 1C), in keeping with our previous findings (Doble et al., 2007). Stable expression of wild-type, but not kinase-dead, GSK-3β normalized cytosolic β-catenin levels. The TCF4DN protein was readily detected through western blotting with a Myc tag antibody (Figure 1C) or a TCF4-specific antibody (Figure S1D). TCF1DN was also easily detected with a TCF1 antibody (Figure S1E). The protein levels of TCF4DN and TCF1DN were markedly higher than their endogenous counterparts (Figures S1D and S1E). TCF1, TCF3, TCF4, and LEF1 were all detectable through western blotting with lysates from mESCs maintained in standard medium (Figure S1D). LEF1 protein levels were dramatically decreased and TCF1 levels were slightly lower in DKO-TCF4DN mESCs, compared to their levels in DKO mESCs (Figure S1D). By contrast, the amount of TCF3 protein was not altered by the expression of TCF4DN in DKO mESCs (Figure S1D). The pluripotent markers Oct4, Nanog, and Sox2 were expressed at similar levels in WT and DKO mESCs (Figures S1F and S1G). DKO-TCF4DN mESCs displayed modestly higher levels of Nanog protein (Figure S1F) and transcript (Figure S1G) compared to WT and DKO mESCs.
The steady-state level of GSK-3β expressed in the DKO-GSK-3β (WT) and DKO-GSK-3β-K85A cell lines was quantitated through densitometry (Figure S1C). The amount of GSK-3β in DKO-GSK-3β cells was approximately 50% of the total amount of GSK-3 detected in WT mESCs. The steady-state levels of GSK-3β in DKO-GSK-3β-K85A mESCs was approximately four times less than that observed in DKO-GSK-3β mESCs. This probably reflects a requirement for GSK-3 autophosphorylation in directing the proper folding of GSK-3, which confers enhanced stability (Lochhead et al., 2006).
The morphology of DKO mESCs (Figure 1D) was identical to that of our previously generated DKO cell line. DKO-GSK-3β mESCs had a colony morphology resembling WT mESCs, whereas cells expressing GSK-3β-K85A or TCF4DN formed colonies reminiscent of DKO mESCs (Figure 1D). There were no obvious differences in the cloning efficiencies of FLP-in cell lines expressing GSK-3β variants, TCF1DN, or TCF4DN. We present representative data from individual clones for most of our FLP-in cell lines. Multiple clones were generated for each type of cell line. As expected for FLP-in cell lines, transgene expression levels and clone properties were found to be equivalent in independent clones expressing the same transgene.
Dominant-Negative TCF4 and TCF1 Effectively Repress TCF Activity in DKO mESCs
The high level of TCF activity detected in the DKO cell line was reduced by ~50-fold through stable expression of GSK-3β (Figure 1E). There was not a marked difference between the levels of TCF reporter activity assayed in true WT (E14K) or DKO-GSK-3β mESCs, whereas DKO-GSK-3β-K85A mESCs displayed equivalent TCF activity to that detected in DKO mESCs (Figure 1E). Stable expression of TCF4DN in DKO mESCs was remarkably efficient in normalizing TCF reporter activity (Figure 1E), as was stable expression of TCF1DN (Figure S1H).
To determine whether stable expression of TCF4DN efficiently blocked the transcription of endogenous TCF-responsive genes, we assessed transcript levels of Axin2, Brachyury, and Cdx1, TCF target genes highly upregulated in DKO mESCs (Figure 1F; Doble et al., 2007). The stable expression of TCF4DN in DKO mESCs reduced the expression of TCF target genes to levels that were equivalent to those observed in DKO-GSK-3β mESCs. Suppression of Brachyury and Cdx1 expression was also attained by overexpressing TCF1DN (Figure S1I).
Neither TCF4DN nor TCF1DN Rescue the DKO Differentiation Blockade
We tested the differentiation capacity of DKO cell lines expressing GSK-3β, TCF1DN, or TCF4DN by using embryoid body (EB) and teratoma assays, as previously described (Doble et al., 2007). We examined the expression of pluripotency markers Oct-4, Sox-2, and Nanog, as well as the neurectoderm marker β-III-tubulin, via immunofluorescent staining of whole EBs harvested after 14 days of differentiation. DKO EBs contained numerous cells expressing Oct-4, Sox-2, and Nanog, and no cells expressing β-III-tubulin, whereas EBs generated from DKO-GSK-3β mESCs contained extensive regions of β-III-tubulin staining but lacked cells expressing Oct-4, Sox-2, and Nanog (Figure 2A). DKO-TCF4DN EBs displayed the same staining pattern as DKO EBs for Oct-4, Sox-2, Nanog, and β-III-tubulin (Figure 2A). DKO-TCF1DN EBs also displayed extensive regions of Oct-4 and Nanog staining and lacked β-III-tubulin and GFAP staining. This result was virtually indistinguishable from that obtained in a parallel experiment done with DKO-TCF4DN EBs (Figure S2A).
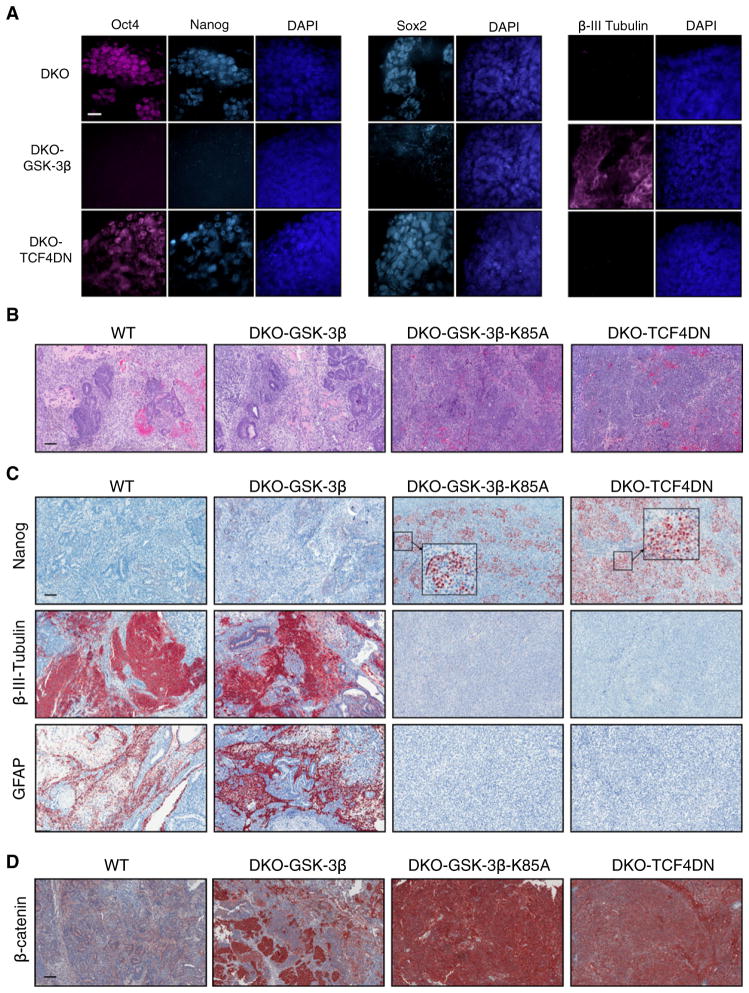
Figure 2. Attenuation of Wnt Target Gene Activation in DKO mESCs, by Stably Expressing TCF4DN, Fails to Rescue Their Impaired Differentiation.
(A) Retention of the pluripotency markers Oct4, Nanog, and Sox2 as well as expression of the neuronal marker β-III-tubulin was determined by immunofluorescent staining of the indicated EBs, maintained under differentiating conditions for 14 days.
(B) H&E staining of 3-week teratomas derived from WT (E14K), DKO-GSK3β, DKO-GSK3β-K85A, and DKO-TCF4DN mESCs.
(C) Immunohistochemical staining of teratomas revealed retention of the pluripotency marker Nanog and absence of neural tissue, as determined by staining for β-III-tubulin and GFAP, in teratomas derived from DKO-TCF4DN mESCs.
(D) β-catenin is maintained at low levels in teratomas derived from WT mESCs, whereas some regions of high β-catenin levels are observed in DKO-GSK3β teratomas, resulting from the loss of GSK3β transgene expression and reversion to DKO phenotype. In contrast, β-catenin levels remain high in DKO-GSK3β-K85A and DKO-TCF4DN teratomas.
Scale bars represent 20 μm (A) or 200 μm (B–D). See also Figure S2.
Control WT teratomas contained examples of all three germ lineages in H&E-stained sections, as did teratomas generated from DKO-GSK-3β mESCs (Figure 2B). By contrast, DKO-GSK-3β-K85A or DKO-TCF4DN teratomas were composed of carcinoma-like solid nodular structures with scant intervening stroma (Figure 2B). There were no obvious histological differences between DKO, DKO-GSK-3β-K85A, DKO-TCF4DN, or DKO-TCF1DNteratomas (Figure 2B;FigureS2B). Immunohistochemical staining of teratoma sections for Nanog and β-III-tubulin supported our findings in EBs. Control (WT) teratomas lacked Nanog but stained extensively for β-III-tubulin and GFAP (Figure 2C). The same pattern of staining was observed in DKO-GSK-3β teratomas (Figure 2C). DKO-GSK-3β-K85A teratomas lacked staining for β-III-tubulin or GFAP, but did stain extensively for Nanog. DKO-TCF4DN teratomas displayed the same pattern of Nanog, β-III-tubulin, and GFAP staining as DKO-GSK-3β-K85A teratomas, with perhaps even more retention of Nanog-positive cells (Figure 2C). Teratomas derived from DKO-TCF1DN mESCs were virtually indistinguishable from those obtained from DKO mESCs (Figure S2C). Our Oct-4 antibody was incompatible with immunohistochemistry, but we were able to detect Oct-4 in western blots with lysates from DKO, DKO-GSK-3β-K85A, and DKO-TCF4DN (but not WT or DKO-GSK-3β) teratomas (Figure S2D).
DKO-GSK-3β-K85A and DKO-TCF4DN teratomas had extremely high levels of β-catenin in all cellular compartments, in contrast to WT teratomas, which had much less intense staining (Figure 2D). DKO-GSK-3β teratomas contained large regions with β-catenin staining resembling that of WT teratomas, but they also contained regions where β-catenin levels were quite high in all cellular compartments (Figure 2D), suggesting limited loss of transgene expression during the 3 week period of teratoma generation.
DKO-TCF4DN mESCs Self-Renew in the Absence of Serum and LIF
In addition to examining their differentiation potential through EB and teratoma analyses, we characterized the self-renewal properties of DKO and DKO-TCF4DN mESCs and found that both lines could be maintained indefinitely (nearly 1 year to date) in basal N2B27 medium (Ying and Smith, 2003). Wild-type or DKO-GSK-3β mESCs could not be passaged in N2B27 more than twice before succumbing to differentiation and death, whereas DKO and DKO-TCF4DN mESCs could initially be passaged at split ratios of 1:5 (first two passages) and could then be passaged at a reduced split ratio (1:2 or 1:3). With prolonged culture in N2B27, the growth rate of DKO and DKO-TCF4DN mESCs decreased dramatically, as did their adherence to gelatin-coated tissue culture dishes. Still, both cell lines were propagated as loosely adherent spheres, amenable to disaggregation with Accutase and replating as single-cell suspensions every 2–3 weeks at a split ratio of 1:2 to 1:3. Incredibly, the DKO-TCF4DN mESCs, maintained in N2B27 for more than five passages, still retained expression of Oct-4, Sox2, and Nanog as assessed by western blotting, whereas DKO mESCs lost expression of Nanog and Sox2 (Figure S3A). Intriguingly, the loss of Nanog and Sox2 expression in DKO mESCs could be reversed by passaging the cells four times in standard medium containing serum and LIF (“DKO-R;” Figure S3A). Immunofluorescent staining of DKO-TCF4DN mESCs maintained in N2B27 revealed that a subpopulation of cells was doubly positive for Oct-4 and Nanog (Figure S3B). Some of the spheres comprising DKO mESCs gave rise to strongly adherent, tightly packed sheets of small cells, whereas DKO-TCF4DN mESCs did not produce cells with this phenotype (Figure S3C).
DKO and DKO-TCF4DN mESCs Do Not Differentiate in a Defined Neural Differentiation Assay
We assessed the neural differentiation potential of DKO and DKO-TCF4DN mESCs through an established assay (Ying and Smith, 2003). Whereas WT and DKO-GSK3β mESCs yielded cells with a neural morphology, DKO and DKO-TCF4DN mESCs did not (Figure 3A). Western blot (Figure 3B) and qRT-PCR analyses (Figure 3C) at 9 and 14 days after the initiation of neural differentiation revealed that WT and DKO-GSK3β mESCs lost the expression of Oct-4 and Nanog at the protein level and gained the expression of the neural marker β-III-tubulin. By contrast, DKO and DKO-TCF4DN mESCs maintained expression of Oct-4 and Nanog and failed to express β-III-tubulin at detectable levels (Figures 3B and 3C). Expression of the GSK3β and TCF4DN transgenes were maintained throughout the assay, even in the absence of drug selection (Figure 3B). WT and DKO-GSK3β, but not DKO or DKO-TCF4DN mESCs, exhibited a dramatic loss of Oct-4, Nanog, and Sox2 transcripts, concomitant with markedly increased expression of the neural markers β-III-tubulin, Map2, and Pax6 (Figure 3C).
Figure 3. Attenuation of β-Catenin/TCF Target Gene Activation in DKO mESCs Does Not Restore Their Ability to Give Rise to Neurectoderm in a Defined Neural Differentiation Assay.
(A) Morphology of DKO, WT, DKO-GSK3β, and DKO-TCF4DN mESCs 9 days after the initiation of neural differentiation in N2B27 medium.
(B and C) The indicated mESC lines were subjected to western blot (B) and qRT-PCR (C) analyses at 9 and 14 days after being seeded in N2B27 medium. WT and DKO-GSK3β mESCs exhibited robustly increased expression of the neuronal markers β-III-tubulin, Map2, and Pax6 and reduced expression of Oct-4, Nanog, and Sox2, whereas DKO and DKO-TCF4DN mESCs retained Oct-4, Nanog, and Sox2 and failed to express β-III-tubulin, Map2, and Pax6. Bars represent n = 3 ± SEM.
See also Figure S4.
Stable Knockdown of β-Catenin Expression in DKO-TCF4DN mESCs Eliminates the Blockade to Neurectodermal Differentiation
To determine whether high levels of β-catenin, acting independently of TCF-mediated gene activation, might underlie the DKO phenotype, we used stable expression of short-hairpin siRNAs (shRNAs) to knock down β-catenin in DKO-TCF4DN mESCs. Two different shRNA constructs were highly effective at reducing cytosolic β-catenin in DKO-TCF4DN mESCs, to levels just slightly higher than those detected in DKO-GSK-3β mESCs (Figure 4A). Two negative control cell lines were ineffective in reducing cytosolic β-catenin levels (β-sh ctrl #1 and #2, respectively) (Figure 4A). β-catenin transcript levels were reduced to approximately 25% of the levels detected in DKO-TCF4DN mESCs by both β-catenin-specific shRNAs (Figure 4B). Microscopic observation revealed that the reduction in β-catenin levels changed the gross morphology of the DKO-TCF4DN mESC colonies. Colonies of DKO-TCF4DN-β-sh (ctrl #1 and #2) mESCs remained highly refractile and individual cells were tightly packed in the colonies, whereas colonies with reduced β-catenin expression were less refractile and were comprised of cells that tended to flatten out (Figure 4C). Immunofluorescent staining for β-catenin confirmed that the total levels of β-catenin were reduced in cells expressing the shRNAs targeting β-catenin (Figure 4D). The nuclear β-catenin immunoreactivity observed in DKO-TCF4DN-β-sh ctrl mESCs was dramatically reduced in cells expressing the β-catenin-specific shRNAs, whereas the junctional β-catenin levels remained high (Figure 4D). DKO-TCF4DN-β-sh mESCs retained strong nuclear staining for Nanog (Figure 4D).
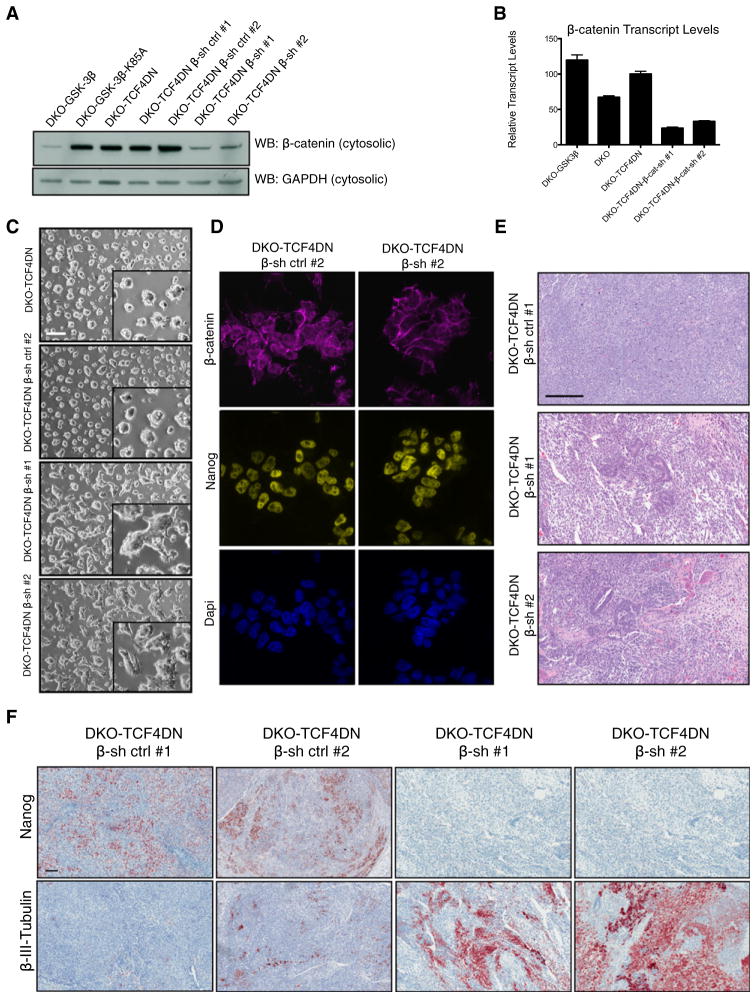
Figure 4. Knockdown of β-Catenin in DKO mESCS Expressing TCF4DN Partially Rescues Differentiation into the Neural Lineage.
(A) Stable expression of β-catenin-specific short hairpin RNAs (shRNAs) reduces cytosolic β-catenin protein levels to approximately that of DKO-GSK3β mESCs. Two negative controls were assayed, including empty vector (β-sh cont. #1) and β-catenin scrambled shRNA (β-sh cont. #2) alongside two distinct β-catenin-specific shRNAs (β-sh #1 and β-sh #2).
(B) Validation of β-catenin knockdown by qRT-PCR. Bars represent n = 3 ± SEM.
(C) Morphology of DKO-TCF4DN β-sh ctrl #1 and DKO-TCF4DN β-sh #2 mESCs grown on gelatin-coated culture dishes. Inset highlights the flatter morphology of β-catenin knockdown DKO-TCF4DN mESCs. Scale bar represents 200 μm.
(D) Immunofluorescent staining of β-catenin knockdown DKO-TCF4DN mESCs reveals a significant reduction in the cytosolic and nuclear β-catenin pools as a result of shRNA expression.
(E) H&E staining of DKO-TCF4DN-β-sh ctrl #1, DKO-TCF4DN-β-sh #1, and DKO-TCF4DN-β-sh #2 teratomas. Scale bar represents 200 μm.
(F) Immunohistochemical analyses of teratomas derived from β-catenin knockdown DKO-TCF4DN mESCs reveals that suppression of β-catenin alleviates the differentiation blockade to the neural lineage. Scale bar represents 100 μm.
See also Figure S5.
We used the teratoma assay to assess the differentiation capacity of DKO-TCF4DN-β-sh mESCs. It was evident from H&E-stained sections that knocking down β-catenin in DKO-TCF4DN mESCs resulted in a dramatic change in the ability of these cells to differentiate. Unlike DKO-TCF4DN-β-sh control mESCs, which formed very homogeneous teratomas resembling DKO teratomas, DKO-TCF4DN-β-sh teratomas displayed examples of differentiated tissues of all three germ layers (Figure 4E). DKO-TCF4DN-β-sh control teratomas were indistinguishable from DKO-TCF4DN teratomas when assayed for retention of immunohistochemical staining for Nanog and lack of positive staining for β-III-tubulin (compare Figures 2C and 4F). By contrast, DKO-TCF4DN-β-sh teratomas did not contain Nanog-positive cells but did have large regions of β-III-tubulin-positive staining (Figure 4F). The effectiveness and persistence of β-catenin knockdown was apparent by staining DKO-TCF4DN-β-sh teratoma sections for β-catenin (Figure S5A).
We attempted to differentiate DKO-TCF4DN-β-sh mESCs into neurons by using the defined N2B27 conditions described above. Despite retention of β-catenin knockdown, the DKO-TCF4DN-β-sh cells did not give rise to cells expressing neural markers under these specific conditions (Figure S4), suggesting that factors present in the microenvironment of teratomas are required for neural differentiation to occur.
Knockdown of β-Catenin in DKO mESCs Does Not Require Synergism with TCF4DN to Elicit Its Effects on Differentiation
To determine whether the rescue of neurectoderm differentiation obtained with β-catenin knockdown required the synergistic suppression of TCF target gene expression, we took advantage of the reversibility of the Flp-in system. We used transient Flp expression to “Flp-out” the cassette encoding TCF4DN in DKO-TCF4DN-β-sh cell lines. We confirmed the absence of TCF4DN and the retention of β-catenin shRNAs in Flp-out clones through western blotting (Figure S5B). The extent of β-catenin knockdown in Flp-out clones expressing either of the two β-catenin shRNAs was unchanged from that which we observed in the parental cell lines (Figure S5B).
We used the teratoma assay to determine whether reduction of β-catenin levels in DKO mESCs would be sufficient to rescue blocked neuronal differentiation. DKO-TCF4DN Flp-out teratomas expressing a negative control shRNA displayed high levels of Nanog immunostaining and no staining for β-III-tubulin (Figure S5C). By contrast, DKO-TCF4DN Flp-out teratomas with normalized β-catenin levels did not retain Nanog and differentiated readily to neurectoderm as assessed by β-III-tubulin staining (Figure S5C). Thus, suppression of β-catenin levels alone was sufficient to rescue neurectodermal differentiation of DKO mESCs in this assay.
Overexpression of Stabilized β-Catenin in WT mESCs Inhibits Neuronal Differentiation and Delays Loss of Pluripotency
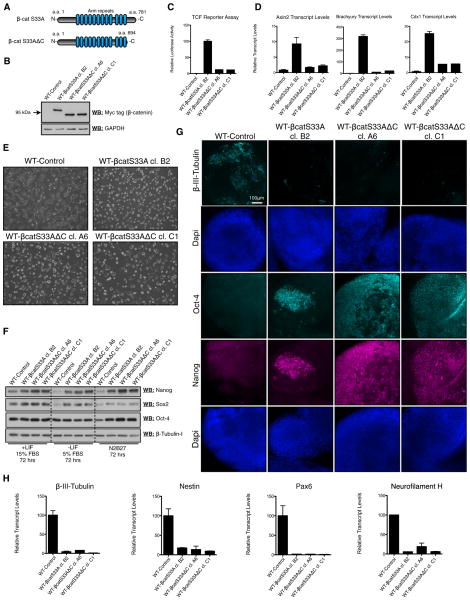
To determine whether overexpression of stabilized β-catenin in wild-type mESCs would phenocopy DKO mESCs, we generated stable cell lines overexpressing stabilized (S33A) β-catenin (Liu et al., 2002; Takao et al., 2007). We obtained several independent cell lines in which stabilized β-catenin levels were equivalent to those detected in DKO mESCs (Figure S6A). Selected cell lines expressing high levels of β-cateninS33A yielded TCF reporter activities (Figure S6B) and transcript levels of the TCF target genes Axin2, Brachyury, and Cdx1 (Figure S6C), which were even higher than in DKO mESCs (Figure S6B).
β-cateninS33A-expressing mESCs were morphologically indistinguishable from DKO mESCs (Figure 5A). They also displayed impaired neuronal differentiation, based on immunuo-fluorescent staining for β-III-tubulin (Figure 5B) and qRT-PCR analyses (Figure 5C) of neuronal markers in EBs after 14 days of differentiation. Teratomas generated from β-cateninS33A mESCs also displayed a dramatic reduction in detectable neuronal tissue (Figure 5D).
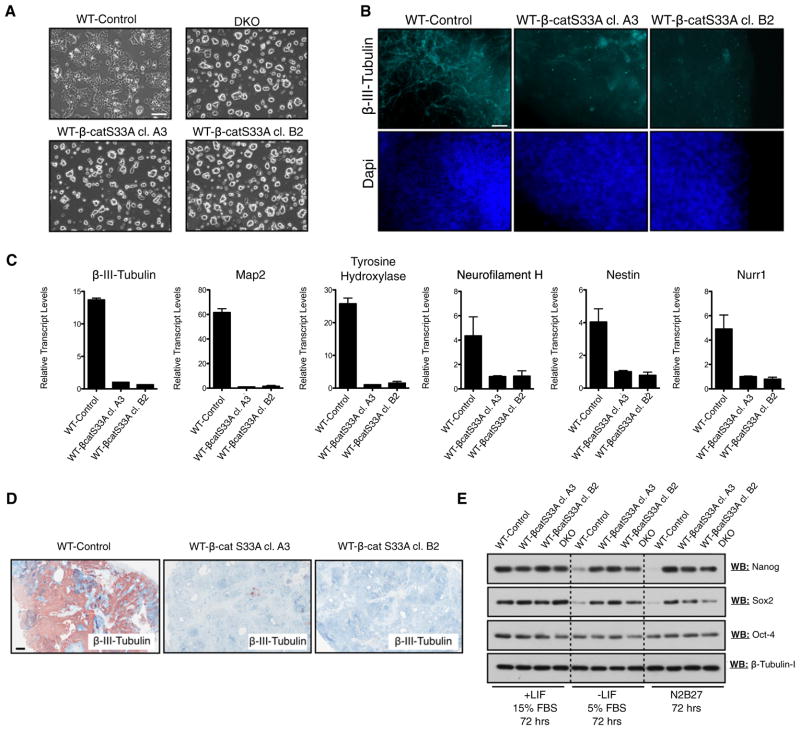
Figure 5. Expression of Stabilized β-Catenin in WT mESCs Inhibits Neural Differentiation and Prolongs the Retention of Pluripotency.
(A) Morphology of WT mESCs expressing stabilized β-catenin (WT-β-catS33A, independent clones A3 and B2), relative to control mESCs stably transfected with empty vector (WT-Control) and DKO mESCs. Scale bar represents 100 μm.
(B) Immunofluorescent staining of day 14 EBs reveals that β-catenin-overexpressing EBs fail to generate neural tissue, as determined by staining for the neural marker β-III-tubulin. Scale bar represents 20 μm.
(C) qRT-PCR analyses of several neural markers in day 14 EBs derived from control and β-catenin-overexpression mESCs demonstrates that β-catenin overexpression inhibits the expression of neural-specific genes. Bars represent n = 3 ± SEM.
(D) Teratomas derived from β-catenin-overexpressing mESCs failed to generate neural tissue in vivo, as determined by immunohistochemical staining for β-III-tubulin. Scale bar represents 500 μm.
(E) β-catenin overexpression prolongs the retention of the pluripotency markers (Oct-4, Nanog, and Sox2) under conditions promoting differentiation (72 hr incubation).
See also Figure S6.
We also carried out short-term differentiation experiments to dissect early loss of pluripotency events. We found that protein levels of Nanog and Sox2 were almost undetectable in WT control cells after 72 hr in EB/differentiation medium or N2B27 basal medium (Figure 5E). By contrast, DKO and WT-β-cateninS33A mESCs strongly retained protein expression of Nanog, Sox2, and Oct-4 after the same treatment (Figure 5E).
A β-Catenin Mutant Lacking Its TCF-Transactivating Domain Inhibits Neural Differentiation and Sustains Pluripotency
We stably overexpressed a C-terminal truncation mutant of β-catenin (β-cateninS33AΔC) that lacks the domain necessary for transactivation of β-catenin/TCF target genes (Figures 6A and 6B; Kolligs et al., 1999; Takao et al., 2007). β-catenin-S33AΔC overexpression activated a TCF reporter 10-fold less efficiently than β-cateninS33A (Figure 6C) and only weakly induced the expression of the β-catenin/TCF target genes Axin2, Brachyury, and Cdx1 (Figure 6D). Strikingly, β-catenin-S33AΔC mESCs appeared morphologically indistinguishable from those expressing β-cateninS33A (Figure 6E).
Figure 6. A β-Catenin Truncation Mutant Lacking Its TCF Transactivation Domain Sustains Pluripotency.
(A) Schematic of full-length β-catenin (β-catS33A; aa 1–781) and the C-terminal deletion mutant (β-catS33AΔC; aa 1–694) used in this study.
(B) Relative protein levels of myc-tagged β-catS33A and β-catS33AΔC transgenes in mESC lines.
(C and D) TCF reporter assays (C) and qRT-PCR analysis of the β-catenin/TCF targets Axin2, Brachyury, and Cdx1 (D) demonstrate that β-catS33AΔC activates β-catenin/TCF signaling ~10-fold less than full-length β-catS33A in mESC lines.
(E) Morphology of WT-Control, β-catS33A, and β-catS33AΔC mESCs grown on gelatin-coated dishes.
(F) Expression of β-catS33AΔC in WT mESCs sustains the retention of pluripotency markers under conditions promoting differentiation (72 hr incubation).
(G) Immunofluorescent staining reveals that stable expression of β-catS33AΔC in WT mESCs inhibits the expression of the neural marker β-III-tubulin and promotes the retention of Oct-4 and Nanog in day 10 EBs.
(H) qRT-PCR analyses of several neural markers in day 10 EBs derived from the indicated control and β-catS33A overexpression mESCs demonstrates that β-catS33AΔC overexpression inhibits the expression of neural-specific genes.
(C, D, and H) Bars represent n = 3 ± SEM.
To address whether β-cateninS33AΔC mESCs exhibited sustained pluripotency and blocked differentiation, we carried out short-term differentiation experiments (Figure 6F), as was done previously for mESCs expressing β-cateninS33A (Figure 5E). After 72 hr of culture in either EB or N2B27 medium, β-catenin-S33AΔC mESCs retained the expression of Oct-4, Sox2, and Nanog, whereas WT control mESCs displayed highly reduced levels of Sox2 and Nanog. Moreover, EBs generated from β-cateninS33AΔC mESCs had sustained expression of Oct-4 and Nanog and lacked β-III-tubulin expression, after 10 days of differentiation, as determined by immunofluorescent staining (Figure 6G) and qRT-PCR analyses (Figure 6H). Collectively, these data further support that transactivation of β-catenin/TCF target genes is not requisite for β-catenin to block differentiation and maintain the pluripotent state of mESCs.
Stabilization of β-Catenin Induces the Formation of β-Catenin/Oct-4 Complexes
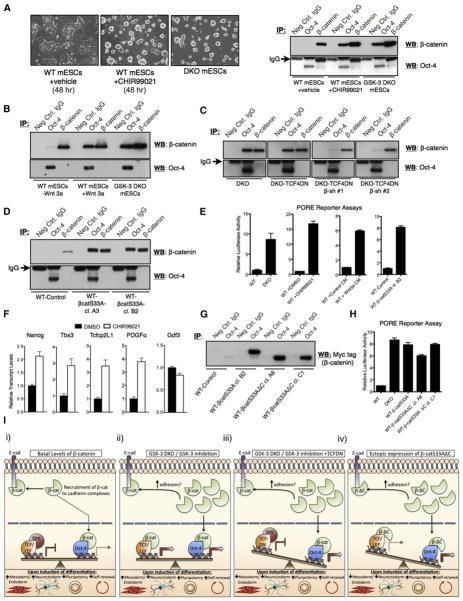
It has been previously suggested that Oct-4 and β-catenin can interact (Takao et al., 2007) and we sought to determine whether such an interaction could underlie, in part, the profound inability of DKO mESCs to differentiate efficiently. We used coimmunoprecipitation experiments to probe β-catenin/Oct-4 interactions under a variety of conditions. Oct-4 immunoprecipitates prepared from lysates of WT mESCs did not contain readily detectable levels of β-catenin, as determined by western blotting (Figure 7A). Strikingly, by using lysates from DKO mESCs, or wild-type mESCs treated for 48 hr with the GSK-3 inhibitor CHIR99021, β-catenin was easily detectable in Oct-4 immunoprecipitates (Figure 7A). Reciprocal coimmunoprecipitations with a β-catenin antibody contained a small amount of detectable Oct-4, which did not change appreciably in lysates obtained from WT, DKO, or CHIR99021-treated WT mESCs (Figure 7A). One possible explanation for this result is that the antibody used for β-catenin immunoprecipitation binds an epitope on β-catenin that precludes its efficient coimmunoprecipitation with Oct-4. CHIR99021 treatment dramatically altered the morphology of WT mESC colonies on gelatin-coated plates, such that they resembled colonies formed by DKO mESCs (Figure 7A).
Figure 7. Stabilized Full-Length or C-Terminal Truncated β-Catenin Forms a Complex with Oct-4 that Is Coincident with Increased Oct-4 Activity.
(A) Morphology of WT mESCs after treatment with CHIR99021 (15 μM for 48 hr; left). Western blots for β-catenin and Oct-4 after control, Oct-4, and β-catenin immunoprecipitates from the indicated cell lines (right).
(B) β-catenin coimmunoprecipitates with Oct-4 from WT mESC lysates after overnight stimulation with Wnt3a-CM.
(C) β-catenin coimmunoprecipitates with Oct-4 from DKO and DKO-TCF4DN mESC lysates, but negligibly from lysates of DKO-TCF4DN mESCs expressing β-catenin-specific shRNAs.
(D) β-catenin coimmunoprecipitates with Oct-4 from lysates derived from WT mESCs expressing stabilized (S33A) β-catenin.
(E) Wnt/β-catenin pathway activation by various means induces the activation of an Oct-4 reporter (PORE).
(F) WT mESCs treated with CHIR99021 for 24 hr displayed a significant increase in the transcript levels for four of five Oct-4 target genes, as assessed by qRT-PCR (p < 0.05, unpaired t test).
(G) Coimmunoprecipitation analysis demonstrates that β-catS33AΔC retains the ability to interact with Oct-4.
(H) Increased activation of an Oct reporter (PORE) in WT mESCs stably expressing both full-length β-catS33A and β-catS33AΔC.
(I) Model of β-catenin action in mESCs. (i) In the absence of Wnt pathway activation, β-catenin is predominantly recruited to cadherin complexes. A small amount of nuclear β-catenin associates with some Oct-4-regulated loci. Under conditions permissive of differentiation, mESCs undergo efficient multilineage differentiation. (ii) Under conditions where the signaling pool of β-catenin is stabilized, nuclear β-catenin coactivates the expression of various genes in concert with either TCF or Oct-4. In this scenario, mESCs exhibit enhanced self-renewal, in part because of β-catenin’s interactions with Oct-4, and mesendoderm-restricted differentiation, resulting from high levels of TCF target gene expression caused by β-catenin-mediated transactivation. (iii) Attenuation of β-catenin/TCF target gene activation, via ectopic expression of a dominant-negative TCF protein (TCFDN), impairs differentiation into all germ layer lineages, including neurectoderm, but enhances retention of pluripotency markers. (iv) mESCs expressing a truncation mutant of stabilized β-catenin (β-ΔC), lacking the C-terminal residues necessary for the efficient transactivation of β-catenin/TCF target genes, exhibit enhanced self-renewal and impeded exit from the pluripotent state. These cells are highly refractory to neurectoderm differentiation.
We examined whether activation of the Wnt/β-catenin signaling pathway with Wnt3a could enhance the formation of Oct-4/β-catenin complexes. A low level of β-catenin/Oct-4 coimmunoprecipitation was detected with WT mESC lysates (Figure 7B). However, β-catenin and Oct-4 formed an easily detectable complex after overnight treatment of WT mESCs with Wnt3a-conditioned medium (CM) (Figure 7B). Stable expression of TCF4DN in DKO mESCs did not attenuate the interaction between Oct-4 and β-catenin, whereas reducing β-catenin levels in DKO-TCF4DN mESCs through shRNA-mediated knockdown reduced the amount of β-catenin complexed with Oct-4 (Figure 7C). Overexpression of stabilized β-catenin (S33A) in wild-type mESCs also resulted in increased coimmunoprecipitation between Oct-4 and β-catenin (Figure 7D). Our ability to detect Oct-4 in β-catenin immunoprecipitates was highly dependent on which β-catenin antibody was used, as is evident from Figure 7A using a mouse monoclonal antibody, versus Figures 7B–7D using a rabbit polyclonal antibody. Oct-4 protein levels in whole-cell lysates from WT and GSK-3-null mESCs are equivalent (Figure S1F). Thus, the increased Oct-4/β-catenin coimmunoprecipitation detected in mESCs with activated Wnt/β-catenin signaling, well exemplified by DKO mESCs, was not due to increased levels of Oct-4 protein.
β-Catenin Enhances Oct Activity
To determine whether β-catenin stabilization through various means could affect Oct activity, we utilized a dual-luciferase-based Oct reporter assay based on six tandem repeats of the palindromic Oct factor recognition element (PORE) (Tomilin et al., 2000). The Oct reporter was efficiently activated by CHIR99021 (~15-fold), Wnt3a-CM (~6-fold), overexpression of β-cateninS33A (~8-fold), or genetic ablation of GSK-3α/β (~8-fold) (Figure 7E). We also observed an increase in transcript levels for the direct Oct-4 target genes Nanog, Tbx3, Tcfcp2L1, and PDGFα (Chen et al., 2008; Mathur et al., 2008; Sharov et al., 2008) upon treatment of wild-type mESCs with CHIR99021, although the expression of another target gene, Gdf3, was unchanged (Figure 7F). Importantly, β-cateninS33AΔC retained the ability to interact with Oct-4 (Figure 7G) and activated an Oct reporter (PORE) with efficiency similar to that of full-length β-catenin (Figure 7H).
DISCUSSION
Taken together, our findings suggest that β-catenin, acting independently of TCF/LEF factors, can reinforce the pluripotent status of mESCs and impair their efficient differentiation. The role of Wnt/β-catenin signaling in ESC pluripotency has been somewhat controversial. Long-term Wnt/β-catenin signaling promotes mesendodermal differentiation of ESCs (Bakre et al., 2007; Lindsley et al., 2006), whereas short-term treatment of ESCs with the GSK-3 inhibitor BIO or Wnt-3a enhances pluripotency of mouse and human ESCs (Sato et al., 2004). Expression of Wnt ligands and receptors has been detected through in situ hybridization in the inner cell mass of mouse blastocysts, and active β-catenin has been detected in the nuclei of mouse blastocysts via immunofluorescence visualization (Kemp et al., 2005; Xie et al., 2008), suggesting that Wnt/β-catenin signaling plays a physiological role in the tissues from which ESCs are derived.
It has been reported that Wnt/β-catenin signaling can synergize with LIF/Stat3 signaling to sustain pluripotency in mESCs (Ogawa et al., 2006). The role of LIF/Stat3 signaling in embryonic stem cell maintenance has been linked to the blockade of mesendodermal differentiation (Ying et al., 2003). Our model, in which β-catenin directs mesendodermal differentiation through TCF-mediated signaling and promotes pluripotency through a TCF-independent mechanism, can help to explain the observed synergism between LIF and Wnt/β-catenin signaling.
Recently, it has been discovered that GSK-3 inhibitors (CHIR99021 or BIO) greatly enhance the ability to establish de novo mESC cell lines from C57BL/6 blastocysts (Gertsenstein et al., 2010; Kiyonari et al., 2010; Sato et al., 2009). Previously, it had been very difficult to obtain germline-competent mESC lines from C57BL/6 mice via standard mESC culture conditions. De novo embryonic stem cell isolation from rats, which has been notoriously difficult, has also been facilitated with GSK-3 inhibitor supplementation (Buehr et al., 2008; Li et al., 2009). Taken together, and given the strong link between GSK-3 inhibition and β-catenin accumulation/activation, these studies strongly support a role for stabilized β-catenin in the positive regulation of pluripotency in mESCs. It is also important to note that the “ground state” of mESC pluripotency requires defined medium, which must be supplemented with an inhibitor of GSK-3 (Ying et al., 2008).
Whereas it is widely assumed that most of the signaling functions of β-catenin rely on its interaction with TCFs, β-catenin can bind several other nuclear proteins to elicit biological effects (Le et al., 2008). These alternative β-catenin signaling modes could explain some of our observations. We provide evidence suggesting that β-catenin’s interaction with the pluripotency regulator Oct-4 at least partially underlies its effects on sustaining pluripotency. Our qRT-PCR data reveal that not all Oct-4 target genes are upregulated under conditions that elevate active β-catenin levels. Thus, only a subset of Oct-4 target genes, including the key pluripotency regulator Nanog, is probably coregulated by β-catenin. The precise mechanism through which β-catenin selectively activates specific Oct-4 targets remains to be identified.
Of note, two recent studies endeavoring to elucidate the Oct-4 protein interaction network in mESCs failed to isolate β-catenin as a high-confidence Oct-4 interactor (Pardo et al., 2010; van den Berg et al., 2010). This is probably because both studies were performed in the absence of Wnt pathway stimulation, conditions under which the majority of cellular β-catenin would be membrane associated and spatially isolated from nuclear Oct-4.
We cannot discount the possibility that β-catenin knockdown may have influenced E-cadherin-mediated cell adhesion in our system (Nelson, 2008). Reduced E-cadherin-mediated intercellular adhesion has recently been reported to reduce Nanog levels and to promote mESC differentiation (Chou et al., 2008). Consistent with this, E-cadherin cell-cell contacts are required for the induction of pluripotency in iPSCs (Chen et al., 2010). Thus, β-catenin’s junctional role in DKO mESCs could partly regulate the ability of the cells to exit the pluripotent state, independent of its nuclear functions.
Through western blot analyses, we were able to detect all four members of the TCF/LEF family in mESCs expressing GSK-3 or lacking it entirely. Based on our TCF reporter assays and TCF target gene analyses, our dominant-negative strategies with TCF1DN and TCF4DN appear to have efficiently blocked β-catenin/TCF/LEF activity contributed by all family members, which is not unexpected given that the DNA binding domains of the TCF family members are nearly identical (Arce et al., 2006). The best-studied TCF/LEF family member in mESCs is TCF3, which acts as a transcriptional repressor of Nanog and possibly other genes involved in mESC self-renewal (Pereira et al., 2006; Tam et al., 2008; Yi et al., 2008). TCF3 is atypical in that its repressive function appears to be retained in the presence of stabilized β-catenin (Pereira et al., 2006). Thus, the highly elevated TCF activity detected in DKO cells is probably mediated by other TCF/LEF family members, suggesting that they are the primary effectors of Wnt/β-catenin signaling in mESCs. Stable expression of dominant-negative LEF1 (analogous to TCF1DN and TCF4DN used in our study) in wild-type mESCs does not appear to have a profound effect on the ability of the mESCs to self-renew in standard serum-containing or fully defined serum-free medium (Ying et al., 2008). Thus, although mESCs harbor all TCF family members, they probably do not play an active role in sustaining pluripotency but may be poised to relay Wnt signals promoting differentiation.
Our experiments revealed that DKO cells are capable of proliferating indefinitely in serum-free medium, although they lose protein expression of Nanog and Sox2, suggesting that they are no longer pluripotent. Intriguingly, the DKO cells can be returned to a pluripotent state by switching back to medium with serum and LIF, suggesting that extrinsic factors are sufficient to “reprogram” them. The loss of Nanog and Sox2 proteins from DKO cells maintained in N2B27 medium was prevented, at least in a subset of cells, by blocking TCF activity through expression of TCF4DN. This suggests that TCF-mediated gene activation plays a prodifferentiation role. Indeed, sustained Wnt-β-catenin signaling has been shown to direct mESCs into mesendodermal progenitors, in part through TCF-mediated activation of brachyury and sox17 transcription (Bakre et al., 2007).
Based on our collective data, we propose a model whereby β-catenin signaling plays a supportive role, in part through its interaction with Oct-4, in the maintenance of ground state pluripotency (Figure 7I). This role of β-catenin does not involve its transactivation of TCF/LEF-mediated transcription, which serves to promote mesendodermal differentiation. Given that β-catenin has been linked to the self-renewal of somatic stem cells and cancer-initiating cells (reviewed in Fodde and Brabletz, 2007), our findings have important implications not only for embryonic stem cell biology but also for cancers arising from dysregulation of Wnt/β-catenin signaling.
EXPERIMENTAL PROCEDURES
Generation of a GSK-3α/β Double-Knockout Flp-in Cell Line
The GSK-3β locus, in a previously generated GSK-3α−/− mESC line, was sequentially targeted to create a DKO line compatible with the Flp-in system (Invitrogen).
Immunofluorescence Analysis
Staining of cells grown on coverslips was performed as previously described (Doble et al., 2007). See Supplemental Experimental Procedures for staining of embryoid bodies.
Coimmunoprecipitation Assays
Coimmunoprecipitation experiments were performed essentially according to the manufacturer’s instructions for Dynabeads Protein G (Invitrogen).
Neural Differentiation Assays
The neural differentiation experiment was performed as described previously (Ying and Smith, 2003).
Self-Renewal Assay
Cells in standard, serum-containing medium were disassociated with Accutase (Innovative Cell Technologies), washed with PBS, and resuspended in serum-free N2B27 medium (see Neural Differentiation Assay section). Serum was not used subsequently in any of the cell maintenance procedures. Cells were plated on gelatin-coated dishes and maintained at 37°C in a humidified incubator with 5% CO2. Cells were passaged when subconfluent with Accutase. The medium was changed every 2–3 days.
Supplementary Material
Acknowledgments
We thank Melissa Coubrough, Shravanti Rampalli, and Mary Jo Smith for their technical assistance and advice. We thank the following investigators who generously provided plasmid reagents: Dr. Randall Moon (TCF reporter), Dr. Bert Vogelstein (TCF4DN), Drs. Marian Waterman and Hans Clevers (TCF1DN), Dr. Hans Schöler (Oct reporter), and Dr. Jun-ichi Miyazaki (pCAGG). Funding was provided by the Canadian Institutes of Health Research to B.W.D. (MOP85057, MOP89359, MOP102490) and K.F.K. (postdoctoral fellowship), the Canada Research Chairs Program (B.W.D.), the Ontario Ministry of Research and Innovation (B.W.D. and K.F.K.), the Ontario Ministry of Training, Colleges, and Universities (D.Y.N.), and the Canada Foundation for Innovation (B.W.D.).
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, and one table and can be found with this article online at doi:10.1016/j.stem.2010.12.010.
References
- Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–7504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- Aubert J, Dunstan H, Chambers I, Smith AG. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol. 2002;20:1240–1245. doi: 10.1038/nbt763. [DOI] [PubMed] [Google Scholar]
- Bakre MM, Hoi A, Mong JCY, Koh YY, Wong KY, Stanton LW. Generation of multipotential mesendodermal progenitors from mouse embryonic stem cells via sustained Wnt pathway activation. J Biol Chem. 2007;282:31703–31712. doi: 10.1074/jbc.M704287200. [DOI] [PubMed] [Google Scholar]
- Bechard M, Dalton S. Subcellular localization of glycogen synthase kinase 3beta controls embryonic stem cell self-renewal. Mol Cell Biol. 2009;29:2092–2104. doi: 10.1128/MCB.01405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone HK, Damiano T, Bartlett S, Perry A, Letchford J, Ripoll YS, Nelson AS, Welham MJ. Involvement of GSK-3 in regulation of murine embryonic stem cell self-renewal revealed by a series of bisindolylmaleimides. Chem Biol. 2009;16:15–27. doi: 10.1016/j.chembiol.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying QL, Smith AG. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Cajánek L, Ribeiro D, Liste I, Parish CL, Bryja V, Arenas E. Wnt/beta-catenin signaling blockade promotes neuronal induction and dopaminergic differentiation in embryonic stem cells. Stem Cells. 2009;27:2917–2927. doi: 10.1002/stem.210. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Chen T, Yuan D, Wei B, Jiang J, Kang J, Ling K, Gu Y, Li J, Xiao L, Pei G. E-cadherin-mediated cell-cell contact is critical for induced pluripotent stem cell generation. Stem Cells. 2010;28:1315–1325. doi: 10.1002/stem.456. [DOI] [PubMed] [Google Scholar]
- Chou YF, Chen HH, Eijpe M, Yabuuchi A, Chenoweth JG, Tesar P, Lu J, McKay RD, Geijsen N. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell. 2008;135:449–461. doi: 10.1016/j.cell.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: Tricks of the trade for a multitasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, Yu X, Cheng L. Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 2005;23:1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Gertsenstein M, Nutter LMJ, Reid T, Pereira M, Stanford WL, Rossant J, Nagy A. Efficient generation of germ line transmitting chimeras from C57BL/6N ES cells by aggregation with outbred host embryos. PLoS ONE. 2010;5:e11260. doi: 10.1371/journal.pone.0011260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegele L, Ingold B, Naumann H, Tabatabai G, Ledermann B, Brandner S. Wnt signalling inhibits neural differentiation of embryonic stem cells by controlling bone morphogenetic protein expression. Mol Cell Neurosci. 2003;24:696–708. doi: 10.1016/s1044-7431(03)00232-x. [DOI] [PubMed] [Google Scholar]
- Kemp C, Willems E, Abdo S, Lambiv L, Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005;233:1064–1075. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- Kielman MF, Rindapää M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S, Taketo MM, Roberts S, Smits R, Fodde R. Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet. 2002;32:594–605. doi: 10.1038/ng1045. [DOI] [PubMed] [Google Scholar]
- Kiyonari H, Kaneko M, Abe SI, Aizawa S. Three inhibitors of FGF receptor, ERK, and GSK3 establishes germline-competent embryonic stem cells of C57BL/6N mouse strain with high efficiency and stability. Genesis. 2010;48:317–327. doi: 10.1002/dvg.20614. [DOI] [PubMed] [Google Scholar]
- Kolligs FT, Hu G, Dang CV, Fearon ER. Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol Cell Biol. 1999;19:5696–5706. doi: 10.1128/mcb.19.8.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le NH, Franken P, Fodde R. Tumour-stroma interactions in colorectal cancer: Converging on beta-catenin activation and cancer stemness. Br J Cancer. 2008;98:1886–1893. doi: 10.1038/sj.bjc.6604401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Lluis F, Pedone E, Pepe S, Cosma MP. Periodic activation of Wnt/beta-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell. 2008;3:493–507. doi: 10.1016/j.stem.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Lochhead PA, Kinstrie R, Sibbet G, Rawjee T, Morrice N, Cleghon V. A chaperone-dependent GSK3beta transitional intermediate mediates activation-loop autophosphorylation. Mol Cell. 2006;24:627–633. doi: 10.1016/j.molcel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He XC. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young RA, Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur D, Danford TW, Boyer LA, Young RA, Gifford DK, Jaenisch R. Analysis of the mouse embryonic stem cell regulatory networks obtained by ChIP-chip and ChIP-PET. Genome Biol. 2008;9:R126. doi: 10.1186/gb-2008-9-8-r126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- Pardo M, Lang B, Yu L, Prosser H, Bradley A, Babu MM, Choudhary J. A. expanded Oct4 interaction network: Implications for stem cell biology, development, and disease. Cell Stem Cell. 2010;6:382–395. doi: 10.1016/j.stem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Yi F, Merrill BJ. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol. 2006;26:7479–7491. doi: 10.1128/MCB.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Sato H, Amagai K, Shimizukawa R, Tamai Y. Stable generation of serum- and feeder-free embryonic stem cell-derived mice with full germline-competency by using a GSK3 specific inhibitor. Genesis. 2009;47:414–422. doi: 10.1002/dvg.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Masui S, Sharova LV, Piao Y, Aiba K, Matoba R, Xin L, Niwa H, Ko MSH. Identification of Pou5f1, Sox2, and Nanog downstream target genes with statistical confidence by applying a novel algorithm to time course microarray and genome-wide chromatin immunoprecipitation data. BMC Genomics. 2008;9:269. doi: 10.1186/1471-2164-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm MP, Bone HK, Beck CG, Bourillot PY, Schreiber V, Damiano T, Nelson A, Savatier P, Welham MJ. Regulation of Nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J Biol Chem. 2007;282:6265–6273. doi: 10.1074/jbc.M610906200. [DOI] [PubMed] [Google Scholar]
- Takao Y, Yokota T, Koide H. Beta-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2007;353:699–705. doi: 10.1016/j.bbrc.2006.12.072. [DOI] [PubMed] [Google Scholar]
- Tam WL, Lim CY, Han J, Zhang J, Ang YS, Ng HH, Yang H, Lim B. T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells. 2008;26:2019–2031. doi: 10.1634/stemcells.2007-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomilin A, Reményi A, Lins K, Bak H, Leidel S, Vriend G, Wilmanns M, Schöler HR. Synergism with the coactivator OBF-1 (OCA-B, BOB-1) is mediated by a specific POU dimer configuration. Cell. 2000;103:853–864. doi: 10.1016/s0092-8674(00)00189-6. [DOI] [PubMed] [Google Scholar]
- van den Berg DLC, Snoek T, Mullin NP, Yates A, Bezstarosti K, Demmers J, Chambers I, Poot RA. A. Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell. 2010;6:369–381. doi: 10.1016/j.stem.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Tranguch S, Jia X, Zhang H, Das SK, Dey SK, Kuo CJ, Wang H. Inactivation of nuclear Wnt-beta-catenin signaling limits blastocyst competency for implantation. Development. 2008;135:717–727. doi: 10.1242/dev.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Pereira L, Merrill BJ. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells. 2008;26:1951–1960. doi: 10.1634/stemcells.2008-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Smith AG. Defined conditions for neural commitment and differentiation. Methods Enzymol. 2003;365:327–341. doi: 10.1016/s0076-6879(03)65023-8. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith AG. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith AG. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.