Abstract
Crows pay close attention to people and can remember specific faces for several years after a single encounter. In mammals, including humans, faces are evaluated by an integrated neural system involving the sensory cortex, limbic system, and striatum. Here we test the hypothesis that birds use a similar system by providing an imaging analysis of an awake, wild animal’s brain as it performs an adaptive, complex cognitive task. We show that in vivo imaging of crow brain activity during exposure to familiar human faces previously associated with either capture (threatening) or caretaking (caring) activated several brain regions that allow birds to discriminate, associate, and remember visual stimuli, including the rostral hyperpallium, nidopallium, mesopallium, and lateral striatum. Perception of threatening faces activated circuitry including amygdalar, thalamic, and brainstem regions, known in humans and other vertebrates to be related to emotion, motivation, and conditioned fear learning. In contrast, perception of caring faces activated motivation and striatal regions. In our experiments and in nature, when perceiving a threatening face, crows froze and fixed their gaze (decreased blink rate), which was associated with activation of brain regions known in birds to regulate perception, attention, fear, and escape behavior. These findings indicate that, similar to humans, crows use sophisticated visual sensory systems to recognize faces and modulate behavioral responses by integrating visual information with expectation and emotion. Our approach has wide applicability and potential to improve our understanding of the neural basis for animal behavior.
Keywords: American crow, cognition, facial recognition, [F-18]fluorodeoxyglucose–PET imaging, learned fear
A variety of species are able to discriminate between human faces (1–3), and this ability appears to be linked to neural integration of perception, emotion, and memory. Brain imaging studies have revealed that humans use a core recognition system in their sensory cortex (the posterior superior temporal sulcus, the inferior occipital gyrus, and the fusiform gyrus) networked with two extended systems that convey the historical (anterior paracingulate, posterior superior temporal sulcus/temporoparietal junction, anterior temporal cortex, precuneus, and posterior cingulate) and emotional (amygdala, insula, and striatum) significance of the person (4). This network of brain regions that perceive and analyze faces is informed by ventral and dorsal visual pathways—the ventral enabling fine discrimination and the dorsal providing rapid, but coarse, emotional assessment (3). Brain mapping investigations on other species capable of human recognition are extremely limited; however, electrophysiological recordings in the visual cortex of domestic sheep and nonhuman primates have indicated the presence of neurons that respond to human facial information (5).
We demonstrated previously that free-ranging American crows (Corvus brachyrhynchos) discriminate among humans based on facial characteristics, but we could only speculate on the neural basis for this behavior (1, 6). Because birds and mammals share some common sensory and motor circuits (7), we hypothesized that recognition of humans by crows might involve a distributed set of interactive brain regions. Here we use a neuroimaging approach to test this hypothesis and investigate the underlying neuronal circuitry activated in response to the sight of familiar people whom we expect crows to recognize as either threatening or not threatening. To accomplish this goal, 12 adult male crows were captured by investigators wearing identical masks, a process which we had previously demonstrated was sufficient for crows to learn the masks as a “threatening” face (1). Over their 4-wk captivity, crows were fed by caregivers wearing an alternative, “caring,” mask (Fig. 1). We used positron emission tomography (PET) combined with administration of [F-18]fluorodeoxyglucose (FDG) to assess the brain activity of wild crows responding adaptively to these faces. During an uptake phase when FDG accumulates in the brain proportional to regional brain activity, we kept the awake crow in a controlled physiologic condition and showed it one of the following: (i) a person wearing the mask that captured it, (ii) a person wearing the mask that fed it, or (iii) an empty room. Once FDG was predominantly fixed in the brain, the subject could be imaged under anesthesia. The resultant images showed brain activity during the uptake phase. Although previously used for human brain mapping research (8), our experiment adapts this technology to map the response of a bird’s brain to a natural, visual, cognitive task and allows us to image a nonhuman animal responding to a human face (9, 10).
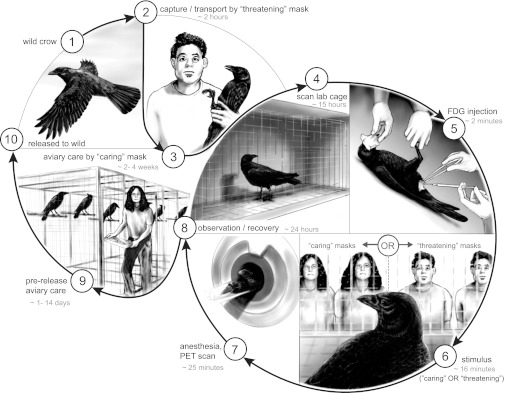
Fig. 1.
Experimental protocol. Different rubber masks, molded from actual people, were used to create threatening and caring faces that single crows responded to during stimulation. During i.p. injection and induction, crow’s faces were covered to prevent them from glimpsing humans.
Results and Discussion
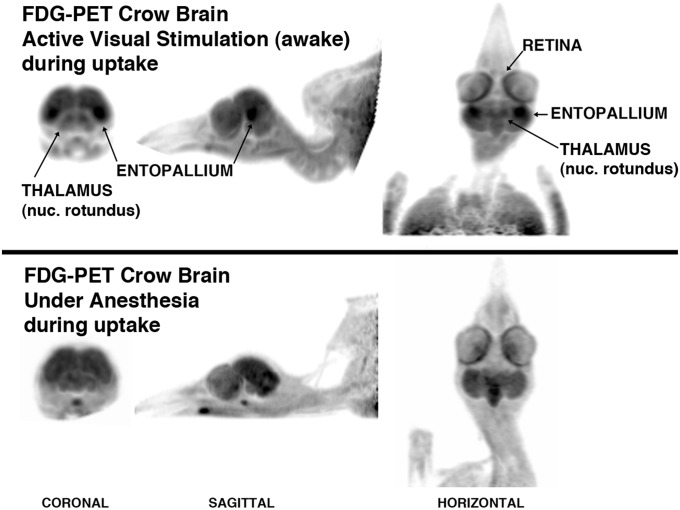
The visual system of birds and primates is supported by perhaps the most advanced and sophisticated neural sensory system known (11). Much of this complexity is evident in the whole-brain responses of crows in our visual discrimination experiments. The pattern of FDG uptake during visual stimulation revealed activation of the crows’ tectofugal visual pathway and a diversity of other forebrain regions (Fig. 2 and Movie S1). Our activation paradigm concentrated neural activity on the central fovea of each retina, stimulating a strong response by the nucleus rotundus of the thalamus and especially its target in the forebrain, the entopallium (Fig. 2). In lateral-eyed birds, such as the crow, this visual network resolves distant, complex, and novel objects, and it is important to visual discrimination tasks; pattern recognition; concept formation that enables categories and individuals to be recognized; and depth perception (12, 13)—all tasks relevant to crows in our experiments.
Fig. 2.
Exemplar of FDG uptake by a crow. (Upper) In this nonquantitative depiction, the FDG-PET brain image has been contrast-enhanced to highlight the fact that the visual network is activated during stimulation following the injection of FDG. (Lower) A similar regional distribution of tracer was not observed in the crow brain that was under anesthesia during the uptake period.
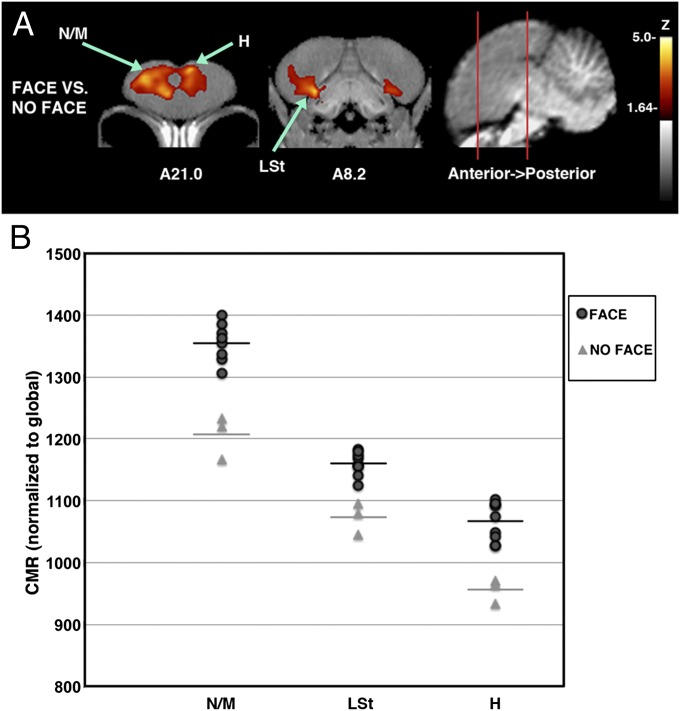
Our results suggest that American crows recognize familiar human faces by evaluating visual sensory information in the context of learned associations. The sight of a familiar human, either threatening or caring, consistently activated the rostral forebrain, including the hyperpallium and a large region in the nidopallium/mesopallium (Fig. 3). Differential activation of the hyperpallium suggests that, in addition to the use of the tectofugal visual pathway, crows used their thalamofugal pathway (directly linking thalamus and hyperpallium) to perceive humans. Activation of the rostral nidopallium, which has strong connections with the entopallium and mesopallium (14) and access to sensory information from both the tectofugal and thalamofugal visual pathways (15), appears important to the recognition of human faces.
Fig. 3.
Differences in brain activation patterns of crows shown familiar human faces vs. no human face. (A) The activation pattern of crows viewing a familiar face (either threatening or caring; n = 9) compared with a group shown an empty room (n = 3) indicated as voxel-wise subtractions converted to group-wise z-scores that have been superimposed onto the MRI template for better anatomical localization. Coronal slices (from anterior to posterior; coordinates refer to Japanese jungle crow atlas; ref. 31) illustrate peak activations (voxels with Z > 1.64 are colored; those with Z > 3.8 are considered significant with associated structures as indicated). (B) Individual values for normalized (global) uptake in each structure that met the threshold for statistical significance on z-score voxel-wise mapping. Horizontal lines indicate group mean. Z values indicated are from peaks in voxel-wise mapping, and P values were derived from one-tailed t tests of volumes of interest (VOIs) centered on peak activation coordinates. Activated structures: N/M: nidopallium/mesopallium, 12.2% increased, Z = 4.25, P = 0.0000142; LSt: lateral striatum, 8.1% increased, Z = 3.99, P = 0.000044; H: hyperpallium, 11.6% increased, Z = 3.80, P = 0.000091.
Crows quickly associated negative (capture) and positive (provision of food) experiences with a human face. In our stimulation protocol, familiar humans were seated and did not behave as expected, neither threatening nor providing care to crows during stimulation. This surprise, or prediction error, may account for the activation of the crows’ lateral striatum (Fig. 3), as it does in a variety of animals, including humans (16). The activated rostral mesopallium, an associative forebrain area that participates in rapid, multimodal learning and is enlarged in crows (17), may be especially important to the association of human faces with reward and punishment. Caudal regions of the nidopallium, mesopallium, and hippocampus—which are important to the recognition of biologically significant conspecifics (18) and executive function (19)—were not consistently activated by the sight of a person.
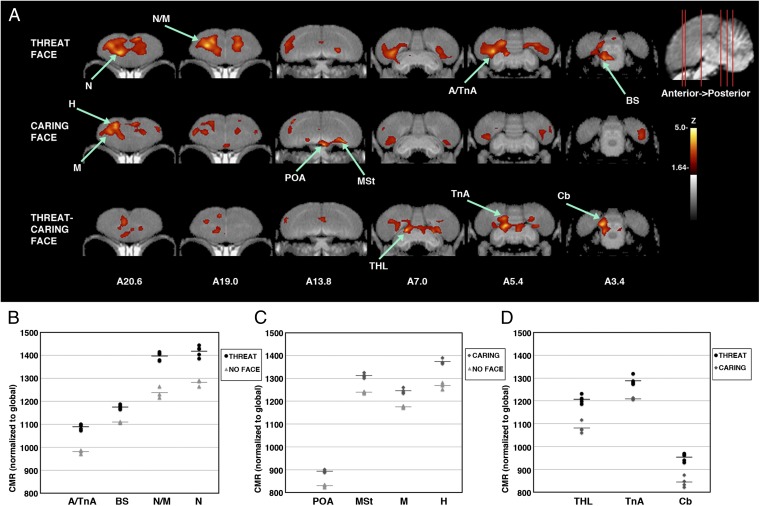
Crows activated different regions when viewing a familiar threatening vs. a familiar caring person. Upon seeing the face of their captor, crows activated their nidopallium/mesopallium, arcopallium including the region containing the nucleus taeniae of the amygdala (TnA), and nuclei in the dorsal thalamus and brainstem (Fig. 4 A–C). This response is commonly elicited by the emotion of fear (20, 21). Definitive identification of nuclei in the thalamus and brainstem was not possible given our resolution and the many small, densely packed nuclei, but likely candidates include those involved in visual pathways (nucleus dorsolateralis posterior thalami and nucleus isthmo-opticus) (22), learning pathways (substantia grisea centralis and locus ceruleus) (23), and emotional motor pathways that control vocal and postural responses to predators (area surrounding tractus occipitomesencephalicus, nucleus reticularis, and trigeminal nucleus) (21, 22). The neural response of crows viewing a human who cared for them daily, albeit in captivity, was distinct from the response of birds to a threatening person. The hyperpallium, mesopallium, preoptic area, and medial striatum were strongly activated (Fig. 4 A and C). These areas are known to be important to associative learning, motivation, and autonomic functions including hunger in vertebrates (17, 24), which suggests that crows perceived the association established between their caretakers and food. In crows, as in other animals including humans, it appears that both the striatum and the amygdala are critical to associative learning, with the former apparently broadly attuned to prediction errors and the latter often attuned to the reliability of threatening cues (16, 20).
Fig. 4.
Brain activation patterns from crows shown human faces that have previously threatened or cared for them. (A) As in Fig. 3, voxel-wise subtractions converted to z-score maps are superimposed to a structural MRI template of the crow brain for better anatomical localization. (Top) The activation pattern of crows viewing a threatening face (n = 5) compared with a group shown an empty room (n = 3). (Middle) The activation pattern of crows shown a caring face (n = 4) compared with the empty room group. (Bottom) Voxel-wise direct comparison of the group shown a threatening face with those shown the caring face (the areas activated by threatening and not caring faces are indicated). Coronal slices (from anterior to posterior; coordinates refer to Japanese jungle crow atlas; ref. 31) illustrate peak activations in one or more group subtractions (voxels with Z > 1.64 are colored; those with Z > 3.8 are considered significant with associated structures as indicated). (B–D) Individual values for normalized (global) uptake in each structure that met the threshold for statistical significance on z-score voxel-wise mapping. Horizontal lines indicate group mean. Z values indicated are from peaks in voxel-wise mapping, and P values were derived from one-tailed t test of VOIs centered on peak activation coordinates. (B) Activated structures for threatening face vs. empty room. A/TnA: arcopallium/nucleus taeniae of the amygdala, 11% increased, Z = 4.42, P = 0.00000425; BS: brainstem nuclei surrounding tractus occipitomesencephalicus including nucleus isthmo-opticus, locus coeruleus, and substantia grisea centralis, 5.9% increased, Z = 4.26, P = 0.0000084; N/M: nidopallium/mesopallium, 12.9% increased, Z = 4.14, P = 0.000020; N: nidopallium, 10.6% increased, Z = 3.93, P = 0.000052. (C) Activated structures for caring face vs. empty room. POA: preoptic area, 7.6% increased, Z = 3.99, P = 0.000011; MSt: medial striatum, 5.9% increased Z = 3.94, P = 0.000052; M: mesopallium, 5.9% increased, Z = 3.87, P = 0.000091; H: hyperpallium, 8.3% increased, Z = 3.81, P = 0.000055. (D) Activated structures for threatening face vs. caring face. THL: dorsal thalamus including dorsolateralis posterior thalami, 11.6% increased, Z = 4.18, P = 0.000019; TnA: nucleus taeniae of the amygdala, 6.5% increased, Z = 4.02, P = 0.000033; Cb: cerebellum, 12.9% increased, Z = 3.99, P = 0.000043.
The neural responses of crows to threatening and caring faces differed in hemispheric bias, or lateralization, as hypothesized for humans. In support of the valence theory of emotional processing (5), crow responses to the caring (positive) face were predominantly left-hemisphere biased, whereas responses to the threatening face were predominantly right-hemisphere biased. These biases were strongest in the limbic and subpallial structures (threatening, amygdalar regions; caring, preoptic area and striatum; Fig. 4 A–C). Lateralization was less consistently right-hemisphere biased in the upper pallium (mesopallium, nidopallium, and hyperpallium) to all familiar faces (Figs. 3A and 4A), perhaps reflecting specialization of the right hemisphere for recognition of familiar social companions (25)—or, alternatively, the tendency of subjects to perch on the right side of the chamber and view the stimulus with their left eye.
Under both experimental conditions and in the field, crows responded to perception of a threatening face with a fixed gaze (quantified as decreased blink rate). In nature, crows blinked less when they looked at threatening people than when they fed in conspecific flocks (Fig. 5B). During our experiments, crows froze, stared at the person, and flashed their conspicuous white nictitating membrane on average 29 times per min (SE = 4.4) upon seeing the threatening face. In contrast, when viewing the caring person, crows stared, blinked 41 times per min (SE = 3.4), and often swallowed (caring, two of four birds; threatening, one of five birds) and even defecated (caring, three of four birds; threatening, two of five birds). Reduced blinking at the sight of the threatening person relative to the caring person was as expected from field observations [Mann–Whitney U = 2.0; P = 0.03 (one-tailed)], but there was substantial variation.
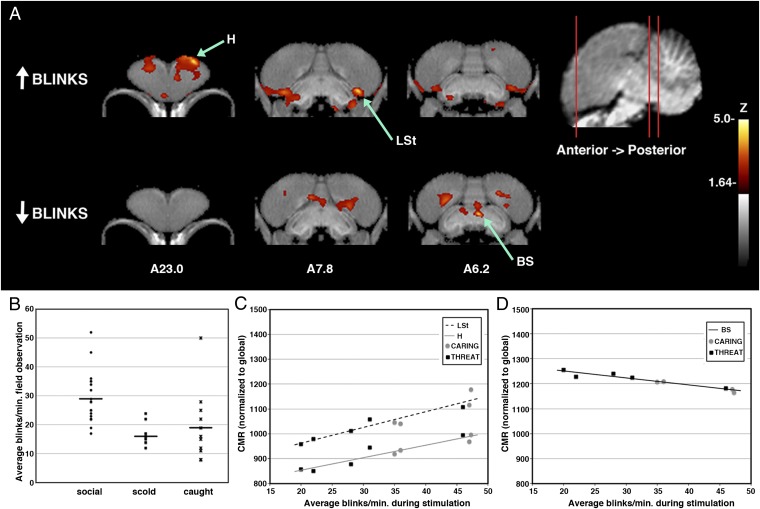
Fig. 5.
Brain activation patterns associated with individual blinking behavior. (A) Voxel-wise linear regression with blink rates in crows shown a familiar face (threatening + neutral; n = 9; recorded during experiments) where the derived correlation coefficients were converted to z-score maps and superimposed to a structural MRI template of the crow brain for better anatomical localization. (Upper) Regions that were correlated positively with blink rates. (Lower) Regions where increased activation was associated with decreased blinking. Coronal slices (from anterior to posterior; coordinates refer to Japanese jungle crow atlas; ref. 31) illustrate peak activation in linear regression (voxels with Z > 1.64 are colored; those with Z > 3.8 are considered significant with associated structures as indicated). (B) Reduced blinking in nature by crows viewing threatening people (social, 19 crows averaged 29 blinks per min, SE = 2.0, while foraging with conspecifics; scold, 11 others averaged 16 blinks per min, SE = 1.1, as they scolded a threatening person; caught, 11 crows averaged 19 blinks per min, SE = 3.6, as we held them during capture; Kruskal–Wallis H(2) = 17.2, P < 0.001). (C) Positive relationships between significant peak activation and blinking. Blinking rates were greatest for birds viewing the caring face (circles). All r > 0.93, P < 0.0001). (D) Negative relationship between significant peak activation and blinking. Blinking rates were least among crows viewing the threatening face (squares ). All r = −0.96, P < 0.00001. H, hyperpallium; LSt, lateral striatum; BS, brainstem nuclei surrounding tractus occipitomesencephalicus, including locus coeruleus.
Individual variation in blink rate was associated with distinct brain activity in the crows that viewed human faces. Increased blinking was correlated with increased activity in the hyperpallium and the lateral striatum near the nidopallium (Fig. 5 A and C). This finding is consistent with processing of visual information by both tectofugal and thalamofugal pathways and integration with expectation from learned associations (15). Reduction in blinking was correlated with increased activity in the brainstem (Fig. 5 A and D). Blinking and associated neural activity varied continuously among individual crows, suggesting that the birds varied in their perception of, or reaction to, risk and rewards associated with human faces.
The greater overall area of activation, including subthreshold pixels in the nidopallium and mesopallium in the group viewing the threatening vs. that viewing the caring face (Fig. 4), may represent heightened arousal and greater involvement of the crow forebrain to resolve negative vs. positive stimuli. Greater consistency in neural responses to the threatening face suggests differential arousal or attention, but the equal number of significant activation peaks between treatments and the similar demeanor of all crows during testing suggest that crows actually used a larger forebrain area to resolve threatening faces compared with caring faces. During stimulation, crows oriented toward the stimulus and did not fly, vocalize, or flick their wings or tails as is typical in agitated birds. This subdued response across treatments was likely due to the confining nature of the small cage we used. All but one bird moved occasionally during the stimulation protocol, either jumping between the cage floor and perch (n = 5) or shifting its head from side to side (n = 7). Most of these birds moved only once. However, a single bird in each treatment frequently moved between perch and floor (threatening, 19 movements; caring, 6 movements), and five birds shifted their heads frequently (threatening: mean = 55.5 shifts during stimulation, SD = 43.1, n = 2; caring: mean = 71.3, SD = 31.7, n = 3).
It is unlikely that differences in brain activity were due to extraneous factors. We eliminated variation in environmental factors known to affect brain activity by holding lighting, noise, time of day, room composition, position of observers, handling, and housing before and after treatment constant across trials. The blinking, swallowing, and defecating behavior of crows discussed above suggested that they perceived the difference in stimuli and that they were attentive to both threatening and caring faces. Documenting the neural responses of birds to a variety of threats and rewards could resolve the relative influence of the stimulus on the extent of forebrain activity.
Our results demonstrate how crows use a diversity of regions from the brainstem to the forebrain to distinguish and adjust their response to individual human faces. This finding is consistent with established and emerging views of how the subpallial limbic network interacts with the integrative forebrain to shape memory-based social behavior in vertebrates (17, 18, 26), including humans (27, 28). The use of cortical sensory processing, the striatum, and the limbic system suggests strong analogy and possible homology between avian and mammalian facial recognition and associative learning systems. Further studies are needed to determine whether the forebrain regions used by crows in our study are functionally analogous to facial recognition regions in humans and other mammals.
Our approach that partners neuroscientists with ecologists could be used to better understand the neural bases of cognition in widely diverse animals (29). Current knowledge comes primarily from a few, well-studied, often domesticated species. Neuroimaging of wild animals to assess whole brain activity during complex behaviors, although presently limited to activities that can be elicited in a temporary captive setting, add substantially to traditional lesion, stimulation, and tracing studies (9). In vivo imaging and voxel-wise analyses of brain responses can be repeated longitudinally in the same animals, and when experiments are completed, the animals can be returned to the wild. Understanding how wild animals integrate perception, memory, and emotion to behave adaptively may allow researchers to generalize important findings across species and sensory modalities, develop strategies to lower stress in captive animals, shape animal actions to reduce human–wildlife conflicts, and engage the public to appreciate the cognitive capacity of other species.
Methods
We captured crows lured from large roosting and foraging groups on three separate occasions using a netlauncher and selected only large (likely male), black-mouthed (adult) birds to bring into captivity (30). None of the birds had previously been captured, and there was no evidence (presence of previously banded birds) that any of the birds resided in study sites that we have previously used for research. Because multiple groups of crows were captured over the course of the study, we counterbalanced the masks used: The mask that was learned as threatening by some crows was learned as caring by others.
After capture, crows were housed for 4 wk in individual 1 × 2 × 2-m cages in accordance with Institutional Animal Care and Use Committee Protocol 3077-01, Washington Scientific Collection Permit 11-359, and US Scientific Collection Permit MB761139-1 (SI Methods). During this time, crows learned a new caring face, the mask worn at all times by the person feeding them and cleaning their cages. The threatening face was the mask used during the initial capture and when they were caught and moved to the PET laboratory. All masks were faces of actual people with neutral expressions; valence was conferred by our behavior, not by facial features.
The evening before imaging, the test subject was moved to a covered 0.5 × 0.5 × 1-m cage in the imaging facility to acclimate, undisturbed, overnight (Fig. 1 and SI Methods). In the morning, crows were blindfolded, removed from the covered cage, administered 1 mCi of FDG via i.p. injection, returned to the cage for a 2-min rest, and then shown the masked investigators in 1-min on/off blocks for 14 min. After the activation protocol, blindfolded crows were anesthetized with 3% isoflurane in oxygen with a flow rate of 300-800 mL/min and imaged. Activation and image acquisition timing were based on our data from dynamic imaging of an anesthetized crow (SI Methods), which indicated much faster brain FDG uptake and washout than is seen in mammals, including mice (31). Each crow was assigned a treatment at random and scanned only once, precluding within-subject analyses but eliminating possible confounding effects of prior experience with injection and anesthesia.
High-resolution FDG-PET images were acquired using a Siemens Inveon PET system for 10 min from 27- to 37-min post-FDG administration (Fig. S1) followed by an ∼13-min attenuation scan and then reconstructed by using 3D ordered subsets expectation maximization/maximum a posteriori to a spatial resolution of 2.5 mm.
We stereotaxically aligned images to a jungle crow (Corvus macrorhynchos) atlas (32), facilitated by structural MRI of one American crow. Nine affine parameters were estimated and applied to images, for consistent stereotactic transformation of scans from the same subject (33, 34). Alignment precision was estimated to be 2–3 mm. After normalizing to global values, significant regional differences in cerebral metabolic rate were determined by using automated voxel-wise subtraction and Z-statistic mapping (NEUROSTAT) (8). Correlation with blink rate was obtained by a voxel-wise linear regression across all subjects exposed to face stimulation (35). We considered Z values that were >3.8 statistically significant, controlling the type I error rate approximately at P = 0.05 for multiple comparisons in a modified Bonferroni correction commonly used in imaging research (36). Volumes-of-interest (VOIs) for structures with a Z score of >3.8 were applied to individual images, and values were compared across groups by using a t test.
We directly observed blinking at close range during experiments and with the aid of 10× binoculars in the field. In the laboratory, we counted each flash of the white nictitating membrane during each minute of stimulation and calculated the average of these as n = 7 counts per subject as the blink rate. We video-recorded laboratory trials, but resolution was insufficient to count blinking. From August 15 to 22, 2011, in the Seattle area or on nearby Vashon Island, we obtained up to five 1-min counts of individual wild crows blinking under three social settings: (i) as we held them during capture, (ii) eating food within a group of conspecifics and heterospecifics, and (iii) scolding a person who was close to the focal crow’s offspring. As in the laboratory, we averaged all blink counts obtained on a single bird to determine the subject’s blink rate. All blink rates were counted by J.M.M. to eliminate possible variation among observers.
Supplementary Material
Acknowledgments
We thank J. DeLap, L. Seckel, B. Shyrock, I. Palmquist, B. Clucas, D. Perkel, T. Shimuzu, and P. Herscovitch for commenting on the manuscript; G. Garwin, B. Lewellen, and H. Cornell for assisting during experiments; and J. DeLap for drafting Fig. 1. This work was supported by the University of Washington Royalty Research Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206109109/-/DCSupplemental.
References
- 1.Marzluff JM, Walls J, Cornell HN, Withey J, Craig DP. Lasting recognition of threatening people by wild American crows. Anim Behav. 2010;79:699–707. [Google Scholar]
- 2.Davis H. Prediction and preparation: Pavlovian implications of research animals discriminating among humans. ILAR J. 2002;43:19–26. doi: 10.1093/ilar.43.1.19. [DOI] [PubMed] [Google Scholar]
- 3.Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia. 2007;45:75–92. doi: 10.1016/j.neuropsychologia.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Tate AJ, Fischer H, Leigh AE, Kendrick KM. Behavioural and neurophysiological evidence for face identity and face emotion processing in animals. Philos Trans R Soc Lond B Biol Sci. 2006;361:2155–2172. doi: 10.1098/rstb.2006.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornell HN, Marzluff JM, Pecoraro S. Social learning spreads knowledge about dangerous humans among American crows. Proc Biol Sci. 2012;279:499–508. doi: 10.1098/rspb.2011.0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler AB, Cotterill RMJ. Mammalian and avian neuroanatomy and the question of consciousness in birds. Biol Bull. 2006;211:106–127. doi: 10.2307/4134586. [DOI] [PubMed] [Google Scholar]
- 8.Jonides J, et al. Spatial working memory in humans as revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 9.Axmacher N, Elger CE, Fell J. The specific contribution of neuroimaging versus neurophysiological data to understanding cognition. Behav Brain Res. 2009;200:1–6. doi: 10.1016/j.bbr.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Van der Linden A, Van Meir V, Boumans T, Poirier C, Balthazart J. MRI in small brains displaying extensive plasticity. Trends Neurosci. 2009;32:257–266. doi: 10.1016/j.tins.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Zeigler HP, Bischof H-J, editors. Vision, Brain, and Behavior in Birds. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 12.Watanabe S. Effect of lesions in the ectostriatum and Wulst on species and individual discrimination in pigeons. Behav Brain Res. 1992;49:197–203. doi: 10.1016/s0166-4328(05)80165-2. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe S, Mayer U, Bischof H-J. Visual Wulst analyses “where” and entopallium analyses “what” in the zebra finch visual system. Behav Brain Res. 2011;222:51–56. doi: 10.1016/j.bbr.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 14.Atoji Y, Wild JM. Afferent and efferent projections of the central caudal nidopallium in the pigeon (Columba livia) J Comp Neurol. 2009;517:350–370. doi: 10.1002/cne.22146. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu T, Bowers AN. Visual circuits of the avian telencephalon: evolutionary implications. Behav Brain Res. 1999;98:183–191. doi: 10.1016/s0166-4328(98)00083-7. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND. Differential roles of human striatum and amygdala in associative learning. Nat Neurosci. 2011;14:1250–1252. doi: 10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehlhorn J, Hunt GR, Gray RD, Rehkämper G, Güntürkün O. Tool-making new caledonian crows have large associative brain areas. Brain Behav Evol. 2010;75:63–70. doi: 10.1159/000295151. [DOI] [PubMed] [Google Scholar]
- 18.Nishizawa K, Izawa E-I, Watanabe S. Neural-activity mapping of memory-based dominance in the crow: Neural networks integrating individual discrimination and social behaviour control. Neuroscience. 2011;197:307–319. doi: 10.1016/j.neuroscience.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Rose J, Colombo M. Neural correlates of executive control in the avian brain. PLoS Biol. 2005;3:e190. doi: 10.1371/journal.pbio.0030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 21.Saint-Dizier H, et al. Subdivisions of the arcopallium/posterior pallial amygdala complex are differentially involved in the control of fear behaviour in the Japanese quail. Brain Res Bull. 2009;79:288–295. doi: 10.1016/j.brainresbull.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Holden AL, Powell TPS. The functional organization of the isthmo-optic nucleus in the pigeon. J Physiol. 1972;223:419–447. doi: 10.1113/jphysiol.1972.sp009856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veenman CL, Karle EJ, Anderson KD, Reiner A. Thalamostriatal projection neurons in birds utilize LANT6 and neurotensin: A light and electron microscopic double-labeling study. J Chem Neuroanat. 1995;9:1–16. doi: 10.1016/0891-0618(95)00057-e. [DOI] [PubMed] [Google Scholar]
- 24.Balthazart J, Ball GF. Topography in the preoptic region: Differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salva OR, Regolin L, Mascalzoni E, Vallortigara G. Cerebral and behavioural asymmetries in animal social recognition. Comparative Cognition & Behavior Reviews. 2012;7:110–138. [Google Scholar]
- 26.Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeDoux JE, Phelps EA. In: Handbook of Emotions. 3rd Ed. Lewis M, Haviland-Jones JM, Barrett LF, editors. New York: Guilford; 2008. pp. 159–179. [Google Scholar]
- 28.Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18:166–172. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinchy M, Schulkin J, Sheriff MJ, McGowan PO, Boonstra R. The neurological ecology of fear: Insights neuroscientists and ecologists have to offer one another. Frontiers in Behavioral Neuroscience. 2011;5:1–6. doi: 10.3389/fnbeh.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dos Anjos L, Debus SJS, Madge SC, Marzluff JM. In: Handbook of Birds of the World. del Joyo J, Eliott A, Christie DA, editors. Barcelona: Lynx Edicions; 2009. pp. 494–641. [Google Scholar]
- 31.Fueger BJ, et al. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med. 2006;47:999–1006. [PubMed] [Google Scholar]
- 32.Izawa E-I, Watanabe S. In: Integration of Comparative Neuroanatomy and Cognition. Hoffman MA, Watanabe S, editors. Tokyo: Keio Univ Press; 2007. pp. 215–273. [Google Scholar]
- 33.Minoshima S, et al. In: Quantification of Brain Function, Tracer Kinetics and Image Analysis in Brain PET. Uemura K, Lassen NA, Jones T, Kanno I, editors. Tokyo: Excerpta Medica; 1993. pp. 409–415. [Google Scholar]
- 34.Cross DJ, et al. Statistical mapping of functional olfactory connections of the rat brain in vivo. Neuroimage. 2004;23:1326–1335. doi: 10.1016/j.neuroimage.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 35.Petrie EC, et al. Preclinical evidence of Alzheimer changes: Convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol. 2009;66:632–637. doi: 10.1001/archneurol.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worsley KJ, Marrett S, Neelin P, Evans AC. In: Quantification of Brain Function Using PET. Myers R, Cunningham V, Bailey D, Jones T, editors. San Diego: Academic; 1995. pp. 327–333. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.