In 1500 B.C., when the author of the Egyptian papyrus Ebers first described an obscure illness that later became known as diabetes mellitus (from Greek diabetes meaning siphon and Latin or Greek mel meaning honey) (1), he could hardly have envisioned the pandemic of metabolic disease that would take place at the end of the 20th century through the beginning of the 21st century. According to the International Diabetes Federation, diabetes affects ∼366 million people worldwide (2). This number is projected to rise to 552 million by 2030 (2). Additionally, there were 280 million people with impaired glucose tolerance in 2011 (2); this number is projected to increase to 398 million in 2030 (2). Thus, by 2030, close to 1 billion people are expected to have abnormal glucose tolerance. The epidemic of diabetes is accompanied by epidemics of obesity and atherosclerotic heart disease and may have at its roots an insulin-resistant state called the metabolic syndrome (3). Why is this epidemic of metabolic disease taking place now? In PNAS, Cai et al. (4) propose one possible explanation.
The contribution of genetics to diabetes is important (5). However, aside from epigenetic effects and the microbiome, it is unlikely that the genetic makeup of humankind has changed significantly in the past 3–4 decades. Therefore, it is generally accepted that the “unholy triumvirate” of obesity, diabetes, and atherosclerosis is probably due to overnutrition, which is spreading in both the developed and developing worlds (6, 7). When they consume more calories than are expended, humans deposit the excess of energy as adipose tissue. This tissue is an active endocrine organ (8), which, if present in excess, contributes to the development of insulin resistance (9). In susceptible individuals with a genetic deficiency of insulin secretion in pancreatic β-cells, this process ultimately leads to diabetes (Fig. 1). The mechanisms by which excessive adipose tissue accumulation, particularly in the intraabdominal space, leads to insulin resistance are still poorly understood but may involve increased production of inflammatory markers [e.g., TNF-α (9–11)] and inadequate production of certain insulin-sensitizing adipokines (e.g., adiponectin) (12). Inflammation is thought to be a key underlying component of these processes (9–11). What triggers inflammation?
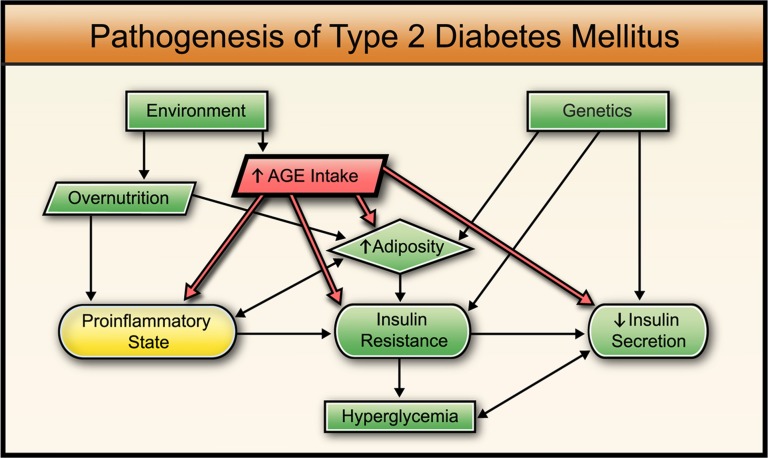
Fig. 1.
Traditional view (green) of the pathogenesis of diabetes mellitus describes a combination of genetic and environmental factors leading to obesity, insulin resistance, reduced insulin secretion, and hyperglycemia. A more recent approach includes a proinflammatory state contributing to insulin resistance (yellow). The current hypothesis also includes the effects of AGEs (red), which induce a proinflammatory state, increase adiposity, induce insulin resistance, and inhibit insulin secretion (details and references are provided in main text).
In a series of elegant and convincing experiments in mice, Cai et al. (4) now may have found a possible “missing link.” They demonstrate that advanced glycation end products (AGEs) are responsible for inducing an inflammatory state, which, in turn, leads to the development of insulin resistance and hyperglycemia.
AGEs are formed in tissues and circulation through nonenzymatic glycation of proteins and lipids, and they are also present in abundance in the modern diet (13). The most familiar pre-AGE is glycosylated hemoglobin, a marker widely used for diagnosing and assessing progress in the treatment of diabetes (14). Glycosylated hemoglobin is a predictor of devastating micro- and macrovascular complications of diabetes (retinopathy, leading to blindness; nephropathy, leading to the loss of renal function; and vasculopathy and neuropathy, contributing to the amputation of extremities) (15, 16). New tests measuring AGEs in circulation, including those absorbed from the diet, have been developed and are poised to enter clinical practice (13). These may offer greater advantages than glycosylated hemoglobin toward early detection and treatment of diabetes as well as prediabetes.
Cai et al. (4) fed a diet enriched in methyl-glyoxal (MG) derivatives (highly reactive glycating agents) to mice that were maintained on isocaloric diets with the control animals by pair-feeding, such that confounding by overnutrition was avoided. After several generations, MG-fed mice developed increased adiposity and insulin resistance, manifested by increased glucose and insulin levels on intravenous glucose tolerance tests and abnormalities in the insulin-receptor signaling cascade (tyrosine-phosphorylated insulin receptor, IRS-1, IRS-2, and Akt). Insulin-stimulated 2-deoxyglucose uptake by adipose tissue and skeletal muscle was reduced in MG-fed mice. Macrophages and adipocytes shifted to a proinflammatory phenotype, manifested by elevated levels of TNF-α, CD11c, and MCP-1 in adipocytes, stromal vascular cells, and peritoneal macrophages. These changes developed in the third generation of MG-fed mice at a much younger age than in the control mice. In this way, a metabolic picture that confers the high risk for diabetes and atherosclerotic disease in humans was duplicated.
If confirmed in human studies, this contribution to our understanding of the pathogenesis of metabolic disease would have global implications, because it may help establish a novel approach to preventing type 2 diabetes, an approach that focuses on AGEs. Evidence from The Diabetes Prevention Program has demonstrated that the risk for diabetes in high-risk individuals can be reduced by lifestyle changes (weight reduction through diet and exercise) (17). However, in practice, these measures are difficult to carry out consistently. Circulating AGE levels can be reduced without altering the caloric content of meals simply by changing the way the food is prepared, that is, by using less heat and more water (e.g., stewing instead of frying) (18). However, even these relatively simple dietary changes may be difficult to introduce into large populations effectively because they involve a change in behavior. Additional measures may need to be applied at the level of the food industry, tied in with a multilevel educational campaign akin to that used against smoking. Medications that can reduce AGE absorption from food need to be developed, and initial steps in this direction have already been taken (13, 19). Finally, Cai et al. (4) demonstrate that a system involving AGE receptor-1 (which clears AGEs from circulation) and a survival factor, SIRT-1 (which inhibits inflammation and enhances production of adiponectin and fat mobilization), was greatly suppressed in insulin’s target tissues (white adipose tissue, skeletal muscle, and liver) of MG-fed mice. Discovering the ways to reactivate this system may help reduce the risk of metabolic disease.
Cai et al. describe an AGE-centered system that contributes to the increased risk for developing insulin resistance and diabetes in experimental animals.
Of course, many unanswered questions remain. The one that intrigues me the most stems from the finding of increased adiposity in MG-treated mice compared with control animals. If the diets were isocaloric, how could this excess state of stored energy occur in the experimental group? Cai et al. (4) point to the marked suppression of SIRT-1 in adipose tissue and propose that excess AGEs may have impaired lipolysis. It is conceivable that glycoxidized fat (AGE fat), present in large excess in adipose tissue, is less energy-active. Alternatively, could AGEs have affected mitochondrial function in the brown fat, thereby reducing nonshivering thermogenesis (20, 21)? Could a high-AGE diet affect the level of physical activity (remember how you feel after an AGE-replete Thanksgiving dinner)? Another unanswered question has to do with metabolic changes peaking in the third generation. Are epigenetic influences at play in this process? Clearly, additional work needs to be done to answer these and many other questions to understand fully the AGE-based approach to preventing metabolic disease.
In summary, Cai et al. (4) describe an AGE-centered system that contributes to the increased risk for developing insulin resistance and diabetes in experimental animals (Fig. 1). Taken together with previous studies that demonstrated a negative effect of AGEs on insulin secretion (13), these findings may lead to the development of new approaches to preventing and treating diabetes and related disorders. Perhaps most importantly, the work by Cai et al. (4) will allow investigators to look in new ways at the “old” disease that has reached epidemic proportions—to look beyond overnutrition.
Acknowledgments
I thank Drs. Md Usman Ali and Julie Islam for help with literature review; Courtney McKenna and Shen Wei for help with art work; Dr. Barnett Zumoff and Kathy Baer for editorial assistance; and Marilyn Jefferson for expert secretarial work.
Footnotes
The author declares no conflict of interest.
See companion article on page 15888.
References
- 1.Zajac J, Shrestha A, Patel P, Poretsky L. The main events in the history of diabetes mellitus. In: Poretsky L, editor. Principles of Diabetes Mellitus. New York: Springer; 2010. pp. 3–16. [Google Scholar]
- 2.International Diabetes Federation (2011) IDF Diabetes Atlas. Brussels, Belgium: International Diabetes Federation. 5th ed. Available at www.idf.org/diabetesatlas.
- 3.Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: A culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2276–2283. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- 4.Cai W, et al. 2012. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci USA 109:15888–15893.
- 5.Manning AK, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44:659–669. doi: 10.1038/ng.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135(1):61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 9.Harford KA, Reynolds CM, McGillicuddy FC, Roche HM. Fats, inflammation and insulin resistance: Insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc Nutr Soc. 2011;70:408–417. doi: 10.1017/S0029665111000565. [DOI] [PubMed] [Google Scholar]
- 10.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 12.Turer AT, Scherer PE. Adiponectin: Mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 13.Vlassara H, Striker GE. AGE restriction in diabetes mellitus: A paradigm shift. Nat Rev Endocrinol. 2011;7:526–539. doi: 10.1038/nrendo.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inzucchi SE. Diagnosis of diabetes. N Engl J Med. 2012;367:542–550. doi: 10.1056/NEJMcp1103643. [DOI] [PubMed] [Google Scholar]
- 15.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 16.Turner RC. The UK Prospective Diabetes Study, a review. Diabetes Care. 1998;21(Suppl 3):C35–C38. doi: 10.2337/diacare.21.3.c35. [DOI] [PubMed] [Google Scholar]
- 17.Knowler WC, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vlassara H, Woodruff S, Uribarri J, Striker GE. 2011. The AGE-Less Way. Amazon.com.
- 19.Piperi C, Adamopoulos C, Dalagiorgou G, Diamanti-Kandarakis E, Papavassiliou AG. Crosstalk between advanced glycation and endoplasmic reticulum stress: Emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab. 2012;97(7):1–12. doi: 10.1210/jc.2011-3408. [DOI] [PubMed] [Google Scholar]
- 20.Silva JE. Physiological importance and control of non-shivering facultative thermogenesis. Front Biosci (Schol Ed) 2011;3:352–371. doi: 10.2741/s156. [DOI] [PubMed] [Google Scholar]
- 21.Tews D, Wabitsch M. Renaissance of brown adipose tissue. Horm Res Paediatr. 2011;75:231–239. doi: 10.1159/000324806. [DOI] [PubMed] [Google Scholar]