Abstract
The epidemics of insulin resistance (IR) and type 2 diabetes (T2D) affect the first world as well as less-developed countries, and now affect children as well. Persistently elevated oxidative stress and inflammation (OS/Infl) precede these polygenic conditions. A hallmark of contemporary lifestyle is a preference for thermally processed nutrients, replete with pro-OS/Infl advanced glycation endproducts (AGEs), which enhance appetite and cause overnutrition. We propose that chronic ingestion of oral AGEs promotes IR and T2D. The mechanism(s) involved in these findings were assessed in four generations of C57BL6 mice fed isocaloric diets with or without AGEs [synthetic methyl-glyoxal-derivatives (MG+)]. F3/MG+ mice manifested increased adiposity and premature IR, marked by severe deficiency of anti-AGE advanced glycation receptor 1 (AGER1) and of survival factor sirtuin 1 (SIRT1) in white adipose tissue (WAT), skeletal muscle, and liver. Impaired 2-deoxy-glucose uptake was associated with marked changes in insulin receptor (InsR), IRS-1, IRS-2, Akt activation, and a macrophage and adipocyte shift to a pro-OS/inflammatory (M1) phenotype. These features were absent in F3/MG− mice. MG stimulation of 3T3-L1 adipocytes led to suppressed AGER1 and SIRT1, and altered InsR, IRS-1, IRS-2 phosphorylation, and nuclear factor kappa-light chain enhancer of activated B cells (Nf-κB) p65 acetylation. Gene modulation revealed these effects to be coregulated by AGER1 and SIRT1. Thus, prolonged oral exposure to MG-AGEs can deplete host-defenses AGER1 and SIRT1, raise basal OS/Infl, and increase susceptibility to dysmetabolic IR. Because exposure to AGEs can be decreased, these insights provide an important framework for alleviating a major lifestyle-linked disease epidemic.
Keywords: glycotoxins, obesity, aging
The current epidemic of type 2 diabetes (T2D) is preceded by insulin resistance (IR), which is linked to increased oxidant stress (OS), inflammation (Infl), and overnutrition (1–3). One source of OS/Infl is industrially processed food, which is increasingly a large part of the modern diet and delivers an excess of thermally accelerated protein- and lipid-associated prooxidant advanced glycation and lipoxidation endproducts (AGEs and ALEs, respectively) (4–7). Although thermal treatment improves food safety, flavorful AGEs may spur overnutrition, which contributes to the metabolic syndrome and diabetes. AGEs, previously best known for their role in diabetes- and age-related complications, have recently been implicated in IR, β-cell damage, and diabetes (T2D and T2D) (8–10).
The effects of AGEs are normally countered by AGE degrading systems and anti-AGE receptors, i.e., AGER1 (11–13). The in vivo protective functions of AGER1 were recently linked to SIRT1, an NAD+-dependent deacetylase and survival factor, based on data showing that AGER1-tg mice are protected against fat-diet–induced vascular disease and abnormal glucose homeostasis (14–16). SIRT1 represses inflammatory signals, enhances adiponectin production, and improves insulin efficiency and fat mobilization (16, 17). Because AGER1 and SIRT1 were independently found to be suppressed in chronic OS conditions such as diabetes, aging, or AGE overload (15, 18–21), and both were enhanced after AGE restriction in T2D patients (18), we hypothesized that AGER1 and SIRT1 could be coregulated by orally absorbed prooxidant AGEs. The mechanism(s) by which specific AGEs contribute to chronic disease is only partly elucidated, owing to the heterogeneity of AGEs (10, 20).
Methyl-glyoxal (MG) is a highly reactive α-dicarbonyl and is among the best-known glycation agents linked to diabetic or age-related cell injury (23–26). Common MG derivatives such as methylglyoxal-imidazolone-H1 (MG-H1) increase OS in vitro/in vivo (24–28) and are elevated in serum of humans with T1D and T2D (23, 29). MG derivatives are also elevated in “normal” individuals who consume AGE-rich diets (30). MG derivatives can be reduced by following a diet made low in AGEs by simply limiting thermal exposure (18, 20, 23). Lowering AGE intake prevents several high-OS conditions in mice and humans (8, 18). Evidence that MG-AGEs in food are pathogenic was first obtained from a study in which mice fed a low-AGE, nutritionally normal diet developed systemic levels of AGEs and OS similar to those seen in diabetes or aging (27).
The current report extends these studies and demonstrates that MG-AGEs, common in thermally treated foods can profoundly alter the organism’s inflammation/insulin axis, in the absence of genetic susceptibility or excess intake of nutrients. Namely, sustained exposure of multiple generations of wild-type C57BL6 mice to a nonheated, isocaloric diet, where the content of AGEs was increased by a single synthetic MG-AGE (MG+), results in severe AGER1 and SIRT1 deficiency, an adipocyte and macrophage shift to a pro-OS/inflammatory phenotype, adiposity, and early insulin resistance.
Results
MG+ Diet Promotes Weight Gain, Adiposity, and Metabolic Changes in WT Mice.
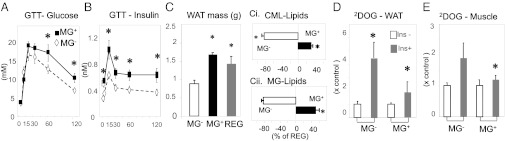
During multigenerational mouse studies, we noted no differences in food intake, growth characteristics, or fecundity in founder (Fo), and F1-F3 of either the MG+ or MG− group. Gradual increases in both serum CML and MG-H1-like derivatives were observed between Fo and F3 of 18 mo MG+ mice; whereas, the opposite trend was noted in Fo-F3 of MG− mice (Fig. S1 B and C). Together with increased plasma 8-isoprostanes and vascular cell adhesion protein 1 (VCAM-1), and the lower plasma adiponectin levels in MG+ and Reg-diet fed mice (Table 1, Fig. S1 D and E), these data indicated that systemic OS was progressively and prematurely elevated in MG+ mice in contrast to MG− mice. Also, 18 mo F3/MG+ mice had higher fasting insulin and leptin levels than the F3/MG− group (Table 1), and displayed abnormal glucose and insulin responses to i.p. glucose tolerance test (IGTT; Fig. 1 C and D), whereas these remained normal in MG− mice.
Table 1.
Characteristics of wild-type C57BL6, F3 mice
| Groups | MG− | MG+ | REG |
| No./group | 12 (6F/6M) | 12 (6F/6M) | 9 (5F/4M) |
| Body weight (g) | 32.1 ± 0.74*,† | 37.1 ± 1.2 | 37.6 ± 1.7 |
| Food intake (g/day) | 4.7 ± 1.4 | 4.9 ± 0.7 | 5.0 ± 1.2 |
| Food CML intake (U/day) | 16.0 × 104*,† | 24.4 × 104 | 30.0 × 104 |
| Food MG intake (nmol/day) | 0.66 × 104*,† | 1.6 × 104 | 1.3 × 104 |
| Serum CML (sCML, U/mL) | 28 ± 2.3 *,† | 43.1 ± 1.5 | 40.8 ± 4.2 |
| Serum MG (sMG, nmol/mL) | 0.84 ± 0.1†,‡ | 1.66 ± 0.17 | 1.43 ± 0.09 |
| Fasting blood glucose (mg/dL) | 67.3 ± 5.1 | 68.1 ± 5 | 81.7 ± 3.4 |
| Fasting insulin (nmol/L) | 0.27 ± 0.01*,† | 0.51 ± 0.03 | 0.47 ± 0.04 |
| Adiponectin (μg/mL) | 12.0 ± 0.4‡ | 8.42 ± 0.58 | 8.0 ± 1.1 |
| Leptin (nm/mL) | 12.8 ± 0.23*,‡ | 17.3 ± 0.51 | 17.0 ± 0.9 |
| Adipo/leptin ratio | 0.94 ± 0.17‡ | 0.49 ± 0.11 | 0.47 ± 0.12 |
| White fat CML (U/g) | 0.9 ± 0.1 × 104*,† | 4.0 ± 0.2 × 104 | 3.3 ± 0.2 × 104 |
| White fat MG (nmol/g) | 44.5 ± 5.1*,† | 190.8 ± 9.8 | 143 ± 9 |
[MG−] denotes mice on non-thermally-treated low-AGE diet; [MG+] denotes mice on a MG-supplemented low-AGE diet, [REG] denotes control mice on standard (thermally treated) diet. Data are means ± SEM.
*P < 0.01 between MG− and MG+ mice;
†P < 0.01 between MG− and REG mice, and
‡P < 0.05.
Fig. 1.
MG+ diet leads to altered glucose homeostasis, adiposity, and AGE-lipids in adult MG+ F3 C57BL6 mice. (A) Blood glucose and (B) insulin levels, after a glucose tolerance test (GTT) (n = 6/group); (C) WAT mass, (Ci) lipid-CML and (Cii) Lipid-MG derivatives (n = 10/group); (D) glucose uptake by WAT adipocytes; and (E) by skeletal muscle from 18 mo MG+ F3 and MG− mice; n = 5–7/group. Data are shown as means ± SEM *P < 0.05 vs. MG− fed mice.
Despite isocaloric pair feeding, the F3/MG+ and Reg mouse body weights were higher than in MG− mice (Table 1). Moreover, the WAT weight in MG+ and Reg mice was nearly twofold greater than in MG− mice (Fig. 1C). In addition, white adipose tissue (WAT) fat-associated AGEs [Nε-carboxymethyllysine (CML) and MG] in MG+ were three- to fourfold higher than in MG− mice (Table 1, Fig. 1 Ci and Cii).
Furthermore, insulin-stimulated 2-deoxyglucose uptake by WAT and skeletal muscle was attenuated in MG+ F3 mice (by 55% and 40%, respectively) compared with MG− F3 mice (Fig.1 D and E) (27, 28).
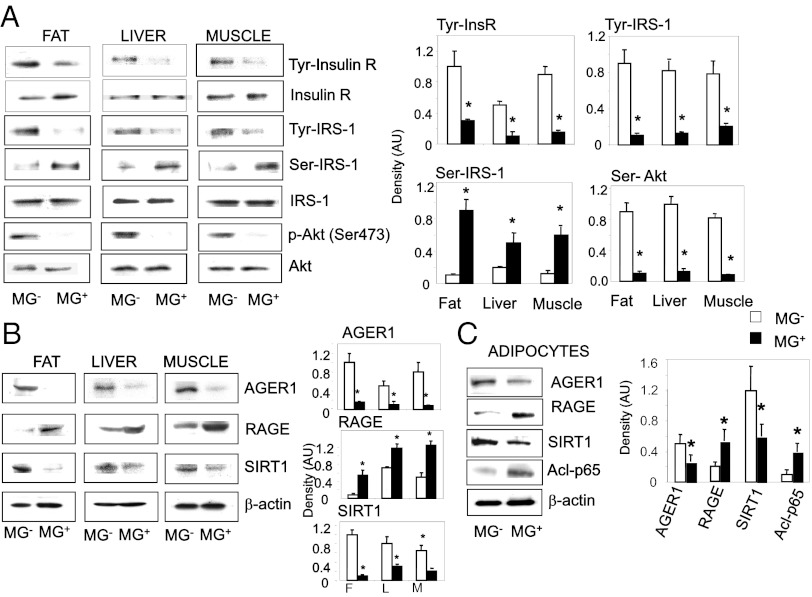
Evaluation of isolated adipocytes, stromal vascular cells from WAT, as well as in peritoneal macrophages, revealed an enhanced proinflammatory pattern in all cell types from MG+ mice relative to MG− (Fig. S2). Namely, mRNA levels of TNF-α, CD11c, and MCP-1 were higher, but those of IL-10, CD206, and PPAR-γ were reduced. Furthermore, the mRNA levels of SIRT1 and AGER1 in macrophages and WAT adipocytes were also reduced in MG+ mice (Fig. S2) Key insulin-signaling targets such as tyr-phosphorylated insulin receptor (InsR), insulin receptor substrate 1 (IRS1), and Ser-473-phosphorylated Akt levels were markedly suppressed in all three tissues from MG+, relative to MG− mice in insulin-sensitive tissues; including fat, liver, and skeletal muscle of MG+ mice. In contrast, Ser/threonine phosphorylated IRS1, a negative regulator of insulin signaling, was significantly enhanced, compared with MG− mice (Fig. 2A), suggesting that insulin action was impaired in MG+ mice.
Fig. 2.
(A) MG+ diet alters insulin receptor (InsR), IRS-1, and Akt activity levels in insulin-sensitive tissues. After an insulin injection (30 min), liver, fat (WAT), and skeletal muscle tissue lysates from 18 mo MG+ and MG− F3 mice were immunoprecipitated (IP) with anti-InsR, anti-IRS1, or anti-Akt antibodies and immunoblotted (IB) with anti-phospho-tyrosine, anti-phospho(Ser307)-IRS-1, or anti-phospho(Ser473)-Akt antibodies. (B) MG+ diet suppresses AGER1 and Sirt1, and enhances RAGE and acetylated Nf-KB p65 levels in whole WAT, liver, and skeletal muscle, and (C) in primary WAT adipocytes. Whole tissue or primary adipocyte lysates from MG+ and MG− mice (n = 3–5/group) were subjected to Western blotting, by the respective antibodies. Densitometric data (means ± SD, n = 3 experiments) indicates the ratios of phosphorylated to total protein (A) or protein to β-actin (B and C), *P < 0.05 vs. MG− mice.
SIRT1 protein levels were also suppressed in fat, liver, and muscle from F3/MG+ mice, but not from F3/MG− mice (Fig. 2B). Tissues from F3/MG+ mice also had reduced AGER1, and enhanced RAGE protein levels, compared with F3/MG− tissues (Fig. 2B), consistent with findings in older mice fed Reg diet (22). Furthermore, levels of AGER1 and SIRT1 were suppressed in primary adipocytes isolated from MG+ WAT, and NF-κB acetyl-p65 and RAGE levels were increased, compared with adipocytes from MG− mice (Fig. 2C), suggesting the presence of a proinflammatory state in MG+ WAT adipocytes.
Adipocytes Require AGER1 for both Metabolic and Inflammatory Insulin Actions During Exposure to AGEs.
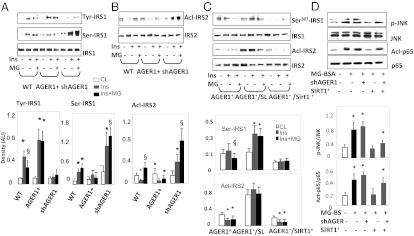
Differentiated 3T3-L1 adipocytes with either overexpressed (AGER1+) or silenced AGER1 (shAGER1) were studied to assess AGER1’s effects on functional targets of insulin. Insulin-stimulated Tyr-phospho-IRS1 in AGER1+ cells was enhanced after 72 h of exposure to MG-AGEs but both ser-307-phospho-IRS1 and acetyl-IRS2 were reduced, compared with WT cells (Fig. 3 A and B). AGER1 silencing led to a decrease in Tyr-phospho-IRS1 but an increase in ser-307-phospho-IRS1 and in acetylated IRS2 (Fig. 3A). These effects were consistent with the data from WAT adipocytes (Fig. 2C). They indicate that, under external MG-AGEs stimulation, AGER1 can support the metabolic, as well as counter the inflammatory actions of insulin, both which are regulated by SIRT1. There was a dose-dependent suppression of SIRT1 protein, and AGER1 protein expression in WT and shAGER1 cells, after prolonged exposure to MG, but this pattern was absent in AGER1+ cells (Fig. S3), suggesting that SIRT1 expression and function are partly AGER1 dependent.
Fig. 3.
AGER1 is required for insulin metabolic and antiinflammatory activity in differentiated 3T3-L1 adipocytes exposed to MG-AGEs. WT 3T3-L1 cells transduced (AGER1+) or silenced for AGER1 (shAGER1) were preincubated with (+) or without (-) MG-BSA (60 μg/mL) for 72 h, and stimulated with insulin (100 nM) for 10 min. Cell lysates were subjected to (A) IP with anti-IRS-1 or IB with anti-phospho-tyrosine and anti-Ser307 IRS-1 antibodies, or (B) IP with anti-IRS-2 antibodies and IB with anti-acetylated-lysine antibody (Acl-IRS-2). Densitometry data (means ± SD, n = 3) show the ratio of phosphoryl- or acetyl-protein to total protein. *P < 0.05 vs. control; §, P < 0.01 vs. insulin-treated. (C) Adipocyte AGER1 cooperatively interacts with SIRT1 under MG-AGE stimulation. 3T3-L1 cells AGER1-transduced (AGER1+), cotransfected with SIRT1 (AGER1+/Sirt1+) or pretreated with SIRT1 inhibitor Sirtinol (10 μM) (AGER1+/+SL) were preincubated with (+) or without (-) MG-BSA (60 μg/mL) for 72 h, and stimulated with insulin (100 nM) for 10 min. Cell lysates were subjected to IP with anti-IRS-1, IB with anti- Ser307-IRS-1 antibody or IP with anti-IRS-2 and IB with anti-acetylated-lysine antibodies. *P < 0.05 vs. insulin. (D) The effects of MG-AGEs are mediated on adipocytes are Phospho-JNK and NF-κB p65 acetylation (Acl-p65) were determined in the same 3T3-L1 cells and prepared as described in C by Western blotting. For phospho-JNK, cells were exposed to MG-BSA (60 μg/mL) for 2 h. For NF-κB p65 acetylation, cells were exposed to MG-BSA (60 μg/mL) for 72 h. Density plots show the ratios (means ± SD) of phosphorylated or acetylated to total protein. *P < 0.05 vs. control.
AGER1 and SIRT1 Coregulate Adipocyte Insulin Signaling During MG-AGE Exposure.
Insulin signaling was assessed in AGER1+ 3T3-L1 cells, which either coexpressed SIRT1 (AGER1+/SIRT1+) or in which SIRT1 expression was suppressed. AGER1+/SIRT1+ coexpressing cells had increased IRS1 tyr-phosphorylation, but not IRS1 ser-phosphorylation or IRS2 acetylation (Fig. 3C). However, when SIRT1 activity in AGER1+ cells was diminished by Sirtinol (a SIRT1 inhibitor, 10 μM), the MG effects were not inhibited (Fig. 3A). Further, SIRT1+ transduction was not sufficient to functionally block MG-enhanced phospho-JNK and NF-κB acetyl-p65 after AGER1 silencing (Fig. 3D). Taken together, these findings pointed to a reciprocal cooperative relationship between SIRT1 and AGER1 under MG stimulation.
Adipocyte AGER1 Positively Regulates SIRT1 Expression and NAMPT Activity via ROS Suppression.
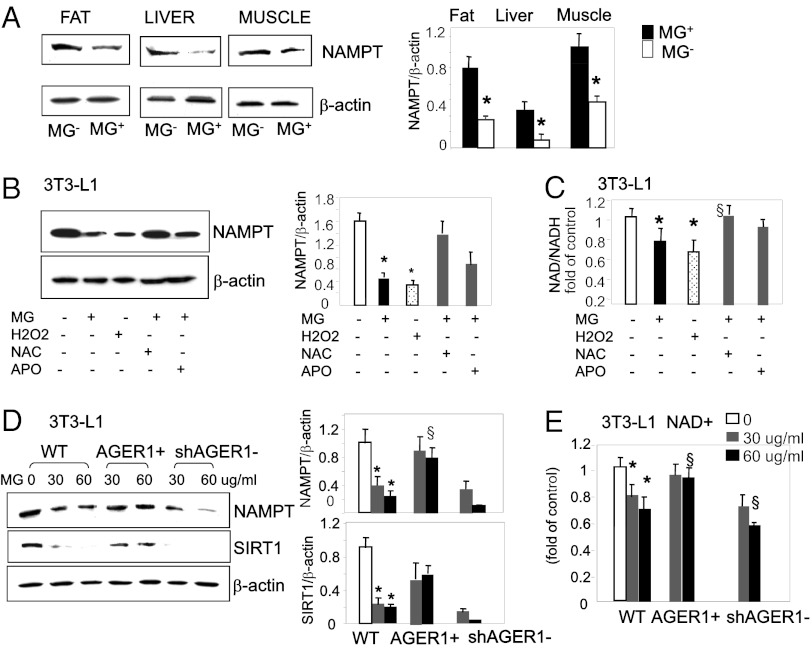
In assessing the mechanism of SIRT1 alteration, we noted that protein levels of nicotinamide phosphoribosyltransferase (NAMPT), a key enzyme regulating the NAD-dependent deacetylase SIRT1, were significantly reduced in WAT, liver, and skeletal muscle from MG+ mice, compared with MG− mice (Fig. 4A). Because MG triggers ROS generation, we examined 3T3-L1 cells after exposure to either MG, or a model oxidant, H2O2 to determine if the reduction of NAMPT levels was ROS dependent. NAMPT protein levels were reduced in these cells after exposure to either MG-AGE, or H2O2 (Fig. 4B), but was largely protected by the antioxidant NAC and partly by apocynin, an NADPH oxidase inhibitor, consistent with ROS, generated in response to MG, from diverse intracellular sources. MG-AGE also compromised the enzymatic activity of NAMPT, [NAD+]:[NADH] ratio, although less effectively than did H2O2, and this effect was significantly inhibited by antioxidants (Fig. 4C). Moreover, both NAMPT protein and [NAD+]:[NADH] levels remained intact in AGER1+ cells, but they were clearly blunted in shAGER1 cells (Fig. 4 D and E). These data indicate that AGER1 inhibits the suppression of NAMPT by MG, largely via an anti-ROS mode of action.
Fig. 4.
MG+ down-regulates NAMPT and NAD+ in insulin-sensitive tissues and adipocytes, via ROS. (A) WAT, liver, and muscle lysates from MG− and MG+ mice were subjected to Western blotting and densitometry of NAMPT protein (n = 3 independent experiments). *P < 0.05 vs. MG+. (B) Differentiated 3T3-L1 cells preexposed to MG-BSA (60 μg/mL) or H2O2 (1 μM), added as positive control, with or without anti-oxidants N-acetylcysteine (NAC, 5 mM) or apocynin (APO, 300 μM) for 72 h. Representative Western blots and density data (n = 3) are shown as means ± SD *P < 0.05 vs. control. §P < 0.05 vs. MG-BSA alone. (C) [NAD+:NADH] ratio in cells treated with MG (60 μg/mL) or H2O2 (1 μM) in the presence or absence of NAC and APO for 72 h. [NAD+:NADH] ratio is shown as fold (mean ± SD) of control (n = 3–5, each in triplicate). *P < 0.05 vs. control. §P < 0.05 vs. MG−BSA alone. (D) AGER1 transduction preserves NAMPT protein expression, and (E) [NAD+:NADH] ratio, during MG treatment. WT, AGER1+, and shAGER1 3T3-L1 cells were exposed to different amounts of MG-BSA for 72 h, and Western blots and densitometry analyses were performed. Data (n = 3–5) show protein to β-actin ratio as means ± SD *P < 0.05 vs. control. §P < 0.05 vs. WT treated with identical MG dosage. For E, data are shown as fold (mean ± SD) of control (n = 3, each in triplicate). *P < 0.05 vs. control. §P < 0.05 vs. WT with identical MG dosage.
Differential Responses to Short- and Long-Term MG Exposure.
The temporal pattern of the responses of adipocytes to extended exposure to AGEs was determined during in vitro MG stimulation of differentiated 3T3-L1 cells. Short-term exposure to MG-BSA (4–8 h) resulted in increases of both AGER1 and RAGE protein levels (by two- to threefold), followed by a plateau for ∼18 h (Fig. S4A). Similarly, SIRT1 and NAMPT protein levels increased, but for a shorter interval (Fig. S4B). During this period there was a modest rise in intracellular ROS (by 0.5-fold) (Fig. S4C), while intracellular AGE (CML and MG) levels did not significantly change (Fig. S4 D and E). After longer MG treatment (24–72 h), and as AGER1 protein levels declined to baseline (Fig. S4A), intracellular ROS and AGE further increased (by two- to threefold) (Fig. S4 C–E); whereas, RAGE protein increased only modestly (by 0.5-fold) (Fig. S4A). Thus, the early responses to MG-AGEs (<24 h) did not involve ROS or AGEs (Fig. S4 C–E) significant enough to trigger changes in inflammatory/insulin axis. As suggested in Figs. 3 and 4, all these occurred only after prolonged exposure to MG-AGEs (>24 h), after the trajectories of a declining AGER1 (as well as SIRT1) crossed over with further rising ROS and AGEs, and, to a lesser extent, RAGE (Fig. S4 A–C). Of note, the short-term (<24 h), modest increases in adipocyte ROS or AGEs did not correlate with the markedly increased RAGE. However, RAGE could contribute to long-term (>24 h) OS-dependent events in adipocytes, namely under conditions of AGER1 depletion, as suggested by silencing RAGE (Fig. S4 Ci and Di).
Discussion
Because it is generally accepted that IR and diabetes are causally linked to increased OS, gaining insight on potential new pro-OS causes is important. The current report identifies a class of pro-OS AGEs as a nontraditional risk factor of IR and T2D in nonobese mice independent of overnutrition. Exposure to an isocaloric diet in which the typical thermally promoted AGEs were largely substituted for by synthetic MG derivatives of a single protein (MG+) caused a phenotypic shift consisting of weight gain, adiposity, and IR. These changes replicate the metabolic syndrome phenotype that is becoming increasingly common in adult humans (1–3). An important feature of this model is an early depletion of defense mechanisms AGER1 and SIRT1, which underlies the elevated state of basal OS, as noted in humans (31). This newly identified link between exogenous AGEs and metabolic dysfunction is supported by the evident activation of inflammation in both macrophages and adipocytes, and the markedly impaired insulin-signaling pathways in insulin-sensitive tissues of MG+ mice. It is notable that this combination of features is virtually absent in genetically identical mice born and raised in an environment differing only with respect to the lower amount of ingested MG-AGEs. These mechanistic findings thus provide an important framework for elucidating the role of abnormal chronic OS in IR and T2D.
The current study addresses IR and T2D in a manner reflected in populations worldwide, which are increasingly affected by these polygenic conditions over the recent decades (1–3). Hence the choice of nonobese WT C57BL6 mice in which the food nutrient content was not manipulated. Several generations of mice were pair fed an isocaloric, nonthermally treated diet, with or without synthetic MG derivatives added at levels similar to those present in standard heat-treated chows (22, 27) and in proportion to those found in human foods (4–7, 18). Also, the nonthermal preparation of the experimental diets largely avoided changes such as of heat-unstable vitamins and antioxidants that may influence disease outcomes. Because MG generates intermediate and terminal AGEs, the MG+ diet was the main source of the excessive MG-H1-, and CML-like substances as well as lipoxidation products (25, 26, 30, 32) found in the circulation and WAT tissue of MG+ mice, including newly formed OS-derived intracellular AGEs. Therefore, the MG+ diet, but not the MG− diet, reproduced an in vivo condition of high basal OS, without the crucial confounder of heat treatment (22, 27).
Increased adiposity, glucose intolerance, and hyperinsulinemia appeared prematurely in F3/MG+ mice, namely 6 mo before its onset in Reg-fed mice (22, 27, 28) and 12 mo before that in F3/MG− mice. Thus, this model reproducibly disassociated dysmetabolic changes from both overnutrition and senescence factors, directing attention to alternative cause(s). Although the MG+ and Reg diets did not contain excessive nutrients, they led to weight gain, reflected in a near doubling of WAT mass in these mice, relative to MG−. The expanded WAT in MG+ or Reg mice was also rich in AGE lipids (by ∼three- to fourfold above MG−). This striking increase reflected a surplus of α-dicarbonyls in MG+ diet interacting with amine-containing, lipoxidized lipids and fatty acids (32–34), aided by the higher intraadipose AGEs/OS in MG+ mice. Given the markedly lower SIRT1 in MG+ WAT, it is possible that the excess intraadipose AGEs could impair normal lipolysis, via suppression of SIRT1, a factor implicated in fatty acid mobilization (35, 36). The net surplus of lipids and lipoxidized lipids could participate in the observed increased fat mass and adiposity in older MG+ (and Reg) mice. Excessive MG-AGEs, by suppressing SIRT1, also led to impaired tissue glucose uptake in MG+ mice. In contrast, preservation of SIRT1 in MG− WAT preserves efficient lipolysis as well as glucose uptake. Because SIRT1 also reduces hepatic gluconeogenesis (37), the low SIRT1 levels in the livers of MG+ mice may contribute to the absence of significant fasting hyperglycemia in these mice, despite elevated fasting plasma insulin levels. The findings may also be partly due to differences in energy expenditure between MG− and MG+ fed animals, a view that warrants exploration.
Chronic MG+ intake was also associated with elevated fasting plasma insulin and leptin and suppressed adiponectin levels in MG+ mice. These findings are consistent with an insulin-resistant state, as seen previously in Reg mice, but not in MG− mice (22, 28). The metabolic changes were associated with cellular changes in insulin-sensitive tissues from MG+ mice in the current study. These were concordant with impaired SIRT1-dependent metabolic insulin-receptor actions, based on decreased tyr-phospho-IR and -IRS-1, but increased Ser-phospho-IRS-1. This pattern was clearly different from that of MG− mice. The same combination of changes in insulin signaling was found in primary adipocytes isolated from WAT of MG+, but was not present in WAT of MG− mice. There was also a marked increase in NF-κB acetylated-p65 in WAT adipocytes from MG+ mice. Together with high Ser-phospho-IRS1, this further highlighted the exacerbated inflammatory insulin pathways in MG+ mice, pointing to their markedly SIRT1-depleted state. These data demonstrate that MG-derived AGEs, present in thermally treated food can profoundly alter the inflammation/insulin axis, in the absence of excess intake of nutrients or genetic susceptibility.
Because WAT consists of a mixture of cells, we separated adipocytes and stromal vascular WAT cells from MG+ mice and found that MG+ WAT was enriched in proinflammatory cells, compared with MG− mice. Moreover, peritoneal macrophages in MG+ mice had high TNF-α and CD11c. However, SIRT1, PPARγ, IL-10, and CD206 mRNA levels were suppressed, consistent with a shift to an activated population (M1), compared with the M2 pattern seen in MG− mice (38, 39). Because AGEs promote macrophage migration and activation (40, 41), it is plausible that the sustained MG influx in MG+ mice mobilizes an M1-enriched macrophage population that can infiltrate WAT and convert it into a pro-OS AGE-lipid–filled reservoir, perpetuating inflammatory and metabolic changes. The consistent absence of this inflammatory profile in both WAT and macrophages in MG− mice underscores the ability of external cytoxic AGEs to progressively foster these changes over time.
The evidence from MG+ mouse WAT cells and macrophages also reveals that AGER1 levels were markedly suppressed. Because AGER1 is a major anti-AGE and anti-OS receptor (12, 13, 20, 28), its suppression may foster AGE-lipid accumulation in WAT and possibly in other tissues. Indeed, as with SIRT1, AGER1 protein levels were suppressed in all three insulin-responsive tissues from MG+ mice, but not in those from MG− mice, raising again the possibility that these factors are coordinately regulated, an observation first made in humans with T2D (18). Studies in differentiated 3T3-L1 cells with genetically modulated AGER1 support the presence of such an interaction. AGER1 transduction, by preserving SIRT1, prevented the MG-mediated NF-κB p65 hyperacetylation, as well as downstream inflammatory events, which preserved the metabolic actions of insulin receptor (17, 38). However, when AGER1 is silenced, cytotoxic AGEs suppress SIRT1-dependent metabolic events of insulin, and/or enhance its inflammatory events, as evidenced by increased JNK activity (16, 21). Alternatively, the protective effects of AGER1 may also require functional SIRT1, because even when AGER1was overexpressed, AGE-induced cytotoxicity was not prevented under conditions that inhibit SIRT1 function.
As SIRT1 is also NAMPT- and NAD+ dependent (15, 16, 18) and both were suppressed in MG+ mouse insulin-sensitive tissues, compared with tissues from MG− mice, we addressed the mechanisms in 3T3-L1 cells. The in vivo findings were reproduced in vitro, indicating that AGEs act upon SIRT1 partly via this enzyme and its activity, which are ROS sensitive (15, 17, 38). The effect of MG-AGEs on NAMPT and NAD+ was inhibited by antioxidants, and, importantly, by AGER1 overexpression. This confirmed both the pro-OS effect of exogenous cytotoxic AGEs and the OS-inhibitory role of this receptor (12, 13, 27).
Because the mouse studies necessarily reflect the compounded effects of prolonged exposure to surplus MG-AGEs, time-dependent in vitro studies on 3T3-L1 cells allowed their partial dissection. Adipocyte AGER1 may initially serve as a shield to the intracellular compartment of WAT against an external oxidant overload, as proposed for other tissues (14, 18, 20, 22, 28). This might help maintain basal OS/Infl in check and via SIRT1 preserve insulin actions. However, under chronically high cytotoxic AGEs, AGER1’s protection is afforded for a limited time frame. The exact in vivo time and mechanism(s) of AGER1 depletion, as in the case of SIRT1, are unknown. These may reflect epigenetic changes, based on new studies of transgenerational exposure to cytotoxic MG-AGEs. We find that MG+ mice show a progressive suppression and MG− mice a progressive increase in AGER1 and SIRT1 levels. In support of the latter, recent clinical evidence indicates that in diabetic humans a lower oral AGE intake can restore these factors to normal and can improve OS/Infl and insulin sensitivity (18), findings with substantial clinical implications.
The long-term disease outcomes noted after exposure of multiple generations of mice to relatively well-defined oral AGEs focuses attention on recent socio/industrial changes through which deleterious agents, common in human foods, may have negatively impacted on human host defense mechanisms. The functional depletion of AGER1 and SIRT1 genes observed in MG+ mice, although likely not restricted to only these genes, may help reassess the convergence of proinflammatory and dysmetabolic diseases in humans. Sustained restriction of the external sources of oxidants can help regain lost gene function and restore native resistance to T2D and other diseases linked to high OS.
Materials and Methods
Reagents.
Details of the reagents are in SI Materials and Methods.
Animals and Treatments.
Details on the animals and treatments are available in SI Materials and Methods. Briefly, Fo 8-wk-old C57BL/6 mice were placed on the standard diet (National Institutes of Health-31 open formula), which had been prepared without heat exposure (low-AGE) (22, 27, 28). The offspring F1-F3 (n = 18–20/group), were assigned to two pair-fed groups after weaning, one received the low-AGE or the same formula with synthetic added MG-BSA (1 mg/g food). The diets were identical in nutritional and caloric content but MG+ diet contained MG derivatives (Fig. S1A), and CML to levels equivalent to standard heat-treated chow, and nearly twice those in MG− diet (Table 1).
AGE Determination.
AGEs in serum, cells, and tissues were determined by a competitive ELISA for CML (4G9 mab) and for MG-derivatives (3D11 mab), cross-reacting with MG-H1 (5) (Fig. S1A). AGE lipids (33, 42) were measured by direct ELISA after tissue lipid extraction.
Glucose Tolerance.
An IGTT was performed at 6-mo intervals after age 12 mo, as described in SI Materials and Methods (22, 27, 28).
Glucose Uptake.
Adipocytes (WAT) isolated from mouse inguinal fat pads and skeletal muscle from the thigh of 18 mo old were used for insulin-stimulated 2-deoxy-[3H] glucose (2DOG) uptake, as described in SI Materials and Methods (43–45).
Cell Culture and Transfection.
The 3T3-L1 preadipocytes (ATCC) after differentiation and transfection with AGER1, shAGER1, or Sirt1 as described (SI Materials and Methods) (44) were stimulated with insulin and with MG-BSA for signaling studies (SI Materials and Methods) (36, 44, 45).
RNA Isolation and Reverse Transcription–PCR, Western Analysis, Immunoprecipitation, [NAD:NADH], and ROS Determination.
Assays were performed as described in SI Materials and Methods (18, 27).
Statistical Analysis.
Data were expressed as means ± SEM. The Student's t test was used to determine the significance between groups. One-way ANOVA with Bonferroni correction analysis was performed for comparisons among the three groups. A probability value of P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by AG23188, HL73417, and MERIT Grant AG-09453 (to H.V.) from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205847109/-/DCSupplemental.
See Commentary on page 15537.
References
- 1.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 2.Knip M, et al. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S125–S136. doi: 10.2337/diabetes.54.suppl_2.s125. [DOI] [PubMed] [Google Scholar]
- 3.Wentworth JM, Fourlanos S, Harrison LC. Reappraising the stereotypes of diabetes in the modern diabetogenic environment. Nat Rev Endocrinol. 2009;5:483–489. doi: 10.1038/nrendo.2009.149. [DOI] [PubMed] [Google Scholar]
- 4.van Boekel MA. Formation of flavour compounds in the Maillard reaction. Biotechnol Adv. 2006;24:230–233. doi: 10.1016/j.biotechadv.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Cai W, et al. Oxidative stress-inducing carbonyl compounds from common foods: Novel mediators of cellular dysfunction. Mol Med. 2002;8:337–346. [PMC free article] [PubMed] [Google Scholar]
- 6.Koschinsky T, et al. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA. 1997;94:6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birlouez-Aragon I, et al. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am J Clin Nutr. 2010;91:1220–1226. doi: 10.3945/ajcn.2009.28737. [DOI] [PubMed] [Google Scholar]
- 8.Vlassara H, Striker GE. AGE restriction in diabetes mellitus: A paradigm shift. Nat Rev Endocrinol. 2011;7:526–539. doi: 10.1038/nrendo.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Z, et al. Advanced glycation end products inhibit glucose-stimulated insulin secretion through nitric oxide-dependent inhibition of cytochrome c oxidase and adenosine triphosphate synthesis. Endocrinology. 2009;150:2569–2576. doi: 10.1210/en.2008-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coughlan MT, et al. Advanced glycation end products are direct modulators of β-cell function. Diabetes. 2011;60:2523–2532. doi: 10.2337/db10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabbani N, Thornalley PJ. Glyoxalase in diabetes, obesity and related disorders. Semin Cell Dev Biol. 2011;22:309–317. doi: 10.1016/j.semcdb.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Cai W, He JC, Zhu L, Lu C, Vlassara H. Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc Natl Acad Sci USA. 2006;103:13801–13806. doi: 10.1073/pnas.0600362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu C, et al. Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc Natl Acad Sci USA. 2004;101:11767–11772. doi: 10.1073/pnas.0401588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torreggiani M, et al. Advanced glycation end product receptor-1 transgenic mice are resistant to inflammation, oxidative stress, and post-injury intimal hyperplasia. Am J Pathol. 2009;175:1722–1732. doi: 10.2353/ajpath.2009.090138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarente L, Franklin H. Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011;364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- 16.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol. 2009;5:367–373. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 17.Yoshizaki T, et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uribarri J, et al. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: Potential role of AGER1 and SIRT1. Diabetes Care. 2011;34:1610–1616. doi: 10.2337/dc11-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He CJ, Koschinsky T, Buenting C, Vlassara H. Presence of diabetic complications in type 1 diabetic patients correlates with low expression of mononuclear cell AGE-receptor-1 and elevated serum AGE. Mol Med. 2001;7:159–168. [PMC free article] [PubMed] [Google Scholar]
- 20.Vlassara H, et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94:4483–4491. doi: 10.1210/jc.2009-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Kreutzenberg SV, et al. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: Potential biochemical mechanisms. Diabetes. 2010;59:1006–1015. doi: 10.2337/db09-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai W, et al. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol. 2008;173:327–336. doi: 10.2353/ajpath.2008.080152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vlassara H, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA. 2002;99:15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornalley PJ. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems—Role in ageing and disease. Drug Metabol Drug Interact. 2008;23:125–150. doi: 10.1515/dmdi.2008.23.1-2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu BF, et al. Methylglyoxal induces apoptosis through activation of p38 mitogen-activated protein kinase in rat mesangial cells. Kidney Int. 2003;63:947–957. doi: 10.1046/j.1523-1755.2003.00829.x. [DOI] [PubMed] [Google Scholar]
- 26.Wu L, Juurlink BH. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension. 2002;39:809–814. doi: 10.1161/hy0302.105207. [DOI] [PubMed] [Google Scholar]
- 27.Cai W, et al. AGER1 regulates endothelial cell NADPH oxidase-dependent oxidant stress via PKC-delta: Implications for vascular disease. Am J Physiol Cell Physiol. 2010;298:C624–C634. doi: 10.1152/ajpcell.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai W, et al. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: Association with increased AGER1 expression. Am J Pathol. 2007;170:1893–1902. doi: 10.2353/ajpath.2007.061281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilhovd BK, et al. Increased serum levels of the specific AGE-compound methylglyoxal-derived hydroimidazolone in patients with type 2 diabetes. Metabolism. 2003;52:163–167. doi: 10.1053/meta.2003.50035. [DOI] [PubMed] [Google Scholar]
- 30.Uribarri J, et al. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–433. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillum MP, et al. SirT1 regulates adipose tissue inflammation. Diabetes. 2011;60:3235–3245. doi: 10.2337/db11-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu MX, et al. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 33.Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H. Lipid advanced glycosylation: Pathway for lipid oxidation in vivo. Proc Natl Acad Sci USA. 1993;90:6434–6438. doi: 10.1073/pnas.90.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Januszewski AS, Alderson NL, Jenkins AJ, Thorpe SR, Baynes JW. Chemical modification of proteins during peroxidation of phospholipids. J Lipid Res. 2005;46:1440–1449. doi: 10.1194/jlr.M400442-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Picard F, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purushotham A, et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 39.Imai S, The NAD. The NAD World: A new systemic regulatory network for metabolism and aging—Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlassara H, Brownlee M, Manogue KR, Dinarello CA, Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: Role in normal tissue remodeling. Science. 1988;240:1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi HK, et al. Advanced glycation end products subspecies-selectively induce adhesion molecule expression and cytokine production in human peripheral blood mononuclear cells. J Pharmacol Exp Ther. 2009;330:89–98. doi: 10.1124/jpet.109.150581. [DOI] [PubMed] [Google Scholar]
- 42.Bucala R, et al. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci USA. 1994;91:9441–9445. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamei N, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 44.Pramfalk C, et al. Insulin receptor activation and down-regulation by cationic lipid transfection reagents. BMC Cell Biol. 2004;5:7. doi: 10.1186/1471-2121-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taha MF, Hedayati V. Isolation, identification and multipotential differentiation of mouse adipose tissue-derived stem cells. Tissue Cell. 2010;42:211–216. doi: 10.1016/j.tice.2010.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.