Abstract
Formation of specific neuronal connections often involves competition between adjacent axons, leading to stabilization of the active terminal, while retraction of the less active ones. The underlying molecular mechanisms remain unknown. We show that activity-dependent conversion of pro–brain-derived neurotrophic factor (proBDNF) to mature (m)BDNF mediates synaptic competition. Stimulation of motoneurons triggers proteolytic conversion of proBDNF to mBDNF at nerve terminals. In Xenopus nerve–muscle cocultures, in which two motoneurons innervate one myocyte, proBDNF-p75NTR signaling promotes retraction of the less active terminal, whereas mBDNF–tyrosine-related kinase B (TrkB) p75NTR (p75 neurotrophin receptor) facilitates stabilization of the active one. Thus, proBDNF and mBDNF may serve as potential “punishment” and “reward” signals for inactive and active terminals, respectively, and activity-dependent conversion of proBDNF to mBDNF may regulate synapse elimination.
Keywords: neuromuscular junction, pro-neurotrophin, synapse competition
The nervous system responds to experience by altering the number and strength of synaptic connections (1). Activity-dependent synaptic competition, a general process seen in many parts of the developing nervous system, plays a critical role in shaping patterns of neuronal connections (2–7). At the neuromuscular junction (NMJ), for example, multiple axons compete for the same postsynaptic muscle cell during early postnatal life until all but one is eliminated (8–10). Extensive experimental data support the view that the more active terminal or “cartel” gets stabilized, whereas less active ones withdraw, resulting in canonical elimination of polyneuronal innervation (8, 11). It is generally believed that this synaptic competition is mediated by a “punishment” or “elimination” signal, produced by the postsynaptic cell, that causes the retraction of the inactive terminals, as well as a “protective” or “reward” signal that stabilizes the active terminal (10–12). Despite significant efforts over decades, the identity of the punishment or reward signals remains unknown (4, 13). This is due at least in part, to the experimental difficulties in manipulating gene expression selectively in one of the competing axons.
Brain-derived neurotrophic factor (BDNF) has been recognized as a key regulator of synapse development and plasticity (14, 15). This is because BDNF is the only neurotrophin indisputably secreted in an activity-dependent manner (15). Indeed, activity-dependent secretion of BDNF has been shown to be critical for hippocampus-dependent memory in human (16, 17). Like all neurotrophins, BDNF is initially synthesized as a precursor (proBDNF), which is subsequently cleaved to generate mature (m)BDNF. proBDNF interacts preferentially with the pan-neurotrophin receptor p75 (p75NTR), whereas mBDNF selectively binds and activates the receptor tyrosine kinase TrkB (18, 19). Cumulative evidence supports a “yin-yang hypothesis,” in which pro- and mBDNF elicit opposite biological effects by activating two distinct receptor systems (20). For example, proBDNF, if not processed, promotes long-term depression (LTD) through the activation of p75NTR in the hippocampus (21, 22). In contrast, mBDNF-TrkB signaling is essential for the early phase of long-term potentiation (E-LTP) (23–25). Moreover, recent studies indicate that a significant proportion of BDNF in the brain is secreted in the proform (26–28), and extracellular conversion of proBDNF to mBDNF by the tissue plasminogen activator (tPA)/plasmin protease system is critical for late-phase LTP (29). The expression of proBDNF and p75NTR in rodents is developmentally regulated, with the highest levels in the first and second postnatal week, correlating well with the timings of synapse formation (28). Therefore, proteolytic cleavage of proBDNF represents an important mechanism by which the opposing cellular actions of proBDNF and mBDNF may be regulated (20).
The opposing nature of proBDNF and mBDNF prompted us to hypothesize that proBDNF and mBDNF might serve as punishment and reward signals, respectively, during synaptic competition at the developing NMJs. In this study, we developed a triplet system that allows alteration of gene function in one of two distinctly labeled axons that innervate a single, unlabeled myocyte. Our study suggests that proBDNF serves as a general “punishment signal” that causes p75NTR-expressing motor terminals to retract, whereas at the active terminal, secretion/activation of extracellular protease(s) converts proBDNF to mBDNF, which serves as a reward signal to stabilize the terminal.
Results
Activity-Dependent Synaptic Competition in Xenopus Nerve–Muscle Cocultures.
Xenopus nerve–muscle coculture system was used to study the activity-dependent synaptic competition. We developed a cell-culture system, in which an unlabeled myocyte was innervated by two spinal neurons (one labeled in green and the other in red, respectively; Fig. 1A). This was accomplished by injecting either FITC-dextran (green) or rhodamine-dextran (red) into a single dorsal animal blastomere at the 8- or 16-cell stage and mixing the neural tubes from embryos that were injected with two different fluorophores to prepare dissociated nerve–muscle cocultures (30). Instead of employing electrical stimulation using a glass electrode, which might cause mechanical damages on neurons, we stimulated one of the spinal neurons by local photolysis of caged glutamate (MNI-glutamate; 50 μM) using a multiphoton microscope (31). Photo-uncaging of caged glutamate at a neuronal soma induced a marked potentiation of synaptic transmission, which lasted for more than 60 min (Fig. S1A). This synaptic potentiation was completely blocked by the sodium channel blocker tetrodotoxin (TTX) (1 μM), demonstrating the requirement of action potentials (Fig. S1A). Furthermore, synaptic potentiation was only observed when a laser beam was applied within 25 μm from the neuronal cell body, suggesting that the photo-uncaging occurred locally (Fig. S1B).
Fig. 1.
Activity-dependent synaptic competition in culture. (A) Schematic diagram (Left) and confocal image (Right) showing a triplet in which a spherical myocyte (M) (indicated by white dotted lines) is innervated by two spinal neurons (N), one labeled with FITC (green) and one labeled in rhodamine (red), in nerve–muscle coculture. (B) Time course of synaptic competition. A higher magnification of A is shown. Stimulation of the red neuron, by photo-uncaging of MNI-glutamate in the soma area using a two-photon laser, caused the unstimulated (green) axon terminal to retract from the synaptic target (indicated by a yellow arrow, upper row). In contrast, the axon terminal (red) from the stimulated neuron did not retract, but elongated a little (white arrows, middle row). The phase and two-color fluorescence images of the triplet at multiple time points (lower row). (Scale bar: 10 μm.) The phase (Bottom) and two-color fluorescence images (Top and Middle) of the triplet at “0” and “120” min are shown. (C) Quantification of axonal retraction and elongation measured 120 min after stimulation of one of the neurons in the triplets. *P < 0.01.
A brief episode (250 ms) of photolysis of MNI-glutamate was applied to one of the two neurons, and the resulting morphological changes in synaptic terminals from both neurons, which were innervating a single myocyte, were monitored by dual-color, time-lapse confocal imaging. Upon stimulation of a red neuron, the axon terminal of the unstimulated neuron (green terminal) gradually withdrew from the previously innervated muscle, whereas the terminal of the stimulated neuron (red terminal) remained stable and occasionally extended (Fig. 1B and Movie S1). Conversely, stimulation of a green neuron triggered the retraction of the red terminal (Fig. S2). Because stimulation of one neuron always resulted in the retraction of the unstimulated neuron, regardless of whether it was red or green, we randomly stimulated neurons in subsequent experiments based on the convenience of photo-uncaging. We performed 11 experiments involving preferential photolysis of one neuron. In all 11 cases, the axon terminals of the unstimulated neurons retracted to varying degrees. In six cases, axon terminals of stimulated neurons showed slight expansion or elongation (Fig. 1 B and C). These results suggest that a postsynaptically derived local punishment signal may trigger synaptic retraction.
Activity-Dependent Cleavage of Secreted proBDNF at NMJ.
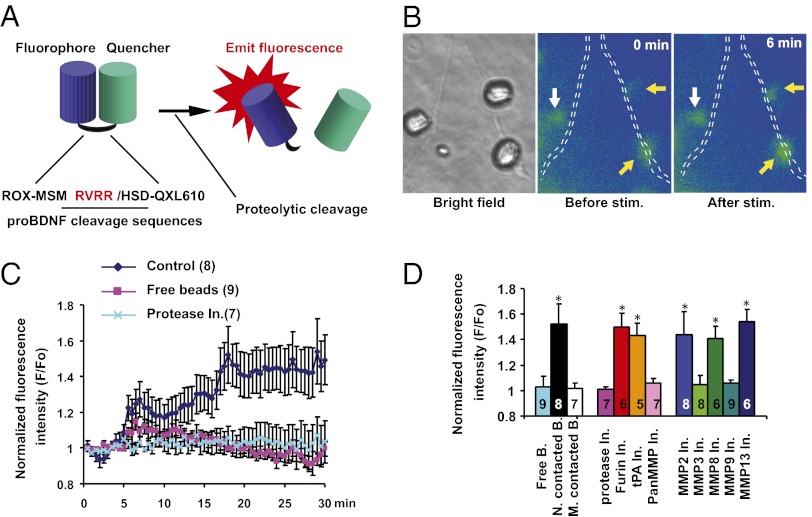
Given that proBDNF and mBDNF could elicit opposite effects, we hypothesized that proBDNF and mBDNF might serve as punishment and reward signals, respectively. Furthermore, we hypothesized that the proteolytic conversion of proBDNF to mBDNF might be essential for synaptic competition and elimination at the developing NMJs. First, we tested whether neuronal activity could activate proteases to process proBDNF to mBDNF using a fluorogenic proteolytic beacon assay. The fluorogenic beacon consisted of synthetic peptides, harboring the propeptide cleavage sequences (MSMRVRR↓HSD) within proBDNF, and these synthetic peptides were flanked by a fluorophore at the N terminus and a quencher at its C terminus (Fig. 2A). Small size peptides allowed both a fluorophore and a quencher within proximity, thereby quenching the fluorescence via fluorescence resonance energy transfer (FRET). Upon cleavage of peptides by a protease(s), which specifically recognizes the proBDNF cleavage sequence, the fluorogenic beacon would emit the fluorescence signal because of the separation of a fluorophore from a quencher (Fig. 2A). In addition, we immobilized the fluorogenic beacon to polystyrene beads to minimize diffusion in culture medium.
Fig. 2.
Activity-dependent cleavage of proBDNF detected by fluorogenic probes. (A) Schematic diagram depicting the design of a fluorogenic indicator of proBDNF cleavage. A peptide containing the proBDNF cleavage site was placed between a fluorophore and a quencher. Upon proteolytic cleavage, the quencher was dissociated from the fluorophore, leading to emission of fluorescence. (B) Sample images of fluorogenic probes in phase (Left) and fluorescence (Right) before and after neuronal stimulation. Polystyrene beads soaked with fluorogenic probes were positioned on (yellow arrows) or near (white arrows) an axonal process (indicated by white dotted lines), respectively, by micromanipulation. A neuronal cell body was stimulated by photolysis of caged-glutamate (MNI-glutamate) with a UV laser. Note that after stimulation, only beads on, but not near, the axonal processes showed an increase in fluorescence. (C) Time course of cleavage-dependent increase in fluorescence intensity upon stimulation of spinal neurons. Fluorescence intensities measured on contacted beads or free beads were normalized to that at “0” time point and presented as ratios (F/F0) over time. (D) Quantification of relative fluorescence intensities in beads 30 min after stimulation of neurons under various conditions. Concentrations of protease inhibitors (In.): general protease inhibitor mixture, 50 μM; pan MMP inhibitor, 60 μM; tPA inhibitor, 10 μM; furin inhibitor, 40 μM; MMP2 inhibitor, 60 μM; MMP3 inhibitor, 50 nM; MMP8 inhibitor, 20 nM; MMP9 inhibitor, 50 μM; MMP13 inhibitor, 80 nM. B, beads; M, muscle cell; N, neuron.
Next, we investigated whether stimulation of neurons could activate proteases at the nerve terminals. The fluorogenic beacons were placed on the axonal process or termini of spinal neurons, and neurons were subsequently stimulated either by an electrical stimulation or by photo-uncaging of MNI-glutamate (Fig. 2B, bright field). After stimulation, the fluorescence intensity of the beads on the axon terminals or axonal processes (e.g., yellow arrows in Fig. 2B) greatly increased (Fig. 2 B and C), suggesting cleavage of the peptide by proteases secreted from the axon. In contrast, no fluorescence change was observed from beads, which were not in contact with the axonal process or termini (Fig. 2B, “free beads,” white arrow). Time-lapse imaging indicated that the increase in fluorescence occurred relatively quickly: the first surge came within minutes, and the florescence increase reached its peak within 20 min (Fig. 2C). Pretreatment with a mixture of protease inhibitors prevented the increase in fluorescence from beads on axon terminals (Fig. 2D). Remarkably, inhibitors for tPA and furin, the extracellular and intracellular proteases known to cleave proBDNF in the brain (29, 32), did not inhibit the fluorescence increase (Fig. 2D). In contrast, matrix metalloprotease (MMP) inhibitors successfully blocked the stimulus-induced increase in fluorescence. Further analyses indicated that MMP3 and MMP9, but not MMP2, MMP8, or MMP13, could increase fluorescence signal on the fluorogenic beacons (Fig. 2D). Finally, when the beacon-containing beads were placed on muscle cells, stimulation of muscle cells did not cause an increase in fluorescence (Fig. 2D), indicating that muscle cells do not secrete proteases that could process proBDNF.
We further examined whether postsynaptic muscle cells secrete proBDNF upon stimulation. Because of unavailability of a proBDNF-specific ELISA and limited number of muscle cells in the Xenopus cocultures (<1,000 myocytes), it was not feasible to measure proBDNF secretion from muscle cells using existing biochemical techniques. Thus, we used cell surface immunostaining to measure proBDNF secretion, given that proBDNF is positively charged at physiological pH and could be associated with a negatively charged cell membrane upon secretion (27, 33). The muscle cell cultures were depolarized by high-K+ treatment (50 mM) for 5 min, fixed, and processed for cell surface immunofluorescence staining under membrane impermeable conditions. A proBDNF-specific monoclonal antibody was used for cell-surface staining of secreted proBDNF (27). Although proBDNF was barely detectable on the muscle cell surface at rest (control), the proBDNF immunoreactivity increased dramatically (88%) upon depolarization (Fig. S3A). This increase was even more pronounced when extracellular cleavage of proBDNF was blocked by general MMPs inhibitors (indicated as MMP-In. in Fig. S3A).
To measure the protease-mediated conversion of endogenous proBDNF to mBDNF at the neuromuscular synapses, we performed cell-surface immunostaining using a specific antibody against mBDNF. We applied glutamate, instead of high K+, to selectively depolarize neurons, because muscle cells do not express glutamate receptors (34). After neuronal depolarization, cultures were subsequently fixed and processed for surface immunostaining using an mBDNF-specific antibody (27). In control conditions, there was no mBDNF staining (Fig. S3B, Left). Upon glutamate application (10 mM; 5 min), we observed a dramatic, threefold increase in surface mBDNF staining at the synaptic junction (Fig. S3B, Center). This increase in mBDNF immunoreactivity was observed only in myocytes that were innervated by a spinal neuron but not in the neighboring noninnervated myocytes (Fig. S3B, Center). This suggested that the release and/or activation of proteases at the nerve terminal converted proBDNF to mBDNF at the active synaptic terminals. Furthermore, when the cultures were pretreated with the MMP inhibitors, the glutamate-induced increase in mBDNF staining was completely abolished (Fig. S3B, Right). Taken together, these results supported the notion that activity-dependent conversion of proBDNF to mBDNF occurred at the developing Xenopus NMJ in situ.
Synaptic Stabilization During Competition Mediated by mBDNF/TrkB.
Previously, we have shown that exogenous proBDNF triggers synaptic depression and subsequent retraction of axon terminal through p75NTR in Xenopus neuromuscular synapses (35). Based on robust secretion of proBDNF upon synaptic depolarization, we reasoned that active terminals might cleave proBDNF to mBDNF, which protects these active terminals from proBDNF-mediated synaptic depression and retraction. To test this, we knocked down endogenous TrkB in one of neurons in our triplet system using a morpholino (Fig. S4A; also see ref. 36). Control experiments indicated that the expression of the TrkB morpholino in presynaptic neurons blocked the mBDNF-mediated synaptic potentiation (Fig. S4 B and C). When the TrkB morpholino was selectively expressed in one of the spinal neurons in our triplet system, stimulation of the TrkB morpholino-expressing neuron (green) resulted in retraction of both green and red terminals (Fig. 3A, yellow arrows). Quantitative analysis of 10 triplets indicated that both stimulated and unstimulated terminals retracted ∼12 μm within 120 min (Fig. 3C).
Fig. 3.
Synaptic stabilization mediated by mBDNF/TrkB. (A) Down-regulation of TrkB by TrkB-morpholino (TrkB-morp.) led to retraction of both stimulated and unstimulated axon terminals. In a triplet system, stimulation of a neuron expressing TrkB morpholino (green) resulted in retraction of the red axon as well as the green axon. (Scale bar: 10 μm.) (B) Treatment with mBDNF prevented activity-dependent synaptic retraction. The culture was treated with mBDNF before stimulating the soma of a red neuron. The terminals from both red and green neurons remained in the synaptic site without any retraction. (Scale bar: 20 μm.) (C) Quantification of axonal retraction and elongation measured 120 min after stimulation of one of the neurons in the triplets.
Next, we reasoned that if the activation of TrkB by mBDNF was critical to prevent synaptic retraction, supply of exogenous BDNF would prevent synaptic competition. Indeed, in a nerve–muscle culture treated with mBDNF (25 ng/mL) for 2 h, stimulation of the red neuron did not cause retraction of the green terminal (Fig. 3B and Movie S2). Moreover, application of exogenous mBDNF even elicited axonal elongation from the stimulated neurons (Fig. 3 B and C, white arrowheads). These results suggest that mBDNF plays an active role in preventing synaptic retraction of the active terminals through TrkB signaling during synaptic competition.
Synaptic Retraction During Competition Mediated by proBDNF/p75NTR.
To determine whether this activity-based synaptic retraction was mediated through proBDNF/p75NTR signaling, we knocked down endogenous p75NTR by using FITC-conjugated p75NTR siRNA that were targeted to all Xenopus p75NTR isoforms (p75NTRa and p75NTRb) (37). The p75NTR siRNA was introduced into a single neuron in our triplet system using embryo-injection techniques. Western blot analysis revealed significant reduction of endogenous p75NTR protein in neural tubes derived from embryos injected with p75NTR siRNA, but not in those injected with scrambled siRNA for p75NTR (35). We reasoned that the blockade of p75NTR would block synapse elimination of less active neurons. Indeed, when photolysis was applied to the red neuron, the axon terminal of a p75NTR siRNA-expressing neuron did not retract over a long period (Fig. 4A). In seven triplets, four showed no retraction, whereas the other three showed reduced retraction of the unstimulated terminals after 2hrs (Fig. 4 A and C). Furthermore, an axon expressing scrambled siRNA still retracted when the competing axon was stimulated (Fig. S5). Because p75NTR siRNA and p75NTR morpholino target different regions of p75NTR, this experiment confirmed that blockade of p75NTR signaling abolished synaptic retraction. Taken together, these data support the hypothesis that synaptic retraction was mediated by presynaptic p75NTR signaling during synaptic competition.
Fig. 4.
Synaptic retraction mediated by proBDNF/p75NTR. (A) Down-regulation of p75NTR by siRNA prevents activity-dependent synaptic retraction. Fluorescence images show a double-innervated myocyte (white dotted lines) by a neuron expressing p75NTR siRNAs (green) and a rhodamine-labeled control neuron (red). The soma of the red neuron (outside the field) was stimulated by photo-uncaging and the axon terminals from both neurons were monitored by time-lapse microscopy. (Scale bar: 20 μm.) Even after 120 min, the axon terminals (white arrow) of both red and green neurons remained unchanged at the synaptic site. (B) Time-lapse images showing retraction of both stimulated (red) and unstimulated (green) axons in the presence of a mixture of protease inhibitors (50 μM). (Scale bar: 20 μm.) (C) Quantification of axonal retraction and elongation measured 120 min after stimulation of one of the neurons in the triplets.
The results above, together with the finding that neuronal stimulation triggered protease secretion/activation at nerve terminals (Fig. 2), prompted us to speculate that active terminals were spared from synaptic retraction because of proteolytic cleavage of proBDNF. If this were true, then blockade of proBDNF cleavage would trigger retraction of all axon terminals during synaptic competition. To test this hypothesis, we treated nerve–muscle cocultures with a mixture of protease inhibitors for 10 min before neuronal stimulation. These inhibitors were able to block proteases that could cleave proBDNF (Fig. 2D). Remarkably, axon terminals from red and green spinal neurons retracted simultaneously from the synaptic site (Movie S3). Quantitative analysis indicated that both stimulated and unstimulated terminals retracted ∼10 μm (Fig. 4C). Because MMP inhibitors, particularly MMP3/9 inhibitors, blocked activity-induced proBDNF cleavage at nerve terminals (Fig. 2D), we further tested whether inhibition of MMPs could perturb synapse elimination. Indeed, pretreatment with a combination of both MMP3 and MMP9 inhibitors triggered synaptic retraction upon stimulation of the competing axon, indicating that MMP3/9 are the candidate proteases that convert proBDNF to mBDNF at the active synaptic terminal (Fig. S6 and Movie S4). Taken together, these data supported the hypothesis that the activation of presynaptic p75NTR by muscle-derived proBDNF triggered the retraction of less active axonal terminals during synaptic competition.
Discussion
For over a half century, activity-dependent synaptic competition/elimination has been one of the central issues in developmental neurobiology. However, the underlying mechanisms remain unknown. Because the competition depends on activity and occurs locally, the experimental challenge has been to selectively activate one of the competing terminals and to perform molecular manipulation selectively in competing axons (38). In the present study, we modified the Xenopus embryo-injection protocol, so that we could reliably obtain triplets, in which an unlabeled myocyte is innervated by two distinctly labeled axons. Confocal live-cell imaging and local glutamate uncaging using a multiphoton laser enabled us to visualize and trigger synaptic retraction of individual axons in triplets. To demonstrate activity-dependent secretion of proBDNF and its conversion to mBDNF, we performed cell surface immunofluorescence staining on cultured cells, using specific antibodies against proBDNF and mBDNF. FRET-based fluorogenic probes were used to monitor the activation of protease activities locally at axonal terminals in real time. Moreover, embryo injection techniques were used to selectively inhibit TrkB or p75NTR in one neuron, but not in others, in triplets. By combining these techniques, we provided the evidence for a local and activity-dependent activation of specific proteases that could convert proBDNF to mBDNF in neuromuscular synapses. Given that proBDNF and mBDNF often elicit opposite biological effects (20, 28), this finding may help us understand how proBDNF-to-mBDNF conversion is regulated via neuronal activity. Moreover, we found that the blockade of p75NTR signaling attenuated synapse elimination, whereas the blockade of TrkB signaling, or inhibition of proBDNF cleavage by metalloproteases, promoted synaptic retraction of both innervated axon terminals in triplets. Taken together, these findings suggest a model for synapse elimination in which the activity-dependent conversion of proBDNF to mBDNF selectively stabilized active terminals, whereas inactive terminals were eliminated in response to proBDNF and subsequent activation of p75NTR signaling (Fig. S7).
Activity-Dependent proBDNF Cleavage Converts a “Synaptotoxin” to a “Synaptotrophin.”
Two theories have been proposed to explain activity-dependent synaptic competition and elimination. The “synaptotoxin” theory suggests that the postsynaptic cell produces destabilizing or “toxic” signals such as proteases that retrogradely punish and remove presynaptic terminals. This theory (11) was based largely on the observation that inhibition of protease activity (39, 40), particularly thrombin, by the naturally occurring protease inhibitor nexin I (41, 42), attenuated synapse elimination. However, specific target(s) of the proteases have not been identified yet. More importantly, selective protection of the active terminal by local secretion of protease inhibitors has not been demonstrated. It is also unclear what axons are competing for, if the fate of an axon is dependent only on its intrinsic activity (11). An alternative is the “synaptotrophin” hypothesis, which suggests that axons compete with each other for a limited supply of trophic factors derived from the postsynaptic cell. However, this model fails to explain: (i) how the trophic factor is secreted locally from the postsynaptic cell only near the active terminal, but not at the inactive terminal; and (ii) how the active terminal preferentially binds, uptakes, or signals the trophic factor, if any. Neither local secretion nor preferential signaling has been reported at NMJs to support the synaptotrophin hypothesis. Moreover, synaptic elimination is an active process that requires a punishment signal, ultimately resulting in the loss of all, but one protected terminal (13). Thus, the synaptotrophin theory fails to explain how inactive terminals initiate withdrawal and why the absence of trophic support leads to synaptic depression and retraction.
Our data support a model that both the punishment and reward signals are originated from the same molecule, namely BDNF (Fig. S7). In this model, presynaptic activity drives the secretion of proBDNF from postsynaptic muscle cells, which serves as a default “punishment signal” to actively retract afferent terminals through p75NTR. mBDNF, on the other hand, serves as a reward signal for which all terminals compete. A major conceptual breakthrough to the model is the activity-dependent conversion of proBDNF to mBDNF by active axon terminals. Locally secreted proteases at the active terminal not only block the punishment signal (proBDNF), but also generate a reward signal (mBDNF), which stabilizes the terminal by activating TrkB. Further work is necessary to substantiate this model in vivo.
proBDNF-mBDNF As Punishment–Reward Signals in Synapse Elimination.
Using cultured neurons, computational modeling, and knockout animals, two recent studies reported mechanisms for synaptic competition and axonal pruning in the sympathetic nervous system (43, 44). In this system, target-derived NGF, through TrkA, provides protection and prosurvival of active axons, which are further strengthened and stabilized by increasing TrkA transcription. On the other hand, NGF-induced synthesis and secretion of BDNF and neurotrophin-4 (NT-4), through p75NTR, facilitate axonal degeneration and pruning of the competing axons (43–45). Although these experiments were largely carried out in cell culture, the basic premise is quite similar: TrkA mediates reward, whereas p75NTR mediates punishment. There are several important differences between the present study and those published works. First, activity-dependent secretion of the reward (NGF) or punishment signal (BDNF) in the superior cervical ganglia (SCG) neurons was not demonstrated. In the Xenopus neuromuscular system, we showed that BDNF is secreted from postsynaptic muscle cells. Thus, the target cell (myocyte) is an important player in synaptic (as opposed to axonal) competition. Second, it is widely accepted that motoneurons express only TrkB, but TrkC and TrkA expression is controversial. In addition, negligible levels of NGF are detectable in the neuromuscular system (46) and TrkB expression in sympathetic neurons is extremely low (47). Therefore, application of excessive BDNF, even with low affinity, can only bind to p75NTR, triggering axonal pruning. Third, and most importantly, in SCG, two separate ligands (NGF and BDNF) are needed for reward and punishment signals, respectively. At the NMJ, the reward and punishment signals are derived from the same molecule, depending on proteolytic cleavage. Our model suggests that the punishment signal (proBDNF) acts on all competing axons to promote elimination, and the active axon is spared because it activates extracellular protease(s) that convert the punishment (proBDNF) signal to a reward (mBDNF) signal.
Several issues require further investigation. First, what is the identity and source of the protease(s)? Our imaging experiments (Figs. 2 and 3) point to metalloproteases, particularly MMP3 and MMP9, as candidates that cleave proBDNF at the active terminal during synaptic competition in Xenopus NMJ. Fluorogenic beacon experiments suggest that the MMPs are derived from axonal terminal, but not from muscle cells (Fig. 2D). Indeed, these proteases are expressed in motor neurons and are highly enriched at the NMJs (48–50). Further work is necessary to identify the specific proteases required at mouse NMJs in vivo. Second, contrary to our findings, several previous studies have shown that inhibition of protease activity attenuates synaptic elimination (39, 40) and even increases numbers of acetylcholine receptor (AChR) in the postsynaptic muscle (51, 52). One way to reconcile these two sets of data is that inhibition of a protease “X,” the function of which is to degrade MMPs, may result in an enhanced conversion of proBDNF to mBDNF by MMPs, leading to attenuation of synaptic elimination. Moreover, our results suggest that proBDNF is derived from postsynaptic muscle cells but not presynaptic motor neurons. It remains to be established whether muscle cells are the only source of proBDNF or whether other cell types, such as Schwann cells, also produce and secrete proBDNF at the NMJ in vivo. With the currently available technologies, it is not possible to demonstrate the secretion of BDNF (more specifically proBDNF) at the NMJs in vivo. An important future experiment is to demonstrate that proBDNF is both secreted and cleaved locally at the active terminal but not at the inactive terminal in vivo. Finally, activation of p75NTR may be a general mechanism for activity-dependent synapse retraction. Indeed, in sympathetic neurons, BDNF (possibly proBDNF) acts through p75NTR to suppress inactive axons, whereas active axons (depolarized by high K+) of the same neurons are spared (45). It will be extremely important to test whether protease-mediated conversion of proBDNF to mBDNF also contributes to activity-dependent synaptic competition in the central nervous system (CNS), an example being ocular dominance formation within the visual cortex.
Experimental Procedures
Full details are contained in SI Experimental Procedures. Use and care of animals in this study abided by the guidelines of the institutional Animal Care and Use Committee at the National Institutes of Health NIH.
Embryo Injection and Xenopus Nerve–Muscle Coculture.
Xenopus nerve–muscle cocultures were prepared as described (53). Morpholinos, siRNAs, or cDNAs were injected into one of the blastomeres at the 2- to 4-cell or 8- to 16-cell stage as described (53).
Immunocytochemistry, Confocal Microscopy Image Analysis, and Statistics.
Xenopus nerve–muscle cocultures were immunostained, maintained, and analyzed as described previously (35). See SI Experimental Procedures for details. Image analysis was performed by investigators who were blinded to experimental conditions.
Supplementary Material
Acknowledgments
We thank Drs. Phillip Nelson, Eugene Zaitsev, Keri Martinowitch, Jay Chang, and Newton Woo for thoughtful comments and suggestions and Regeneron Pharmaceuticals for providing recombinant BDNF. We also thank Drs. Bruce Carter, Mark Bothwell, Moses Chao, and Phil Barker for antibodies to p75NTR and Louis Reichardt and Moses Chao for antibodies to TrkB. Microscopy imaging was performed at the Porter Neuroscience Center Light Imaging Facility with the assistance of Dr. Carolyn Smith (NIH). This work was supported by the National Institute of Mental Health (NIMH) and National Institute of Child Health and Human Development (NICHD) intramural research programs (B.L.) and grants from the NIH and Muscular Dystrophy Association (MDA) (to B.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207767109/-/DCSupplemental.
References
- 1.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 2.Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- 3.Goda Y, Davis GW. Mechanisms of synapse assembly and disassembly. Neuron. 2003;40:243–264. doi: 10.1016/s0896-6273(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 4.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 5.Taha SA, Stryker MP. Molecular substrates of plasticity in the developing visual cortex. Prog Brain Res. 2005;147:103–114. doi: 10.1016/S0079-6123(04)47008-3. [DOI] [PubMed] [Google Scholar]
- 6.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 7.Debski EA, Cline HT. Activity-dependent mapping in the retinotectal projection. Curr Opin Neurobiol. 2002;12:93–99. doi: 10.1016/s0959-4388(02)00295-7. [DOI] [PubMed] [Google Scholar]
- 8.Lichtman JW, Balice-Gordon RJ. Understanding synaptic competition in theory and in practice. J Neurobiol. 1990;21:99–106. doi: 10.1002/neu.480210107. [DOI] [PubMed] [Google Scholar]
- 9.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt RM, Balice-Gordon RJ. Activity-dependent elimination of neuromuscular synapses. J Neurocytol. 2003;32:777–794. doi: 10.1023/B:NEUR.0000020623.62043.33. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen QT, Lichtman JW. Mechanism of synapse disassembly at the developing neuromuscular junction. Curr Opin Neurobiol. 1996;6:104–112. doi: 10.1016/s0959-4388(96)80015-8. [DOI] [PubMed] [Google Scholar]
- 12.Jennings C. Developmental neurobiology. Death of a synapse. Nature. 1994;372:498–499. doi: 10.1038/372498a0. [DOI] [PubMed] [Google Scholar]
- 13.Snider WD, Lichtman JW. Are neurotrophins synaptotrophins? Mol Cell Neurosci. 1996;7:433–442. doi: 10.1006/mcne.1996.0031. [DOI] [PubMed] [Google Scholar]
- 14.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 15.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 17.Lu B. Pro-region of neurotrophins: Role in synaptic modulation. Neuron. 2003;39:735–738. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- 18.Chao MV, Bothwell M. Neurotrophins: To cleave or not to cleave. Neuron. 2002;33:9–12. doi: 10.1016/s0896-6273(01)00573-6. [DOI] [PubMed] [Google Scholar]
- 19.Ibáñez CF. Jekyll-Hyde neurotrophins: The story of proNGF. Trends Neurosci. 2002;25:284–286. doi: 10.1016/s0166-2236(02)02169-0. [DOI] [PubMed] [Google Scholar]
- 20.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 21.Woo NH, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 22.Rösch H, Schweigreiter R, Bonhoeffer T, Barde YA, Korte M. The neurotrophin receptor p75NTR modulates long-term depression and regulates the expression of AMPA receptor subunits in the hippocampus. Proc Natl Acad Sci USA. 2005;102:7362–7367. doi: 10.1073/pnas.0502460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korte M, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 25.Patterson SL, et al. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 26.Teng HK, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagappan G, et al. Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc Natl Acad Sci USA. 2009;106:1267–1272. doi: 10.1073/pnas.0807322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, et al. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang PT, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 30.Moody SA. Quantitative lineage analysis of the origin of frog primary motor and sensory neurons from cleavage stage blastomeres. J Neurosci. 1989;9:2919–2930. doi: 10.1523/JNEUROSCI.09-08-02919.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuzaki M, et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seidah NG, Benjannet S, Pareek S, Chrétien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- 33.Blöchl A, Thoenen H. Localization of cellular storage compartments and sites of constitutive and activity-dependent release of nerve growth factor (NGF) in primary cultures of hippocampal neurons. Mol Cell Neurosci. 1996;7:173–190. doi: 10.1006/mcne.1996.0014. [DOI] [PubMed] [Google Scholar]
- 34.Fu WM, Liou JC, Lee YH, Liou HC. Potentiation of neurotransmitter release by activation of presynaptic glutamate receptors at developing neuromuscular synapses of Xenopus. J Physiol. 1995;489:813–823. doi: 10.1113/jphysiol.1995.sp021094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang F, et al. Pro-BDNF-induced synaptic depression and retraction at developing neuromuscular synapses. J Cell Biol. 2009;185:727–741. doi: 10.1083/jcb.200811147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du JL, Poo MM. Rapid BDNF-induced retrograde synaptic modification in a developing retinotectal system. Nature. 2004;429:878–883. doi: 10.1038/nature02618. [DOI] [PubMed] [Google Scholar]
- 37.Hutson LD, Bothwell M. Expression and function of Xenopus laevis p75(NTR) suggest evolution of developmental regulatory mechanisms. J Neurobiol. 2001;49:79–98. doi: 10.1002/neu.1067. [DOI] [PubMed] [Google Scholar]
- 38.Buffelli M, et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 39.Connold AL, Evers JV, Vrbová G. Effect of low calcium and protease inhibitors on synapse elimination during postnatal development in the rat soleus muscle. Brain Res. 1986;393:99–107. doi: 10.1016/0165-3806(86)90069-6. [DOI] [PubMed] [Google Scholar]
- 40.Vrbová G, Fisher TJ. The effect of inhibiting the calcium activated neutral protease, on motor unit size after partial denervation of the rat soleus muscle. Eur J Neurosci. 1989;1:616–625. doi: 10.1111/j.1460-9568.1989.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Fields RD, Festoff BW, Nelson PG. Proteolytic action of thrombin is required for electrical activity-dependent synapse reduction. Proc Natl Acad Sci USA. 1994;91:10300–10304. doi: 10.1073/pnas.91.22.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson PG, Lanuza MA, Jia M, Li MX, Tomas J. Phosphorylation reactions in activity-dependent synapse modification at the neuromuscular junction during development. J Neurocytol. 2003;32:803–816. doi: 10.1023/B:NEUR.0000020625.70284.a6. [DOI] [PubMed] [Google Scholar]
- 43.Deppmann CD, et al. A model for neuronal competition during development. Science. 2008;320(5874):369–373. doi: 10.1126/science.1152677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh KK, et al. Developmental axon pruning mediated by BDNF-p75NTR-mediated axon degeneration. Nat Neurosci. 2008;11(6):649–658. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- 45.Singh KK, Miller FD. Activity regulates positive and negative neurotrophin-derived signals to determine axon competition. Neuron. 2005;45:837–845. doi: 10.1016/j.neuron.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 46.Pitts EV, Potluri S, Hess DM, Balice-Gordon RJ. Neurotrophin and Trk-mediated signaling in the neuromuscular system. Int Anesthesiol Clin. 2006;44:21–76. doi: 10.1097/00004311-200604420-00004. [DOI] [PubMed] [Google Scholar]
- 47.Fagan AM, et al. TrkA, but not TrkC, receptors are essential for survival of sympathetic neurons in vivo. J Neurosci. 1996;16:6208–6218. doi: 10.1523/JNEUROSCI.16-19-06208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kherif S, Dehaupas M, Lafuma C, Fardeau M, Alameddine HS. Matrix metalloproteinases MMP-2 and MMP-9 in denervated muscle and injured nerve. Neuropathol Appl Neurobiol. 1998;24:309–319. doi: 10.1046/j.1365-2990.1998.00118.x. [DOI] [PubMed] [Google Scholar]
- 49.VanSaun M, Werle MJ. Matrix metalloproteinase-3 removes agrin from synaptic basal lamina. J Neurobiol. 2000;43:140–149. [PubMed] [Google Scholar]
- 50.Schoser BG, Blottner D. Matrix metalloproteinases MMP-2, MMP-7 and MMP-9 in denervated human muscle. Neuroreport. 1999;10:2795–2797. doi: 10.1097/00001756-199909090-00018. [DOI] [PubMed] [Google Scholar]
- 51.Werle MJ, VanSaun M. Activity dependent removal of agrin from synaptic basal lamina by matrix metalloproteinase 3. J Neurocytol. 2003;32:905–913. doi: 10.1023/B:NEUR.0000020631.69804.f5. [DOI] [PubMed] [Google Scholar]
- 52.VanSaun M, Herrera AA, Werle MJ. Structural alterations at the neuromuscular junctions of matrix metalloproteinase 3 null mutant mice. J Neurocytol. 2003;32:1129–1142. doi: 10.1023/B:NEUR.0000021907.68461.9c. [DOI] [PubMed] [Google Scholar]
- 53.Je HS, et al. Presynaptic protein synthesis required for NT-3-induced long-term synaptic modulation. Mol Brain. 2011;4:1. doi: 10.1186/1756-6606-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.