Abstract
To produce genetically engineered T cells directed against prostate and breast cancer cells, we have cloned the T-cell receptor recognizing the HLA-A2–restricted T-cell recptor γ-chain alternate reading-frame protein (TARP)4–13 epitope. TARP is a protein exclusively expressed in normal prostate epithelium and in adenocarcinomas of the prostate and breast. Peripheral blood T cells transduced with a lentiviral vector encoding the TARP-TCR proliferated well when exposed to peptide-specific stimuli. These cells exerted peptide-specific IFN-γ production and cytotoxic activity. Importantly, HLA-A2+ prostate and breast cancer cells expressing TARP were also killed, demonstrating that the TARP4–13 epitope is a physiologically relevant target for T-cell therapy of prostate and breast cancer. In conclusion, we present the cloning of a T cell receptor (TCR) directed against a physiologically relevant HLA-A2 epitope of TARP. To our knowledge this report on engineering of T cells with a TCR directed against an antigen specifically expressed by prostate cells is unique.
Keywords: genetic engineering, T-cell receptor transfer
T-cell–based immunotherapy is a promising approach to treat disseminated cancer; however, it has been limited by the difficulty to isolate and expand T cells specific for tumor-associated antigens. Using ex vivo lentiviral gene transfer, patient T cells can be genetically engineered to express a novel T-cell receptor (TCR) or chimeric antigen receptor (CAR). Thereby, they acquire specificity for tumor-associated antigens and hence exert tumor cell cytotoxicity. Indeed, T cells genetically engineered with TCRs or CARs have recently been successfully used in cancer treatment (1–3). In terms of prostate cancer, CARs against the prostate-specific membrane antigen (PSMA) (4, 5) and the prostate stem cell antigen (PSCA) (6) have been evaluated preclinically. Genetically engineered T cells with a TCR directed against a prostate differentiation antigen have not yet been developed.
The TCRγ chain alternate reading-frame protein (TARP) is a protein exclusively expressed in normal prostate epithelium, as well as in adenocarcinomas of the prostate and breast (7, 8). Three HLA-A2–restricted TARP epitopes with relevance for cancer vaccine, and cytolytic T-cell development have been described: TARP4–13 (9), TARP27–35 (9, 10), and TARP29–37 (10). T-helper cell epitopes derived from TARP have also been reported (11) and an antibody fragment against the HLA-A2/TARP29–37 complex has been developed for targeting TARP-expressing cancer cells (12). Antibody responses against TARP have been detected in prostate cancer patient treated with GM-CSF–secreting cellular immunotherapy (13) and early-stage HLA-A2+ prostate cancer patients have circulating CD8+ T cells against the TARP4–13 and TARP27–35 epitopes (14). Taken together, these data indicate that TARP is a relevant immunological target in prostate cancer. We have successfully used tetramers with HLA-A2–restricted TARP peptides to isolate and expand TARP-specific CD8+ T cells ex vivo in the past (9). We have now used limited dilution of TARP-specific T cells and cloned a TCR specific for the HLA-A2–restricted TARP4–13 epitope (FPPSPLFFFL). The coding sequence for the full-length TCRα and -β chains with human variable (V), diverse (D), and joining (J) segments and mouse constant domains (C) was synthesized and cloned into a lentiviral vector. T cells engineered with the unique TARP4–13–specific TCR were found to be specific and efficient in killing TARP-expressing HLA-A2+ prostate and breast cancer cells.
Results
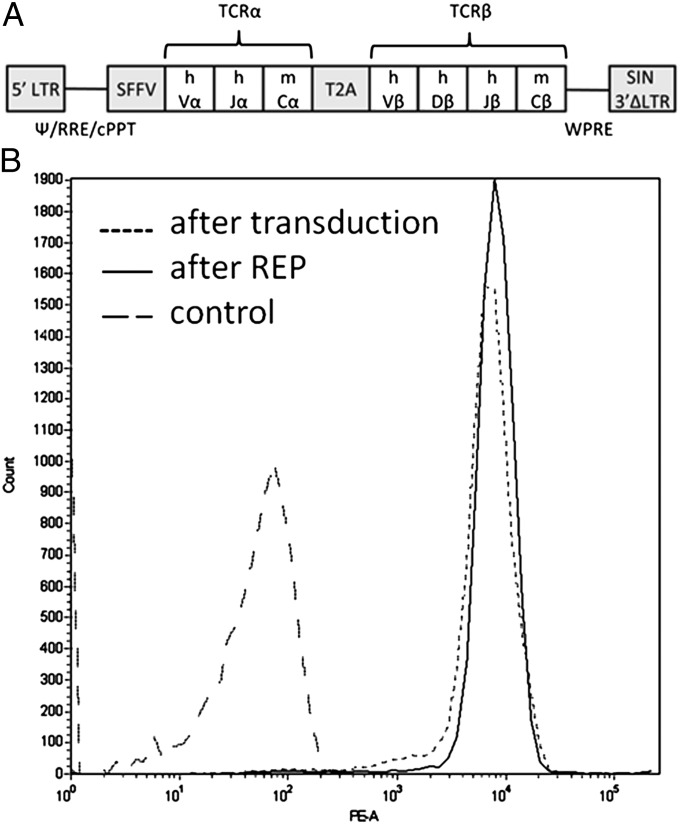
We developed single-cell clones of sorted and long-term stored TARP4–13–specific T cells (9) through limited dilution. RNA was isolated from TARP(P5L)4–13/HLA-A2-dextramer–positive clones that also secreted high levels of IFN-γ against T2 cells pulsed with the TARP(P5L)4–13 peptide. Ten individual clones were sequenced and verified to have identical TCRα chains and identical TCRβ chains. The sequence encoding the developed TCR was synthesized and cloned in a lentiviral vector under transcriptional control of the spleen focus forming virus (SFFV) promoter (Fig. 1A). To ensure equimolar expression of TCRα and TCRβ, the sequence encoding the self-cleaving peptide T2A was introduced in between the two chains. To reduce mispairing with endogenous TCRα and -β chains, the constant domains of the introduced TCRα and -β chains were replaced with mouse counterparts.
Fig. 1.
Illustration of the TARP-TCR expression cassette and TARP-TCR expression in engineered T cells. (A) The encoded TCR, which specifically recognizes the HLA-A2/TARP4–13 complex, has TCRα and TCRβ chains with human (h) variable (V) and mouse (m) constant (C) domains. The TCRα and TCRβ sequences are separated by a sequence encoding a self-cleaving T2A peptide and the SFFV promoter controls expression of the TCR transgene in a lentiviral vector. (B) Peripheral blood lymphocytes were transduced with the TARP-TCR–encoding lentiviral vector and CD3+, CD8+ T cells expressing the TCR were detected by TARP(P5L)4–13/HLA-A2-dextramer after transduction and after REP expanding the T cells. Control cells did not show unspecific binding to the dextramer. The experiment was repeated at least three times and one representative experiment is shown.
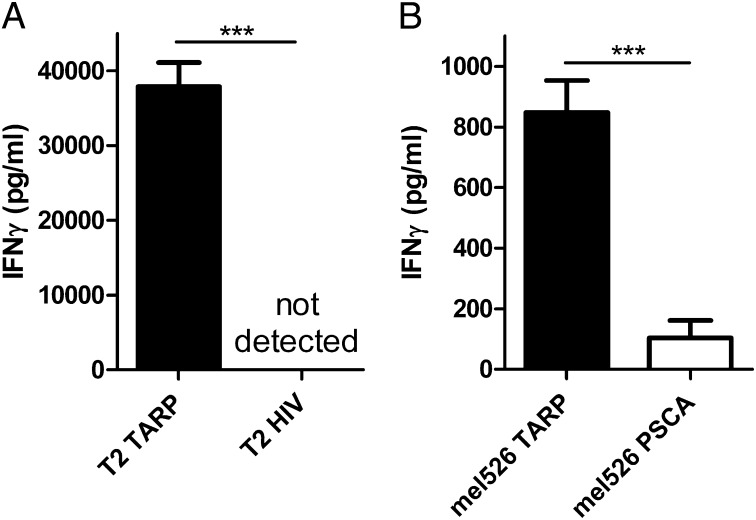
Human peripheral blood CD8+ lymphocytes were transduced with the TARP-TCR–encoding lentivirus and 1 wk later were stained with antibodies against CD3 and CD8 and with the TARP(P5L)4–13/HLA-A2 dextramer. The dextramer positivity (Fig. 1B, after transduction) verifies the specificity of the cloned TCR, and it shows that the introduced TCRα and -β chains pairs efficiently with each other and are expressed on the surface of transduced T cells. The transduced T cells were then rapidly expanded 500-fold and reexamined with the TARP(P5L)4–13/HLA-A2 dextramer. The percentage of TARP-TCR–positive cells and TARP-TCR expression level remained unchanged (Fig. 1B, after REP), indicating stable expression of the transferred TCR. TARP-TCR–engineered T cells produced IFN-γ when cocultured with T2 cells pulsed with the TARP(P5L)4–13 peptide (Fig. 2A, T2 TARP) but not when cocultured with T2 cells pulsed with the irrelevant HIV-1pol476–484 peptide (Fig. 2A, T2 HIV). A peptide-pulsing time of the target cells of 2 h was sufficient for high levels of IFN-γ production from the TARP-TCR–engineered T cells (Fig. S1). Furthermore, the TARP-TCR–engineered T cells specifically produced IFN-γ when cocultured with HLA-A2+ tumor cells transfected with a plasmid encoding the wild-type TARP protein (Fig. 2B, mel526 TARP) but not when cocultured with the same tumor cells transfected with an irrelevant PSCA-expressing plasmid (Fig. 2B, mel526 PSCA). The specific secretion of IFN-γ from TARP-TCR–engineered T cells when mixed with HLA-A2+ tumor cells transfected with a wild-type TARP-expressing plasmid is evidence of intracellular processing of the TARP protein yielding TARP4–13 peptide presentation by HLA-A2. Importantly, this evidence shows that the transferred TCR, which binds the TARP4–13/HLA-A2 complex, recognizes a physiologically relevant peptide epitope.
Fig. 2.
TARP-TCR–modified T cells secrete IFN-γ in response to peptide-specific stimulation. (A) HLA-A2+ T2 target cells were pulsed for 2 h with the TARP(P5L)4–13 peptide (T2 TARP) or the irrelevant HIV-1pol476–484 peptide (T2 HIV) and cocultured overnight with TARP-TCR–engineered T cells. (B) HLA-A2+ tumor cells (mel526) were transfected with a plasmid encoding either the full-length wild-type TARP protein (mel526 TARP) or the irrelevant PSCA protein (mel526 PSCA) and cocultured overnight with TARP-TCR–engineered T cells. An ELISA was used to measure IFN-γ secretion from TARP-TCR–engineered T cells. Asterisks denote significant difference (***P < 0.001, Student t test). Bars represent mean +SD from three experiments.
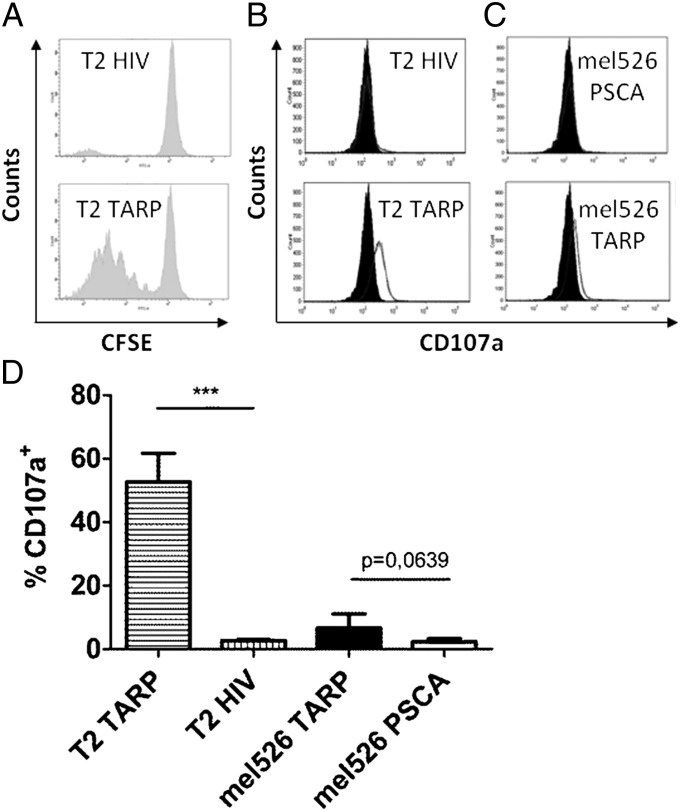
Next, we evaluated the proliferative capacity of the TARP-TCR–engineered T cells. These cells were labeled with 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) and cultured for 5 d with HLA-A2+ target cells presenting either the relevant TARP(P5L)4–13 peptide or the irrelevant HIV-1pol476–484 peptide. We observed efficient and specific proliferation of the TARP-TCR–engineered T cells after stimulation with TARP-presenting target cells (Fig. 3A). The data show that TARP-TCR–engineered T cells can proliferate efficiently upon contact with their cognate antigen.
Fig. 3.
TARP-TCR–modified T cells proliferate and get activated in response to specific stimulation. (A) TARP-TCR–engineered T cells were labeled with CFSE and mixed with T2 target cells pulsed with either the HLA-A2–restricted TARP(P5L)4–13 peptide (T2 TARP) or the irrelevant HLA-A2–restricted HIV-1pol476–484 peptide (T2 HIV). The CFSE-labeled T cells were analyzed for cell proliferation by flow cytometry 5 d later. One representative experiment of three is shown. (B) TARP-TCR–engineered T cells were mixed with T2 target cells pulsed with either the TARP(P5L)4–13 peptide (T2 TARP) or the irrelevant HIV-1pol476–484 peptide (T2 HIV). T cells were analyzed for CD107a expression after 12 h. One representative experiment of two is shown. (C) TARP-TCR–engineered T cells were mixed with mel526 target cells transfected with a plasmid encoding either the full-length wild-type TARP (mel526 TARP) protein or the irrelevant PSCA protein (mel526 PSCA). T cells were analyzed for CD107a expression after 12 h. One representative experiment of two is shown. (D) Percentage CD107a+ of all CD3+ T cells after culture with the indicated target cells. Asterisks denote significant difference (***P < 0.001, Student t test). Bars depict mean +SD of two experiments run in triplicates.
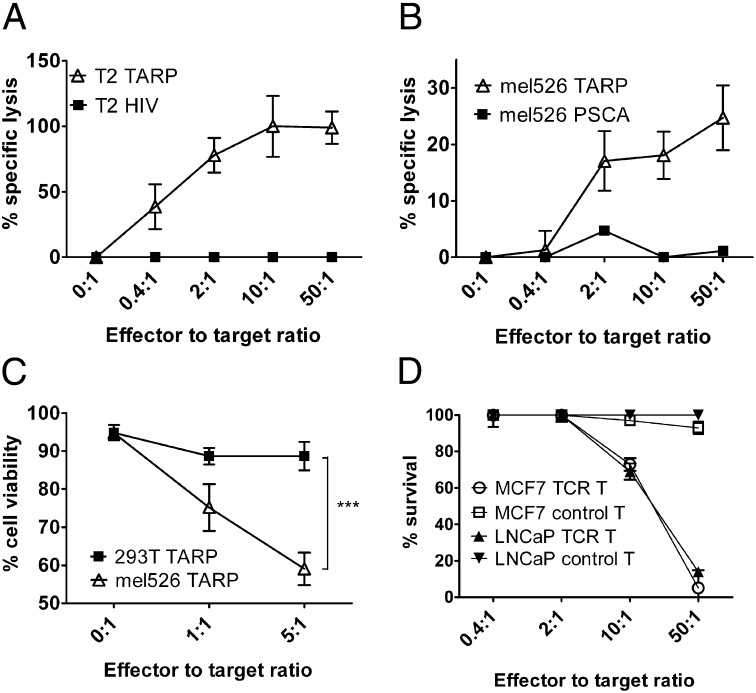
Surface localization of the degranulation marker CD107a, also known as LAMP-1, which is normally found inside granules of T cells, is a sign of cytolytic activity as the T cells release perforin and granzymes from the granules to kill target cells. Specific and significant up-regulation of CD107a was observed on the TARP-TCR–engineered T cells after exposure to target cells pulsed with the TARP(P5L)4–13 peptide (Fig. 3 B and D). A clear—however, not significant—up-regulation was also observed after exposure to target cells overexpressing the TARP protein (Fig. 3 C and D). A chromium release assay confirmed that the TARP-TCR–engineered T cells specifically kill TARP(P5L)4–13 peptide-pulsed T2 target cells (Fig. 4A). Furthermore, using the same assay we were able to demonstrate specific killing of HLA-A2+ tumor cells transfected with a plasmid encoding the full-length wild-type TARP (Fig. 4B). HLA-A2–restricted cytotoxicity was further verified by a flow cytometry-based assay where TARP plasmid-transfected HLA-A2+ mel526 cells were killed but TARP plasmid-transfected HLA-A2− HEK-293T cells were not killed (Fig. 4C).
Fig. 4.
TARP-TCR–modified T cells specifically kill TARP4–13-presenting tumor cells. (A) TARP-TCR–engineered T cells were mixed with 51Cr-labeled T2 target cells, pulsed either with the HLA-A2–restricted TARP(P5L)4–13 peptide (T2 TARP) or the irrelevant HLA-A2–restricted HIV-1pol476–484 peptide (T2 HIV). (B) TARP-TCR–engineered T cells were mixed with 51Cr-labeled mel526 target cells transfected with a plasmid encoding either the full-length wild-type TARP (mel526 TARP) or the irrelevant PSCA protein (mel526 PSCA). Specific killing was assayed in terms of 51Cr release from dead target cells after 4 h. Data represent triplicate samples from one representative experiment of two. (C) TARP-TCR–engineered T cells were mixed with either HLA-A2+ target cells (mel526) or HLA-A2− target cells (HEK-293T) transfected with a plasmid encoding the full-length wild-type TARP protein. Target cell viability was assayed by propidium-iodide staining of CD3− target cells after overnight coculture with TARP-TCR–engineered T cells. Asterisks denote significant difference (***P < 0.001, two way ANOVA). Shown is pooled data from two experiments run in triplicates. (D) The HLA-A2–engineered prostate cancer cell line LNCaP and the IFN-γ pretreated breast cancer cell line MCF7 were transduced with a lentiviral vector encoding firefly luciferase. TARP-TCR T cells or nontransduced control T cells were cocultured for 72 h with luciferase-expressing LNCaP or MCF7 target cells at ratios 0.4:1, 2:1, 10:1, and 50:1. Luciferase expression was then examined as a measure of target cell viability. Luciferase expression from target cells not exposed to T cells was set to 100%.
There is today no appropriate prostate cancer cell line expressing TARP along with high levels of HLA-A2 (15). The prostate cancer cell line LNCaP expresses TARP (15), (Fig. S2A) and is HLA-A2+ (15). However, LNCaP has an intracellular defect in the assembly of the MHC class I molecule (16) and HLA-A2 expression on the cell surface is virtually zero and is not increasing after IFN-γ treatment (15, 16). Herein, we overcame the low HLA-A2 surface expression by engineering LNCaP to express HLA-A2 from a lentiviral vector (Fig. 2SB). The breast cancer cell line MCF7 is both positive for TARP (15) (Fig. S2A) and HLA-A2+ (15). Upon IFN-γ treatment, MCF7 up-regulates its surface expression of HLA-A2 (15), (Fig. S2C). To evaluate whether the TARP-TCR–engineered T cells were able to kill prostate and breast cancer cells that naturally express TARP, we tested their ability to kill LNCaP and MCF7. Killing of target cells by TARP-TCR–engineered T cells was observed at ratios 10:1 and 50:1 (Fig. 4D, TCR T), whereas nonengineered T cells were not able to kill target cells even at 50:1 ratio (Fig. 4D, control T). The data presented in Figs. 3C and 4 B–D further stress that TARP is processed in such a way that the TARP4–13 peptide is presented in the context of HLA-A2 on the surface of target cells, showing that TARP4–13 is a physiologically relevant target for T-cell therapy of prostate and breast cancer.
Discussion
Recent clinical trials for prostate cancer have demonstrated that immunotherapy can lead to improvements in overall survival. These studies include randomized controlled trials with Provenge and PROSTVAC-VF, both of which rely on stimulating the immune system to target prostate proteins (17). Furthermore, the success story of genetically engineered T cells inducing complete remission in patients with otherwise treatment refractory B-cell leukemia (1, 3) indicates that T-cell therapy may lead to efficient new treatment options for patients with incurable cancer. Genetically engineered T cells with CARs against PSMA have recently entered clinical trials (www.clinicaltrials.gov). However, so far TCR-engineered T cells have not yet been developed for prostate cancer.
Herein, we present a unique report on the cloning of a TCR with specificity for a prostate differentiation antigen. The targeted antigen is TARP, a protein exclusively expressed in normal prostate epithelium, as well as in adenocarcinomas of the prostate and breast. TARP may be a particularly good target for T-cell therapy of prostate cancer as we have previously shown that early stage HLA-A2+ prostate cancer patients have circulating T cells against both TARP4–13 and TARP27–35 (14). Until now, circulating T cells against TARP4–13 in cancer patients was taken as indirect evidence that the TARP4–13 peptide is in fact processed from the TARP protein and presented correctly by HLA-A2 molecules to T cells. Herein, we show that the cloned TCR specifically recognizes the TARP4–13 peptide on HLA-A2+ tumor cells transfected to express the full-length wild-type TARP protein, proving that the TARP4–13 peptide is processed and presented. Importantly, we further show that TARP-TCR–engineered T cells can kill both prostate and breast cancer cell lines expressing the TARP antigen. The obtained data are taken as evidence that TARP4–13 is a physiologically relevant T-cell target.
Because every T cell already has a unique TCR, genetic transfer of an exogenous TCRα and TCRβ pair can lead to mispairing with endogenous TCR-α and TCRβ chains. Mispairing gives rise to TCRs with unpredictable specificity and may create TCRs reactive with self-antigens and thereby generate autoreactive T cells. Furthermore, mispaired TCRs may compete for CD3 and thereby reduce the surface expression levels of the correctly paired transferred TCR. Several strategies have been used to avoid this from happening. Cohen et al. (18) successfully demonstrated replacement of the constant domain of the human TCRα and TCRβ chains with the murine counterparts. Cohen et al. also reported that murinized receptors were overexpressed on the surface of human lymphocytes compared with their human counterparts and were able to mediate higher levels of cytokine secretion when cocultured with peptide-pulsed antigen-presenting cells. Preferential pairing of murine constant regions and improved CD3 stability seemed to be responsible for these observations (18). We did not specifically address the issue of mispairing or compared human TCRs with murinized ones, but the finding that rapidly expanded TARP-TCR–engineered T cells have the same high-expression level of correctly formed TCR as before expansion, as shown by dextramer reactivity in Fig. 1B, strongly indicates that misparing is rather low.
In conclusion, we present the cloning of a TCR directed against a physiologically relevant HLA-A2 epitope of an antigen that is highly and specifically expressed by normal prostate epithelial cells, prostate cancer cells, and breast cancer cells. T cells transduced with the TCR can specifically kill appropriate prostate and breast cancer cells and may therefore become important in future development of T therapy for prostate and breast cancer.
Materials and Methods
Cell Lines.
The vector-packaging cell line HEK-293T (HLA-A2−), the breast cancer cell line MCF7 (HLA-A2+) and the melanoma cell line mel526 (HLA-A2+) were grown in DMEM, supplemented with 10% (vol/vol) FBS. The prostate cancer cell line LNCaP (HLA-A2+) was grown in RPMI-1640 supplemented with 10% (vol/vol) FBS, 10 mM Hepes, and 1 mM sodium pyruvate. LNCaP was modified to express relevant levels of HLA-A2 using a lentiviral vector (a kind gift from Richard Morgan, National Cancer Institute, Bethesda, MD). The antigen processing-deficient, HLA-A2+ T2 cell line was grown in RPMI-1640 supplemented with 10% FBS. Primary human peripheral blood mononuclear cells (PBMCs) were cultured in RPMI-1640 supplemented with 10% (vol/vol) human AB serum (our own production), 2 mM l-glutamine, 10 mM Hepes, 1% penicillin/streptomycin (PEST), and 20 μM β-mercaptoethanol. T cells derived from PBMCs were cultured in PBMC medium with the addition of 100 IU/mL of IL-2 (proleukin; Novartis). All cell culture reagents were from Invitrogen except when stated otherwise. Cells were grown under standard cell culture conditions.
Generation of T-Cell Clones.
Tetramer-sorted TARP4–13–specific T cells (9) were plated in 96-well plates at a density of 0.6 cells per well in presence of 50 ng/mL anti-CD3 antibody (OKT-3; Nordic Biosite), 1,000 IU/mL IL-2, and 40,000 irradiated (50 Gy) feeder PBMCs (a pool from three donors). Growing clones were identified by visual inspection after 2 wk, transferred to larger wells, and restimulated in the same way. After an additional restimulation and 4 wk of culturing, reactive clones were identified by IFN-γ ELISA and TARP(P5L)4–13/HLA-A2-dextramer staining (described below).
Isolation and Cloning of TCR Genes.
To clone the T-cell receptor sequence from a TARP4–13–specific T-cell, total RNA was isolated from IFN-γ/dextramer-positive clones using RNeasy Mini Kit (Qiagen). The TCRα and -β chain sequences were obtained by 5′ RACE PCR (SMARTer RACE cDNA Amplification Kit; Clontech) using the TCRα constant domain primer: 5′-GGT GAA TAG GCA GAC AGA CTT-3′; the TCRβ constant domain primer: 5′-GTG GCC AGG CAC ACC AGT GT-3′; and the Advantage 2 polymerase mix (Clontech). The RACE PCR products were purified (PCR purification kit; Qiagen) tailed with 3′ A (Taq polymerase; Invitrogen) and T/A-cloned into pCR2.1 (Invitrogen). Ten clones were sequenced and found to contain one unique TCRα chain and one unique TCRβ chain. The coding sequence for the TARP4–13–specific TCRα and TCRβ chains was initiated with a Kozak sequence (19), codon optimized, and the two chains separated by the sequence for the T2A self-cleaving peptide (20). Furthermore, to improve pairing between the exogenous TCRα and -β chains and reduce mispairing with endogenous human α- and β-chains, mouse constant domains replaced the human ones (18). The synthetic sequence was generated (Genscript) and we then subcloned it into a third generation self-inactivating lentiviral vector under the SFFV promoter to generate pBMN(TARP4–13-TCR). The construct is illustrated in Fig. 1A.
Lentivirus Production.
Vesicular stomatitis virus (VSV)-G pseudotyped lentiviral particles were produced in HEK-293T cells. Cells were transfected with pBMN(TARP4–13-TCR) and the pLP1, pLP2 and pLP/VSVG (Invitrogen) packaging plasmids at a 1:1:1:2 ratio using polyethyleneimine (Sigma-Aldrich). Viral supernatant was collected 48 and 72 h posttransfection, filtered (0.45 μm), and concentrated by ultracentrifugation at 75,000 × g for 90 min at 4 °C using a Sorvall AH629 rotor. The viral pellet was resuspended in PBS and stored at −80 °C until further use. Lentiviral vectors expressing HLA-A2 and firefly luciferase were produced in the same fashion.
Generation of TARP-TCR–Modified T Cells.
PBMCs were isolated from buffy coats of healthy blood donor using Ficoll-Paque (GE Healthcare) and activated for 24–48 h with 100 ng/mL OKT-3 antibody and 100 IU/mL IL2. In some cases, CD8+ T cells were isolated using CD8+ T-cell Isolation Kit (Miltenyi Biotec) according to the manufacturer’s instructions. One million PBMCs or CD8+ T cells were transduced with 40 μL of the concentrated TARP-TCR–coding lentivirus in the presence of 10 μg/mL protamine sulfate (Sigma-Aldrich) and 100 IU/mL IL2 for 4 h. The transduction was repeated 24 h later. Transduced T cells were expanded using the rapid expansion protocol (REP) with OKT-3, IL2 and irradiated feeder cells, as previously described (21).
Flow Cytometry Analysis.
To determine the percentage of TARP4–13-specific CD8+ T cells after transduction, the cells were stained with a phycoerythrin (PE)-conjugated TARP(P5L)4–13/HLA-A2-dextramer (Immudex), a FITC-conjugated anti-CD8 antibody (BioLegend) and an allophycocyanin (APC)-conjugated anti-CD3 antibody (BioLegend).
Peptides, Plasmids, and Preparation of Target Cells.
The HLA-A2-restricted TARP(P5L)4–13 (FLPSPLFFFL) and HIV-1pol476–484 (ILKEPVHGV) peptides were synthesized to a purity of >95% (Genscript). T2 target cells were pulsed with 50 μg/mL TARP peptide or irrelevant HIV-1 peptide for 2 h in presence of 3 μg/mL β2 microglobulin (Sigma-Aldrich) and then used as target cells for evaluation of TARP-TCR-modified T cells. The full-length wild-type TARP-coding sequence was PCR amplified and cloned into pVAX1 (Invitrogen). As a negative control, the full-length PSCA coding sequence was PCR amplified and cloned into pShuttle (Stratagene). Mel526 cells were transfected with the TARP-encoding plasmid or the irrelevant PSCA-encoding plasmid using lipofectamine 2000 (Invitrogen). Twenty-four hours later, the cells were used as target cells for evaluation of TARP-TCR–engineered T cells.
ELISA.
T cells (1 × 105) were mixed with peptide-pulsed T2 cells or plasmid-transfected mel526 cells at a 1:1 ratio and cocultured overnight. Supernatants were collected and IFN-γ ELISA was performed using an ELISA kit (Mabtech) according to the manufacturer’s instructions.
T-Cell Proliferation Assay.
TARP-TCR–modified T cells were labeled with 5 μM CFSE (Invitrogen) for 10 min in PBS and the labeling was stopped by addition of medium containing 10% (vol/vol) human AB serum. The T cells were washed twice and 1 × 105 cells were cocultured with equivalent amount of target T2 cells pulsed with TARP peptide or irrelevant HIV-1 peptide. IL-2 (10 IU/mL) was added to the medium. T-cell proliferation, as detected as dilution of the CFSE dye for each cell division, was analyzed by flow cytometry 5 d later.
Flow Cytometry-Based CD107a Degranulation Assay.
TARP-TCR–modified T cells (105 cells) were mixed with peptide-pulsed (TARP or HIV-1) T2 cells or plasmid-transfected (full-length TARP or PSCA) mel526 cells at a 1:1 ratio and cocultured overnight. For CD107a analysis the percentage of CD107a+ T cells were stained with a FITC-conjugated anti-CD107a antibody (BD Bioscience) followed by flow cytometry analysis.
51Cr Release Cytotoxicity Assay.
T2 target cells (1 × 106) were labeled with 0.25 mCi 51Cr (Perkin-Elmer) for 1 h, washed five times, and pulsed with 50 μg/mL peptide (TARP or HIV-1) for 2 h. Mel526 target cells, transfected with a plasmid encoding either the full-length TARP or PSCA were labeled with 51Cr for 1 h and washed five times. One-thousand target cells were cocultured with TARP-TCR–modified T cells at various effector to target cell ratios for 4 h in U-shaped 96 well microplates. Spontaneous release of 51Cr was assessed by the incubation of target cells in medium alone, and maximum release of 51Cr was determined by the incubation of target cells in 0.1% Triton X-100 (Sigma-Aldrich). Supernatants (100 μL) containing the released 51Cr were collected and mixed with 75 μL Optiphase SuperMix (Perkin-Elmer). Radioactivity was measured with a beta-counter (Wallac 1450 MicroBeta TriLux; Perkin-Elmer). Triplicate wells were averaged and the percentage of specific lysis was calculated as described previously (21). Spontaneous release was set to zero and in case that release from target cells was less than spontaneous release it was also set to zero.
Bioluminiscent Luciferase Reporter Gene-Based Cell Killing Assay.
LNCaP or MCF7 were modified to express luciferase using a lentiviral vector. MCF7 was treated with 1,000 IU/mL IFN-γ for 72 h before coculture with T cells. Ten-thousand target cells (LNCaP or MCF7) were plated in a 96-well plate and cocultured with transduced T cells in ratios of 0.4:1, 2:1, 10:1, or 50:1. After 72 h of coculture, the luciferase expression from viable target cells was measured using Steady-Glo Luciferase Assay System (Promega), according to the manufacturer’s instructions using a luminometer (Wallac Victor 2 Multilabel Counter; Perkin-Elmer). Luciferase acitivity from target cells not exposed to T cells was set as 100% cell viability (survival).
Flow Cytometry-Based Cytotoxicity Assay.
TARP-TCR-modified T cells (105 cells) were mixed at a 1:1 ratio and cocultured overnight with mel526 (HLA-A2+) or HEK-293T (HLA-A2−) cells transfected with a plasmid encoding the full-length TARP protein. T cells were stained with the CD3 marker and excluded from the analysis. Dead target cells were discriminated from live ones by using propidium iodide (BD Biosciences).
Statistics.
Statistical analyses were performed using GraphPad prism software version 5.04. Statistical test for IFN-γ secretion and CD107a expression were performed using paired t test. Statistical analysis for cell viability using propidium iodide staining was performed using two-way ANOVA and Bonferroni´s multiple comparison test. Values of P < 0.05 were considered to be statistically significant.
Supplementary Material
Acknowledgments
The authors thank Dr. Richard Morgan and the members of his laboratory at the National Cancer Institute, Bethesda, MD for sharing reagents and protocols and for training V.H. for 2 wk. This work was supported by the Swedish Cancer Society, Gunnar Nilsson’s Cancer Foundation, and the Marcus and Marianne Wallenberg’s Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209042109/-/DCSupplemental.
References
- 1.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong MC, et al. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1:123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gade TP, et al. Targeted elimination of prostate cancer by genetically directed human T lymphocytes. Cancer Res. 2005;65:9080–9088. doi: 10.1158/0008-5472.CAN-05-0436. [DOI] [PubMed] [Google Scholar]
- 6.Morgenroth A, et al. Targeting of tumor cells expressing the prostate stem cell antigen (PSCA) using genetically engineered T-cells. Prostate. 2007;67:1121–1131. doi: 10.1002/pros.20608. [DOI] [PubMed] [Google Scholar]
- 7.Essand M, et al. High expression of a specific T-cell receptor gamma transcript in epithelial cells of the prostate. Proc Natl Acad Sci USA. 1999;96:9287–9292. doi: 10.1073/pnas.96.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfgang CD, Essand M, Vincent JJ, Lee B, Pastan I. TARP: A nuclear protein expressed in prostate and breast cancer cells derived from an alternate reading frame of the T cell receptor gamma chain locus. Proc Natl Acad Sci USA. 2000;97:9437–9442. doi: 10.1073/pnas.160270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsson B, Tötterman TH, Essand M. Generation of cytotoxic T lymphocytes specific for the prostate and breast tissue antigen TARP. Prostate. 2004;61:161–170. doi: 10.1002/pros.20091. [DOI] [PubMed] [Google Scholar]
- 10.Oh S, et al. Human CTLs to wild-type and enhanced epitopes of a novel prostate and breast tumor-associated protein, TARP, lyse human breast cancer cells. Cancer Res. 2004;64:2610–2618. doi: 10.1158/0008-5472.can-03-2183. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H, et al. Recognition of prostate and breast tumor cells by helper T lymphocytes specific for a prostate and breast tumor-associated antigen, TARP. Clin Cancer Res. 2005;11:3869–3878. doi: 10.1158/1078-0432.CCR-04-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epel M, et al. Targeting TARP, a novel breast and prostate tumor-associated antigen, with T cell receptor-like human recombinant antibodies. Eur J Immunol. 2008;38:1706–1720. doi: 10.1002/eji.200737524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen MC, et al. Antibody responses to galectin-8, TARP and TRAP1 in prostate cancer patients treated with a GM-CSF-secreting cellular immunotherapy. Cancer Immunol Immunother. 2010;59:1313–1323. doi: 10.1007/s00262-010-0858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsberg O, et al. High frequency of prostate antigen-directed T cells in cancer patients compared to healthy age-matched individuals. Prostate. 2009;69:70–81. doi: 10.1002/pros.20858. [DOI] [PubMed] [Google Scholar]
- 15.Carlsson B, Forsberg O, Bengtsson M, Tötterman TH, Essand M. Characterization of human prostate and breast cancer cell lines for experimental T cell-based immunotherapy. Prostate. 2007;67:389–395. doi: 10.1002/pros.20498. [DOI] [PubMed] [Google Scholar]
- 16.Sanda MG, et al. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst. 1995;87:280–285. doi: 10.1093/jnci/87.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal N, Padmanabh S, Vogelzang NJ. Development of novel immune interventions for prostate cancer. Clin Genitourin Cancer. 2012;10:84–92. doi: 10.1016/j.clgc.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984;12:857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szymczak AL, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 21.Carlsson B, Cheng WS, Tötterman TH, Essand M. Ex vivo stimulation of cytomegalovirus (CMV)-specific T cells using CMV pp65-modified dendritic cells as stimulators. Br J Haematol. 2003;121:428–438. doi: 10.1046/j.1365-2141.2003.04300.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.