Abstract
Passive transfer of neutralizing antibodies against HIV-1 can prevent infection in macaques and seems to delay HIV-1 rebound in humans. Anti-HIV antibodies are therefore of great interest for vaccine design. However, the basis for their in vivo activity has been difficult to evaluate systematically because of a paucity of small animal models for HIV infection. Here we report a genetically humanized mouse model that incorporates a luciferase reporter for rapid quantitation of HIV entry. An antibody’s ability to block viral entry in this in vivo model is a function of its bioavailability, direct neutralizing activity, and effector functions.
HIV-1 (HIV), the causative agent of AIDS, represents a formidable global challenge, with the development of an effective vaccine being of paramount importance (1–4). Rapid progress in this area has been hindered in part by lack of a widely available small animal model for HIV entry. Currently available animal models include nonhuman primates and immunodeficient humanized mice, neither of which is readily available or amenable to genetic modifications (5, 6).
Some viral pathogens exhibit a narrow host range, one of those being HIV. HIV’s entry into target cells is mediated by binding of its trimeric envelope spike (gp160) to human CD4 (hCD4) (7) and subsequently to a coreceptor such as human CXCR4 (8) or human CCR5 (hCCR5) (9–11). hCCR5 is of particular interest because it seems to be the primary coreceptor used for transmission (12, 13), as evidenced by the finding that homozygous deletion in the CCR5 allele confers resistance against HIV-1 acquisition (14, 15) and can also lead to long-term control of HIV after stem cell transplantation (16). Finally, HeLa cells and other HIV-resistant cells, including mouse cells, support viral entry when they are engineered to express hCD4/hCCR5/hCXCR4 (17–19).
Here, we describe a hCCR5- and hCD4-expressing luciferase reporter mouse that can be used to measure HIV pseudovirus entry and antibody-mediated protection against initial infection in vivo.
Results
hCCR5-2A-hCD4 Construct.
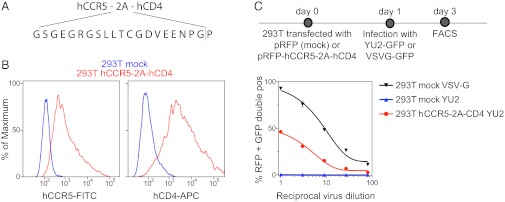
To overcome HIV’s host-restriction at the level of viral entry, we coexpressed hCCR5 and hCD4 on a single poly-protein transcript separated by a ribosomal skip 2A peptide sequence (hCCR5-2A-hCD4) (Fig. 1A) (20). Coexpression of hCCR5 and hCD4 was verified in transfected HEK293T cells (293ThCCR5-2A-hCD4) by flow cytometry (Fig. 1B). The ability of these proteins to support viral entry was confirmed by infection of 293ThCCR5-2A-hCD4 with an HIVYU-2 pseudotyped lentivirus encoding GFP (Fig. 1C). We conclude that the hCCR5-2A-hCD4 mRNA supports cell surface expression of the two proteins that restrict HIV entry into mammalian cells.
Fig. 1.
Construction of hCCR5-2A-hCD4. (A) Schematic diagram of the hCCR5-2A-hCD4 construct showing the sequence of the ribosomal skip 2A peptide sequence. (B) Representative histogram plots showing the surface expression of hCCR5 and hCD4 on 293T cells transfected with hCCR5-2A-hCD4 [allophycocyanin (APC); fluorescein isothiocyanate (FITC)]. (C) Functional expression of hCCR5 and hCD4. 293T cells transfected with hCCR5-2A-hCD4-IRES-RFP were infected with YU2-GFP. Graph shows the percentage of RFP-positive cells that are also GFP positive. Mock-transfected 293T cells served as negative control and infection with VSV-G-GFP as positive control.
Adenoviral Delivery of hCCR5-2A-hCD4.
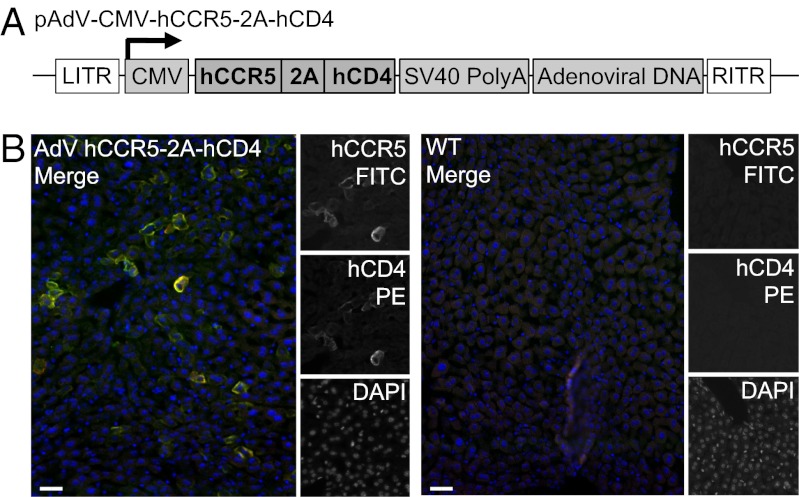
Recombinant adenoviruses provide an established tool for efficient gene delivery and expression (21, 22). To express high levels of the factors that restrict HIV entry in vivo, we used a recombinant human adenovirus (AdV) serotype 5 encoding hCCR5-2A-hCD4 under control of the CMV promoter (AdV-hCCR5-2A-hCD4) (21, 22) (Fig. 2A). AdV serotype 5 targets the liver after i.v. injection (23–26). We confirmed that i.v. administration of this vector resulted in hepatic expression of both hCD4 and hCCR5, as measured by fluorescence microscopy (Fig. 2B). On the basis of immune fluorescence, we estimate that 30–40% of the cells in the liver express hCD4 and hCCR5.
Fig. 2.
Adenoviral delivery of AdV-hCCR5-2A-hCD4. (A) Schematic diagram of the AdV construct used to deliver hCCR5-2A-hCD4. hCCR5-2A-hCD4 is under control of the CMV promoter. (B) hCCR5 and hCD4 were detected in fixed frozen liver sections by immunofluorescence microscopy 1 d after delivery of 1011 adenoviral particles of AdV-hCCR5-2A-hCD4 through the lateral tail vein [fluorescein isothiocyanate (FITC); phycoerythrin (PE)]. Representative images of two experiments are shown. (Scale bar, 30 μm.)
In Vivo Infection with HIVYU-2-Pseudotyped Lentivirus.
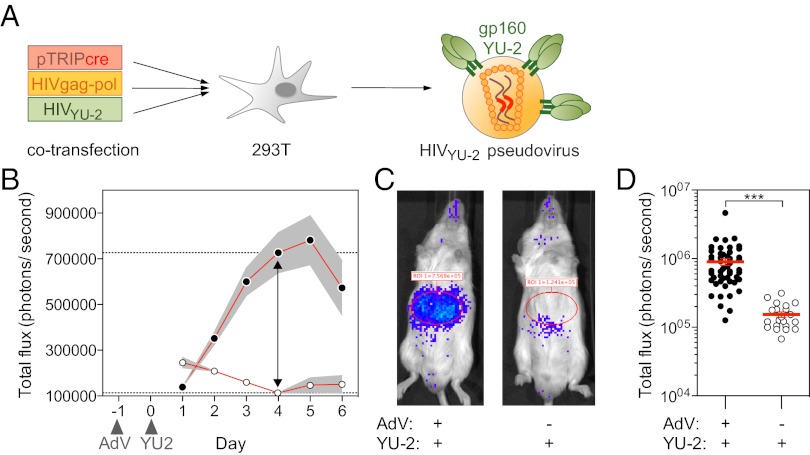
To determine whether hCCR5- and hCD4-expressing mice can be used to measure HIV entry in vivo, we transduced mice that carry an inducible loxP-STOP-loxP luciferase reporter [Gt(ROSA)26Sortm1(Luc)Kaelin] (27, 28) with AdV-hCCR5-2A-hCD4 (HIV-LUCAdV mice). One day later, HIV-LUCAdV mice were challenged i.v. with gp160YU-2-pseudotyped lentivirus (HIVYU-2) encoding Cre recombinase (Fig. 3A). Productive viral uptake by hCCR5-2A-hCD4 expressing cells would result in the expression of Cre recombinase capable of excising the transcriptional stop element and consequently inducing luciferase expression. Bioluminescence activity, imaged in an optical luminometer (IVIS; Caliper Life Sciences), increased longitudinally and peaked between day 4 and 5 after i.v. pseudovirus injection into HIV-LUCAdV mice (Fig. 3 B and C). Despite a limited dynamic range and variation by as much as one order of magnitude between mice, there was a highly significant difference between experimental and control groups (P < 0.0001; Fig. 3D). Taken together, the data indicate that HIV pseudovirus entry can be measured quantitatively in living mice.
Fig. 3.
HIV pseudovirus entry and protection in vivo. (A) Diagram summarizes method for producing HIVYU-2 pseudovirus. (B–D) ROSAfloxSTOP-Luc mice were injected with AdV-hCCR5-2A-hCD4 at day −1, followed by HIVYU-2 injection at day 0. Bioluminescence was acquired longitudinally in B and at day 4 in C and D. (B) Graph shows the mean total photon flux (±SEM) for n = 6 infected mice (filled circles) vs. controls that did not receive the AdV-hCCR5-2A-hCD4 virus, n = 3 mice (open circles). (C) Representative images of mice from B. (D) Dot plot shows total photon flux of mice receiving AdV-hCCR5-2A-hCD4 and HIVYU-2 (filled circles) or HIVYU-2 only (open circles). Each dot represents an individual mouse, error bars represent SEM. ***P < 0.0001.
Neutralizing Human Anti-HIV Monoclonal Antibodies Mediate Protection in HIV-LUCAdV Mice.
Antibodies are key components of most protective vaccines (29, 30) and thus are thought to be essential for protection against HIV infection. In support of this idea, passive administration of potent broadly neutralizing monoclonal antibodies can provide sterilizing immunity against simian/HIV (SHIV) infection in macaques (31–35), and they seem to delay HIV rebound in humans (36, 37). In addition, plasma concentration of anti-HIV IgG antibodies specific for the V1V2 loop region was inversely correlated with infection risk in the recent RV144 vaccine trial (38, 39). However, the mechanisms that mediate the protective effects in RV144 are poorly understood, as exemplified by the finding that the vaccine assessed in the RV144 trial did not elicit broadly neutralizing antibodies (38–40).
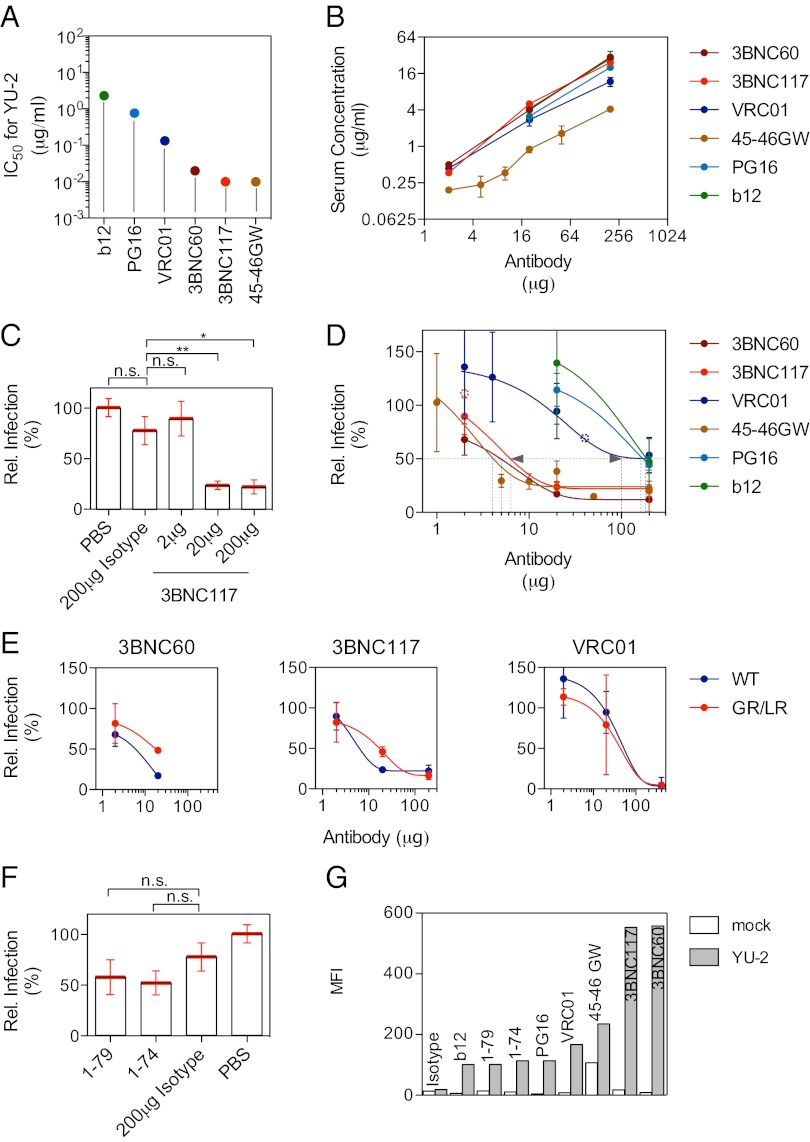
We selected six potent, broadly neutralizing anti-HIV antibodies to examine their effects on HIV entry in vivo. NIH45-46G54W (41), 3BNC117 (42), 3BNC60 (42), VRC01 (43), and b12 (44) all target the CD4 binding site, whereas PG16 (45) targets the V1/V2 loop region. These antibodies have varying levels of neutralizing activity (IC50) in the TZM-bl cell assay against HIVYU-2 in vitro, ranging from 0.01 to 2.30 μg/mL (Fig. 4A). Antibodies were administered individually at doses ranging from 1 μg to 200 μg s.c. 1 d before challenge with HIVYU-2, and luciferase expression was measured 4 d later. Serum antibody levels determined at the time of pseudovirus injection varied among the selected monoclonal antibodies. For example, high levels were achieved after 3BNC117 injection, whereas antibody concentrations for NIH45-46G54W were fourfold lower (Fig. 4B). The different serum antibody levels are in keeping with the half-life of these antibodies, with 3BNC117 having t1/2 = 48.6 h and NIH45-46G54W having t1/2 = 24.1 h after i.v. injection (Fig. S1). In contrast to the HIV neutralizing antibodies that blocked entry with varying degrees of efficacy, an isotype control antibody (mGO53) (46) had no significant effect on entry compared with the PBS control (Fig. 4 C and D). Fifty percent inhibition of entry was achieved after injection of 100–200 μg of VRC01 or PG16 or b12, whereas the same level of inhibition was obtained with as little as 4–6 μg of 3BNC117 or 3BNC60 or NIH45-46G54W. For 3BNC117 and 3BNC60 this equals a serum concentration of ∼1 μg/mL, which is 100 times greater than the in vitro IC50 (Fig. 4B). We conclude that the ability of antibodies to inhibit HIV entry in vivo can be measured directly in HIV-LUCAdV mice.
Fig. 4.
In vitro and in vivo neutralization data. (A) Broadly neutralizing mAbs were tested in the TZM-bl assay for neutralization of HIVYU-2. Plot shows the IC50 in μg/mL against HIVYU-2 for the individual monoclonal antibodies. (B) Serum antibody concentration after 1 d, the time point of pseudovirus injection, as determined by ELISA. The injected antibody amount (μg) is plotted against the determined serum concentration of the monoclonal antibody (μg/mL). (C and D) Relative infection (% compared with PBS control) plotted against the amount of mAb injected (μg) in bar graph (C; six to 17 mice per group, mean ± SEM; *P < 0.05, **P < 0.01) or dose–response curves (D; three to nine mice per group, except open circles represent a single mouse, mean ± SEM). (E) Comparison of the in vivo neutralizing activity of the WT form and GR/LR mutant of 3BNC60, 3BNC117, and VRC01 (three to nine mice per group, mean ± SEM). (F) In vivo neutralizing activity of nonneutralizing antibodies 1-79 and 1-74 (14 to 20 mice per group, mean ± SEM). (G) Binding of antibodies to the envelope of HIVYU-2 expressed on the surface of 293T cells. Graph shows the mean fluorescent intensity (MFI) for the different monoclonal antibodies binding to HIVYU-2 expressed on 293T cells. Mock-transfected cells served as negative control.
Antibody Fc Involvement in Entry Inhibition.
Neutralizing antibodies can protect against HIV infection in vitro in the absence of innate effector cells or complement (47–49). However, the mechanisms by which they mediate protection against HIV in vivo are poorly understood. Complement seems to be essential in antibody-mediated postexposure protection in hu-SCID mice (50), whereas Fc receptors but not complement are important in antibody-mediated preexposure protection in macaques (51), but neither study resolves the question of whether antibodies protect from entry by direct viral clearance or by mediating clearance of infected cells or a combination of both.
To examine the role of antibody effector functions in protection against HIV entry we introduced mutations (G236R/L328R) into the antibody Fc domain that eliminated binding to mFcγRs and complement (Fig. S2) but did not alter antibody binding to the HIV envelope protein or neutralizing activity in vitro (Fig. S3 A–C and Tables S1 and S2) (52). Dose–response experiments were performed to compare the in vivo activity of 3BNC60GR/LR, 3BNC117GR/LR, and VRC01GR/LR to WT controls. GR/LR mutant and WT forms of the individual antibodies were present at similar serum concentrations at the time of pseudovirus injection (Fig. S3D). Mutant 3BNC60GR/LR and 3BNC117GR/LR showed decreased activity at doses of 20 μg of injected antibody compared with the WT form (P = 0.0027 and P = 0.0044, respectively; Fig. 4E). Although statistically significant, these differences were small and not found at higher or lower doses of 3BNC60 or 3BNC117 or at any tested dose of VRC01. Thus, although Fc effector function seems to make a contribution to the inhibition of HIV entry, additional studies will be necessary to fully evaluate this component.
Nonneutralizing Antibodies in Entry Inhibition.
Although plasma concentration of anti-HIV IgG specific for the V1V2 loop region was inversely correlated with infection risk in the recent RV144 vaccine trial, broadly neutralizing antibodies were not found (38, 39). Whether nonneutralizing monoclonal antibodies can mediate protection in vivo remains controversial (53). For example, b6, a phage-derived monoclonal anti-HIV antibody with limited in vitro activity showed little or no protection against vaginal SHIV challenge in macaques (54). However, the macaque experiments were performed on a relatively small number of subjects compared with the RV144 trial and may not have been sufficiently powered to detect effects of the magnitude found in the trial (38, 54). To investigate whether nonneutralizing monoclonal antibodies can reduce viral entry, we selected two antibodies that bind to the HIVYU-2 trimer expressed as soluble protein (55) but that do not reach an IC50 against HIVYU-2 at concentrations of up to 50 μg/mL in vitro: 1-79 targets the V3 loop (55), and 1-74 recognizes an epitope in proximity to the CD4 binding site (55). Both of these antibodies were tested for their ability to block entry in vivo by injecting mice with 200 μg as described above. Neither reduced entry significantly compared with the isotype control (P = 0.1848 and P = 0.0830, respectively; Fig. 4F). When testing 1-79 and 1-74 for binding to the HIVYU-2 trimer expressed on the surface of 293T cells, we found that their binding is relatively weak compared with the neutralizing antibodies examined above (Fig. 4G). Thus, antibodies that bind weakly to the HIV trimer and do not neutralize the virus in the TZM-bl assay seem to have little or no effect reducing HIV entry in vivo. Additional studies with antibodies such as those derived from the RV144 trial will be necessary to fully evaluate the contribution of nonneutralizing antibodies in entry inhibition.
Discussion
Basic understanding of the humoral immune response to HIV has advanced significantly over the last several years. Antibody cloning experiments have revealed that humans can develop potent and broadly active serologic activity to the HIV trimer by producing combinations of antibodies to many different epitopes (55, 56), or by producing unique monoclonal antibodies to specific target sites, such as the CD4 binding site (42, 57). Moreover, broadly neutralizing human monoclonal antibodies can prevent chimeric SHIV infection in nonhuman primate models (31–35), and antibodies seem to be the only correlate of protection in the recent RV144 vaccine trial (38, 39).
However, progress toward an effective antibody-based vaccine has been elusive. One of the significant challenges in this area of research has been the development of adequate animal models to test vaccines or to study the mechanisms of action of broadly neutralizing antibodies. In vitro assays for antibody neutralization such as the TZM-bl assay, which is a reproducible and reliable assay for neutralizing antibody activity, do not take into account Fc-mediated antibody effector function or bioavailability (18). Moreover, antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated viral inhibition (ADCVI) assays, which are commonly used to try to measure effector function in vitro, are far more difficult to reproduce and standardize than the TZM-bl assay and are limited in that they include only a fraction of the effector cells that participate in antibody-mediated effector activity in vivo (49).
Although they cannot be infected with HIV, rhesus macaques are the current gold standard for testing vaccines because they can be challenged with simian immunodeficiency virus (SIV) (58), or with chimeric SHIV (59). However, there are a number of important issues with these models, including the fact that SIV and SHIV do not produce the same disease in rhesus macaques as HIV in humans, nor are they identical to HIV in terms of the requirements for effective vaccination (60). In addition, there is the problem of limited availability of macaques, enormous expense, inability to control for genetic heterogeneity, and finally ethical considerations.
Humanized mice (hu-mice) containing human cell- and, in some cases, tissue-transplants represent an alternative to macaques (reviewed in ref. 61). This model may be particularly useful for pharmacologic studies of anti-HIV drugs (62) and passive immunization (63), but it is limited in some important ways. Adaptive immune responses, and in particular antibody-mediated immune responses to HIV, are not optimal in these mice, and therefore they are not amenable to studies that require vaccination (64–67). Equally important, genetic experiments are not possible in chimeric mice containing mouse and human cells.
In contrast to hu-mice, the HIV-LUCAdV mouse model is fully immunocompetent, providing a platform for combined immunization and challenge studies using the i.v. route of infection. This route of exposure, which occurs primarily among i.v. drug users, represents a growing global health challenge (68). Additional advantages of the HIV-LUCAdV mice include the use of replication-deficient pseudovirus (18) as opposed to infectious virus and the possibility of genetic modifications. However, HIV-LUCAdV mice express hCCR5/hCD4 in hepatocytes, a nonphysiological target of HIV, owing to preferential hepatic expression of the Coxsackie and adenovirus receptor (CAR) in mice (69, 70). Although infection of other cell types might be achieved, it would require transgenic expression of human CAR (hCAR); for example, under control of the ubiquitin promoter using the available hCAR transgenic mouse (71) or targeting hCAR expression to specific cell types using tissue-specific promoters (72). Alternatively, if expression is high enough, transgenic mice expressing hCCR5/hCD4 under a T cell-specific promoter (19) could be used after breeding to Gt(ROSA)26Sortm1(Luc)Kaelin reporter mice. Still another alternative that could be used in combination with humanized mice would be infectious molecular clones that stably express luciferase (Env-IMC-LucR) (73).
Previous experiments in hu-mice and macaques showed that broadly neutralizing antibodies can interfere with HIV infection in vivo (50, 51). However, these experiments could not distinguish whether protection from infection was due to the effects of antibodies on infected cells or to blocking HIV entry directly, or a combination thereof. The availability of HIV-LUCAdV mice allowed for quantitative assessment of potent broadly neutralizing antibodies in vivo and revealed that the antibodies block viral entry directly because this model does not support HIV envelope expression on the surface of infected cells. This means that ADCC would not influence viral control in this system; however, other effector mechanisms, such as Fc-dependent viral uptake by Kupffer cells or other phagocytes, could play a role in viral clearance (74).
A large number of antibodies were compared directly using pseudotyped virus expressing the envelope of HIVYU-2, a difficult-to-neutralize tier 2 virus. The HIV-LUCAdV mouse model made it possible to perform dose–response experiments with a significant number of mice (220 in total), something that would be very difficult to achieve in macaques, for which the number of well-characterized SHIV envelopes and experimental animals are very limited. We find that neutralizing activity is related to both the in vitro activity in TZM-bl assays and to bioavailability in vivo. For example, VRC01 did not perform as well as 3BNC60 after s.c. injection of equivalent doses, likely owing to the lower bioavailability of VRC01 and the weaker in vitro neutralizing potency. Conversely, NIH45-46G54W, which had equal potency in vitro but lower bioavailability than 3BNC117, was similar in its ability to the latter in blocking infection in HIV-LUCAdV mice. The low bioavailability of NIH45-46G54W is probably related to its high levels of polyreactivity, as evidenced by its binding to mock-transfected HEK293T cells (Fig. 4G). Finally, our results suggest an involvement of Fc effector functions in entry protection, and therefore our experiments are in agreement with the macaque SHIV model (51) but extend those findings by revealing the importance of antibody dose on the effect of the antibody Fc region on protection against HIV in vivo. Additional insights might also be gained by evaluation of antibodies targeting glycan residues (75) or antibodies targeting the membrane-proximal external region (74).
In conclusion, HIV-LUCAdV mice resemble the TZM-bl assay system in that they serve as an assay for HIV entry into heterologous cells, with the important additional dimension of doing so in vivo where the effects of antibody effector functions and bioavailability can be measured.
Materials and Methods
Animals.
Gt(ROSA)26Sortm1(Luc)Kaelin (ROSAfloxSTOP-Luc) mice (27) were purchased from The Jackson Laboratory. ROSAfloxSTOP-Luc mice contain the firefly luciferase reporter under control of the ROSA26 promoter. Cre-mediated excision of the transcriptional stop cassette results in luciferase expression. Mice were bred and maintained at the Comparative Bioscience Center at The Rockefeller University according to guidelines established by its Institutional Animal Committee.
AdV Constructs.
hCD4 and hCCR5 were PCR-amplified from pT4B (76) and pc.CCR-5 (9, 77) respectively.
hCD4 forward: GTCGACGCCACCATGAACCGGGGAGTCCCTTT
hCD4 reverse: GCGGCCGCCATTCATTCATTCAAATGGGGCTACATGTCTT
hCCR5 forward: GTCGACGCCACCATGGATTATCAAGTGTCAAG
hCCR5 reverse: GCGGCCGCCATTCATTCATTCACAAGCCCACAGATATTT
hCCR5 and hCD4 were linked by the ribosomal skip T2A peptide sequence (GSGEGRGSLLTCGDVEENPGP) (20) and inserted into pMX-IRES-mCherry (78) using EcoRI and BamHI sites.
The adenoviral construct expressing hCCR5-2A-hCD4 was created using the AdEasy Adenoviral Vector System (Agilent Technologies) according to the manufacturer’s instructions. All plasmid constructs were verified by DNA sequencing.
A detailed description of flow cytometry, production of recombinant AdVs, HIV pseudovirus production and in vivo infection, histological detection of hCCR5 and hCD4, serum antibody ELISA, bioluminescence imaging, neutralization assays, recombinant protein expression and purification, site-directed mutagenesis, gp140 ELISA, surface plasmon resonance, C1q binding and C3 fixation assays, mAb binding to cell surface gp160, immune complex binding assay, and statistical analysis are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Johannes F. Scheid for providing the antibody plasmids of 3BNC60, 3BNC117, 1-74, and 1-79; Alexander Abadir, Kai-Hui Yao, Caroline Eden, and Marcus Dorner for technical assistance; David Bosque and Rachael Labitt for help with mouse colonies; Joseph Sodroski for providing pSVIIIenvYU-2 plasmid; and members of the M.C.N. laboratory for helpful discussions. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH): pT4B from Richard Axel and pc.CCR-5 from Nathaniel Landau. This work was supported by grants from the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery (to M.S.S., P.J.B., J.V.R., and M.C.N.); and NIH Grant AI081677, “Novel Approaches to Vaccine Design” (to J.V.R. and M.C.N.). H.G. was supported by The German National Academic Foundation. F.K. was supported by German Research Foundation Grant KL 2389/1-1. A.P. is a recipient of the Astella Young Investigator Award of the Infectious Disease Society of America and of the Liver Scholar Award from the American Liver Foundation. P.J.B. and M.C.N. are Howard Hughes Medical Institute Investigators.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213409109/-/DCSupplemental.
References
- 1.McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33:542–554. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 3.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 4.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: Good news for an HIV-1 vaccine? Nat Med. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 5.Boberg A, et al. Murine models for HIV vaccination and challenge. Expert Rev Vaccines. 2008;7:117–130. doi: 10.1586/14760584.7.1.117. [DOI] [PubMed] [Google Scholar]
- 6.Denton PW, Garcia JV. Novel humanized murine models for HIV research. Curr HIV/AIDS Rep. 2009;6:13–19. doi: 10.1007/s11904-009-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klatzmann D, et al. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 8.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 9.Deng H, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 10.Trkola A, et al. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 11.Dragic T, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 12.Spijkerman IJ, et al. Lower prevalence and incidence of HIV-1 syncytium-inducing phenotype among injecting drug users compared with homosexual men. AIDS. 1995;9:1085–1092. doi: 10.1097/00002030-199509000-00016. [DOI] [PubMed] [Google Scholar]
- 13.van’t Wout AB, et al. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J Clin Invest. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 15.Liu R, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 16.Hütter G, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 17.Wei X, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seaman MS, et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol. 2010;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Browning J, et al. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc Natl Acad Sci USA. 1997;94:14637–14641. doi: 10.1073/pnas.94.26.14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szymczak AL, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 21.Berkner KL. Development of adenovirus vectors for the expression of heterologous genes. Biotechniques. 1988;6:616–629. [PubMed] [Google Scholar]
- 22.He TC, et al. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alemany R, Curiel DT. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 2001;8:1347–1353. doi: 10.1038/sj.gt.3301515. [DOI] [PubMed] [Google Scholar]
- 24.Leissner P, et al. Influence of adenoviral fiber mutations on viral encapsidation, infectivity and in vivo tropism. Gene Ther. 2001;8:49–57. doi: 10.1038/sj.gt.3301343. [DOI] [PubMed] [Google Scholar]
- 25.Mizuguchi H, et al. CAR- or alphav integrin-binding ablated adenovirus vectors, but not fiber-modified vectors containing RGD peptide, do not change the systemic gene transfer properties in mice. Gene Ther. 2002;9:769–776. doi: 10.1038/sj.gt.3301701. [DOI] [PubMed] [Google Scholar]
- 26.Smith T, et al. In vivo hepatic adenoviral gene delivery occurs independently of the coxsackievirus-adenovirus receptor. Mol Ther. 2002;5:770–779. doi: 10.1006/mthe.2002.0613. [DOI] [PubMed] [Google Scholar]
- 27.Safran M, et al. Mouse reporter strain for noninvasive bioluminescent imaging of cells that have undergone Cre-mediated recombination. Mol Imaging. 2003;2:297–302. doi: 10.1162/15353500200303154. [DOI] [PubMed] [Google Scholar]
- 28.Dorner M, et al. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208–211. doi: 10.1038/nature10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plotkin SA. Vaccines: Correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson Hedestam GB, et al. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol. 2008;6:143–155. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- 31.Hessell AJ, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hessell AJ, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hessell AJ, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 35.Shibata R, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 36.Trkola A, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11:615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 37.Mehandru S, et al. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol. 2007;81:11016–11031. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rerks-Ngarm S, et al. MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 40.McMichael AJ, Haynes BF. Lessons learned from HIV-1 vaccine trials: New priorities and directions. Nat Immunol. 2012;13:423–427. doi: 10.1038/ni.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diskin R, et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011;334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou T, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burton DR, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 45.Walker LM, et al. Protocol G Principal Investigators Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 47.Parren PW, Burton DR. The antiviral activity of antibodies in vitro and in vivo. Adv Immunol. 2001;77:195–262. doi: 10.1016/S0065-2776(01)77018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overbaugh J, Morris L. The antibody response against HIV-1. Cold Spring Harb Perspect Med. 2012 doi: 10.1101/cshperspect.a007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forthal DN, Moog C. Fc receptor-mediated antiviral antibodies. Curr Opin HIV AIDS. 2009;4:388–393. doi: 10.1097/COH.0b013e32832f0a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gauduin MC, Weir R, Fung MS, Koup RA. Involvement of the complement system in antibody-mediated post-exposure protection against human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1998;14:205–211. doi: 10.1089/aid.1998.14.205. [DOI] [PubMed] [Google Scholar]
- 51.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 52.Horton HM, et al. Fc-engineered anti-CD40 antibody enhances multiple effector functions and exhibits potent in vitro and in vivo antitumor activity against hematologic malignancies. Blood. 2010;116:3004–3012. doi: 10.1182/blood-2010-01-265280. [DOI] [PubMed] [Google Scholar]
- 53.Hope TJ. Moving ahead an HIV vaccine: To neutralize or not, a key HIV vaccine question. Nat Med. 2011;17:1195–1197. doi: 10.1038/nm.2528. [DOI] [PubMed] [Google Scholar]
- 54.Burton DR, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci USA. 2011;108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheid JF, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 56.Mouquet H, et al. Memory B cell antibodies to HIV-1 gp140 cloned from individuals infected with clade A and B viruses. PLoS ONE. 2011;6:e24078. doi: 10.1371/journal.pone.0024078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu X, et al. NISC Comparative Sequencing Program Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gardner MB. SIV infection of macaques: A model for AIDS vaccine development. Dev Biol Stand. 1990;72:259–266. [PubMed] [Google Scholar]
- 59.Li J, Lord CI, Haseltine W, Letvin NL, Sodroski J. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J Acquir Immune Defic Syndr. 1992;5:639–646. [PubMed] [Google Scholar]
- 60.Nath BM, Schumann KE, Boyer JD. The chimpanzee and other non-human-primate models in HIV-1 vaccine research. Trends Microbiol. 2000;8:426–431. doi: 10.1016/s0966-842x(00)01816-3. [DOI] [PubMed] [Google Scholar]
- 61.Berges BK, Rowan MR. The utility of the new generation of humanized mice to study HIV-1 infection: Transmission, prevention, pathogenesis, and treatment. Retrovirology. 2011;8:65. doi: 10.1186/1742-4690-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Denton PW, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balazs AB, et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baenziger S, et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2-/-gamma c-/- mice. Proc Natl Acad Sci USA. 2006;103:15951–15956. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gorantla S, et al. Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2-/-gammac-/- mice. J Virol. 2007;81:2700–2712. doi: 10.1128/JVI.02010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe S, et al. Humanized NOD/SCID/IL2Rgamma(null) mice transplanted with hematopoietic stem cells under nonmyeloablative conditions show prolonged life spans and allow detailed analysis of human immunodeficiency virus type 1 pathogenesis. J Virol. 2007;81:13259–13264. doi: 10.1128/JVI.01353-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brainard DM, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009;83:7305–7321. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mathers BM, et al. 2007 Reference Group to the UN on HIV and Injecting Drug Use Global epidemiology of injecting drug use and HIV among people who inject drugs: A systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 69.Fechner H, et al. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- 70.Bergelson JM, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 71.Tallone T, et al. A mouse model for adenovirus gene delivery. Proc Natl Acad Sci USA. 2001;98:7910–7915. doi: 10.1073/pnas.141223398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hurez V, Dzialo-Hatton R, Oliver J, Matthews RJ, Weaver CT. Efficient adenovirus-mediated gene transfer into primary T cells and thymocytes in a new coxsackie/adenovirus receptor transgenic model. BMC Immunol. 2002;3:4. doi: 10.1186/1471-2172-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edmonds TG, et al. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology. 2010;408:1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perez LG, Costa MR, Todd CA, Haynes BF, Montefiori DC. Utilization of immunoglobulin G Fc receptors by human immunodeficiency virus type 1: A specific role for antibodies against the membrane-proximal external region of gp41. J Virol. 2009;83:7397–7410. doi: 10.1128/JVI.00656-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walker LM, et al. Protocol G Principal Investigators Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maddon PJ, et al. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985;42:93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- 77.Morgenstern JP, Land H. Advanced mammalian gene transfer: High titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robbiani DF, et al. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell. 2008;135:1028–1038. doi: 10.1016/j.cell.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.