Abstract
Fumarate and nitrate reduction (FNR) regulatory proteins are O2-sensing bacterial transcription factors that control the switch between aerobic and anaerobic metabolism. Under anaerobic conditions [4Fe-4S]2+-FNR exists as a DNA-binding homodimer. In response to elevated oxygen levels, the [4Fe-4S]2+ cluster undergoes a rapid conversion to a [2Fe-2S]2+ cluster, resulting in a dimer-to-monomer transition and loss of site-specific DNA binding. In this work, resonance Raman and UV-visible absorption/CD spectroscopies and MS were used to characterize the interconversion between [4Fe-4S]2+ and [2Fe-2S]2+ clusters in Escherichia coli FNR. Selective 34S labeling of the bridging sulfides in the [4Fe-4S]2+ cluster-bound form of FNR facilitated identification of resonantly enhanced Cys32S-34S stretching modes in the resonance Raman spectrum of the O2-exposed [2Fe-2S]2+ cluster-bound form of FNR. This result indicates O2-induced oxidation and retention of bridging sulfides in the form of [2Fe-2S]2+ cluster-bound cysteine persulfides. MS also demonstrates that multiple cysteine persulfides are formed on O2 exposure of [4Fe-4S]2+-FNR. The [4Fe-4S]2+ cluster in FNR can also be regenerated from the cysteine persulfide-coordinated [2Fe-2S]2+ cluster by anaerobic incubation with DTT and Fe2+ ion in the absence of exogenous sulfide. Resonance Raman data indicate that this type of cluster conversion involving sulfide oxidation is not unique to FNR, because it also occurs in O2-exposed forms of O2-sensitive [4Fe-4S] clusters in radical S-adenosylmethionine enzymes. The results provide fresh insight into the molecular mechanism of O2 sensing by FNR and iron-sulfur cluster conversion reactions in general, and suggest unique mechanisms for the assembly or repair of biological [4Fe-4S] clusters.
Facultative anaerobic bacteria respond to environmental O2 levels to promote optimal cell growth under aerobic and anaerobic conditions. The best-characterized O2-sensing transcriptional regulator is the Escherchia coli fumarate and nitrate reduction (FNR) protein, which controls the switch between aerobic and anaerobic metabolism by regulating the transcription of hundreds of genes in response to cellular O2 levels (1–3), and has been the subject of several recent reviews (4–7). Although there is currently no crystallographic structure for E. coli FNR, the protein shares sequence homology with the structurally characterized cyclic-AMP receptor class of proteins (8), which comprise a C-terminal helix-turn-helix DNA-binding domain and an N-terminal sensory domain and binds to DNA as a homodimer. However, the sensor domain in E. coli FNR contains five cysteines and mutagenesis studies indicate that four of these cysteines (C20, C23, C29, and C122) are essential for in vivo function (9) and serve as ligands to the [4Fe-4S] and [2Fe-2S] clusters that function in the O2-sensing mechanism (10, 11).
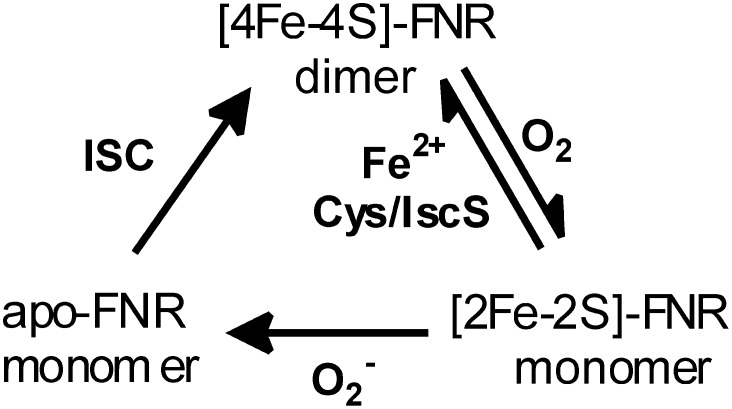
A large part of our current understanding of the mechanism of O2-sensing by FNR comes from the early biochemical and spectroscopic studies of Kiley and colleagues (10, 12–16) (Fig. 1). In vivo and in vitro Mössbauer studies demonstrated the presence of a [4Fe-4S]2+ cluster under anaerobic conditions that is converted to a [2Fe-2S]2+ cluster (60–65% yield in vitro) on exposure to air (10, 12). This cluster conversion is accompanied by a dimer-to-monomer transition and inability of FNR to bind DNA (13, 14). The [4Fe-4S]2+ cluster can be reformed in vitro by anaerobic incubation with excess Fe2+ and cysteine in the presence of catalytic amounts of the cysteine desulfurase, IscS (10). The [2Fe-2S]2+ cluster-bound form of FNR is stable in air for at least 1 h in vitro, but is more rapidly degraded in vivo by superoxide, a byproduct of aerobic metabolism, to yield monomeric apo-FNR (15). Hence, the dominant forms of FNR under anaerobic and aerobic growth conditions are the [4Fe-4S]2+ cluster-containing dimer and the apo monomer, respectively. E. coli has two primary machineries for Fe-S cluster assembly, the iron-sulfur cluster (ISC) system for general Fe-S cluster biosynthesis and the sulfur utilization factor (SUF) system, which operates under oxidative stress and iron-limitation conditions. In vivo studies have clearly demonstrated that the ISC system, but not the SUF system, is required for converting apo-FNR back to [4Fe-4S]2+ cluster-bound FNR upon the onset of anaerobic growth conditions (16).
Fig. 1.
Summary of the in vivo and in vitro cluster and oligomeric state interconversions reported for E. coli FNR.
There are two major unresolved issues involving the mechanism of O2 sensing by FNR. The first concerns the mechanism of O2-induced [4Fe-4S]2+ to [2Fe-2S]2+ cluster conversion and centers on the question of whether the reaction involves metal-based oxidation, sulfur-based oxidation, or a combination of the two. The metal-based oxidation mechanism involves oxidation and release of two cluster irons to yield a [2Fe-2S]2+ cluster, one Fe3+ and one Fe2+ and two S2− ions, proceeding via a [3Fe-4S]+ cluster intermediate (4, 17–20). A sulfur-based oxidation mechanism was proposed based on analytical data showing that only 70% of the sulfide in the original [4Fe-4S]2+ cluster was detectable following O2 exposure, leading to the suggestion that ∼30% had become oxidized (10). However, attempts to detect sulfur oxidation products, such as polysulfide, cysteine persulfide, or sulfinic acid, were unsuccessful (21). The second issue involves the significance of the [2Fe-2S]2+ cluster-bound form of FNR; that is, is it just an intermediate in the conversion of the transcriptionally active [4Fe-4S]-FNR to the transcriptional inactive apo-FNR or can [4Fe-4S]2+ ↔ [2Fe-2S]2+ cluster interconversion occur in vivo and, hence, play a role as a sensor of dynamic changes in cellular O2 levels?
Here we report on resonance Raman, UV-visible absorption and CD, and MS studies of the O2-induced [4Fe-4S]2+ to [2Fe-2S]2+ cluster conversion in E. coli FNR. The results clearly show sulfur-based oxidation and the formation of [2Fe-2S]2+ clusters with one or two cysteine persulfide ligands. In addition, the cluster conversion is shown to be reversible on addition of Fe2+ under anaerobic conditions in the presence of dithiol reagents and the absence of S2−. This result suggests that facile [4Fe-4S]2+ ↔ [2Fe-2S]2+ cluster interconversion can occur, implying that FNR can be responsive to dynamic changes in cellular O2 levels. Moreover, our observation that O2-induced, sulfur-based oxidation of [4Fe-4S]2+ clusters to yield semi-stable cysteine persulfide-ligated [2Fe-2S]2+ clusters also occurs in other O2-sensitive Fe-S proteins, raises the possibility that formation of [4Fe-4S]2+ clusters by the addition of Fe2+ to cysteine persulfide-ligated [2Fe-2S]2+ clusters in the presence of dithiol reagents may provide a major mechanism for the repair or de novo biosynthesis of [4Fe-4S]2+ clusters.
Results
Resonance Raman Characterization of the [4Fe-4S]2+ ↔ [2Fe-2S]2+ Cluster Interconversion in FNR.
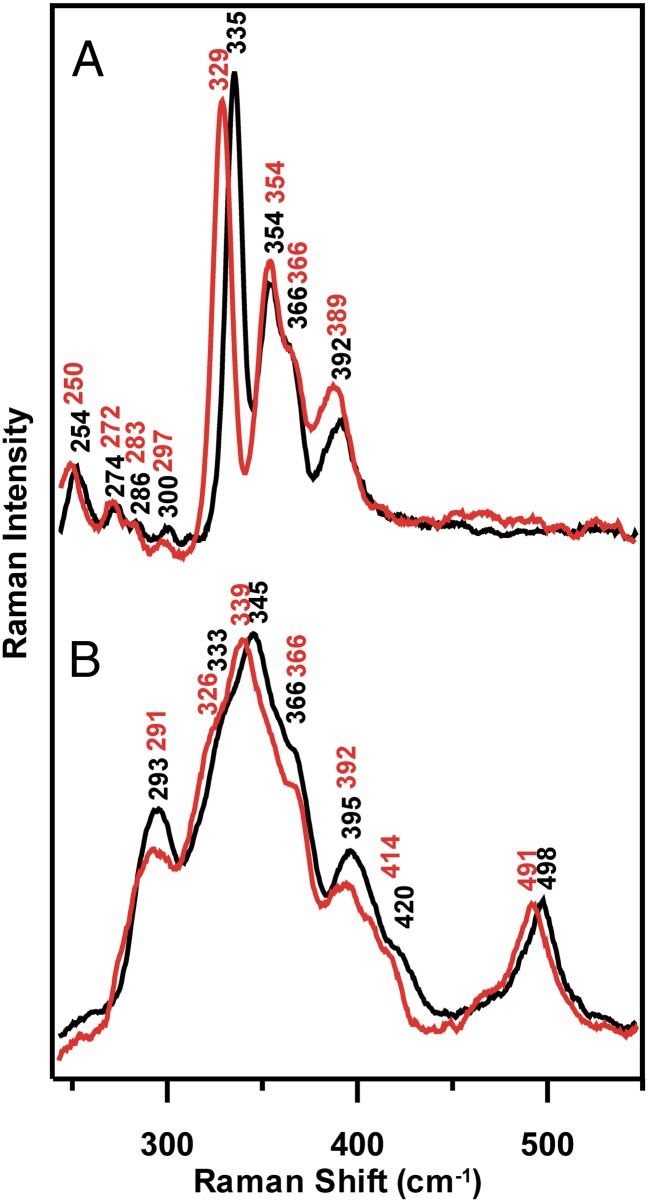
Resonance Raman spectra of the [4Fe-4S]2+ center in FNR anaerobically reconstituted with natural abundance (∼95% 32S, ∼4% 34S) and isotopically enriched (>95%) 34S bridging sulfides are shown in Fig. 2A. The Fe-S stretching frequencies, relative intensities of Raman bands with 458-nm excitation, and the 34S-bridging downshifts are all characteristic of an all cysteine-ligated [4Fe-4S]2+ cluster and are very similar to those reported and assigned by normal-mode analysis for [4Fe-4S]-ferredoxins and appropriate analog complexes (22). To a first approximation, the Fe-S stretching modes can be assigned under effective D2d symmetry as predominantly Fe-S(bridging) and Fe-S(Cys) stretching modes based on the magnitude of the 34S-bridging downshifts, 2–7 cm−1 and <1 cm−1, respectively (Table S1).
Fig. 2.
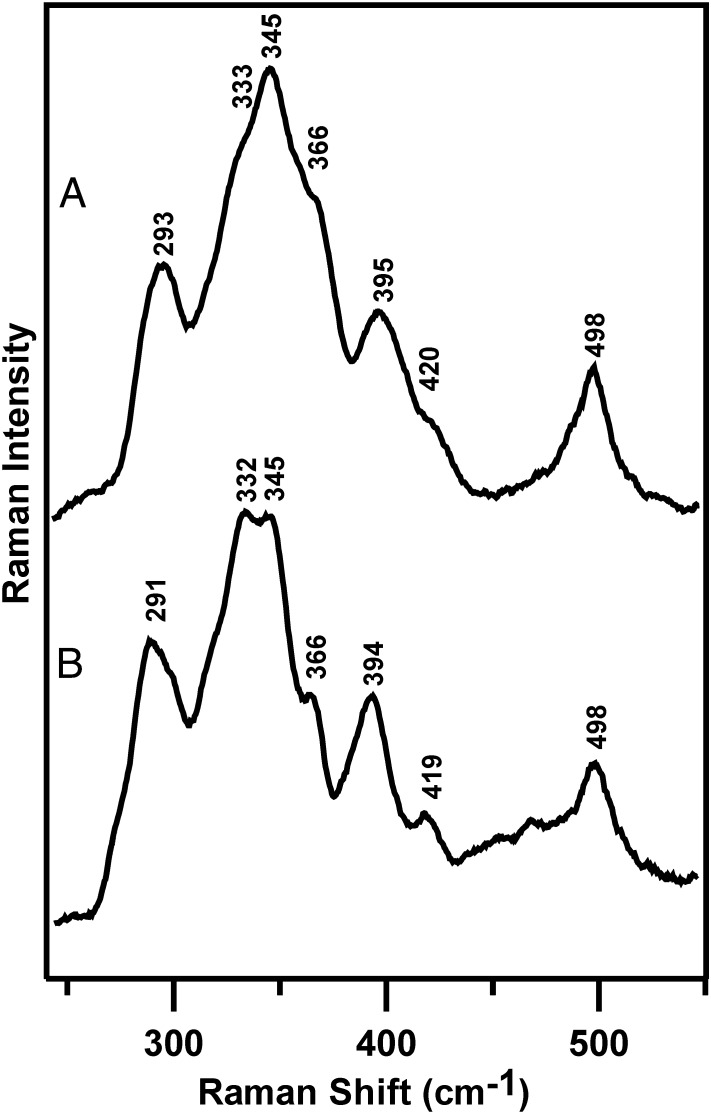
Resonance Raman spectra of the O2-induced [4Fe-4S]2+ to [2Fe-2S]2+ cluster conversion of FNR with natural abundance (black spectra) and 34S-labeled (red spectra) bridging sulfides. (A) [4Fe-4S]-FNR prepared by anaerobic reconstitution. (B) [2Fe-2S]-FNR obtained by exposing the [4Fe-4S]2+-FNR to air for 20 min. The resonance Raman spectra were recorded for samples at 21 K with 140 mW of 457.9-nm laser excitation at the sample, using samples that were ∼2 mM in [4Fe-4S]2+ clusters. Each spectrum is the sum of ∼100 individual scans with each scan involving photon counting for 1 s at a 0.5-cm−1 increment with 7-cm−1 spectral resolution. Bands caused by ice lattice modes have been subtracted from all spectra.
After exposure to air for 20 min, the cluster is converted into a [2Fe-2S]2+ center with an atypical resonance Raman spectrum in the Fe-S stretching region (240–450 cm−1); that is, 10- to 20-fold lower resonance enhancement and three broad and poorly resolved bands centered near 293, 345, and 395 cm−1, compared with [2Fe-2S]2+ cluster-containing ferredoxins, which have six or seven well-resolved and strongly resonantly enhanced bands (23–25) (Fig. 2B). In addition, the [2Fe-2S]2+ center in O2-exposed FNR has a band at 498 cm−1 in the S-S stretching region that is resonantly enhanced with visible excitation into the S-to-Fe3+ charge transfer transitions of the [2Fe-2S]2+ cluster, suggesting assignment to the S-S stretching mode of one or more coordinated cysteine persulfides. Definitive confirmation of a cysteine persulfide-ligated [2Fe-2S]2+ center in O2-exposed FNR is provided by selective 34S labeling of the bridging sulfides in the [4Fe-4S]2+ cluster-bound form of FNR. In contrast to the parent [4Fe-4S]2+ center, bands involving primarily Fe-S(Cys) or Fe-S(bridging) stretching of the [2Fe-2S] center both undergo significant 34S/32S isotope shifts, implying oxidation of some of the bridging sulfides to form Fe-34S-S(Cys) ligands. Moreover, the 7-cm−1 34S/32S isotope shift of the 498 cm−1 band is consistent with the 7–8 cm−1 isotope shifts predicted for a mixed 34S-32S stretching mode based on a simple diatomic oscillator approximation. The Raman data, therefore, demonstrate the presence of cysteine persulfide ligation to the [2Fe-2S] center in O2-exposed FNR and indicate that the cysteine persulfide results from O2-induced S2− to S0 oxidation.
The O2-induced [4Fe-4S]2+ to [2Fe-2S]2+ cluster conversion process is independent of the presence of DTT or GSH, based on very similar resonance Raman spectra in the presence of 3 mM GSH, which approximates to cellular GSH concentration, and 8 mM DTT, after 20 min of air exposure (Fig. S1). Moreover, resonance Raman investigations using 445- and 488-nm excitation of the time course of air exposure of [4Fe-4S]-FNR indicates that the conversion to a cysteine persulfide-ligated [2Fe-2S]2+ center is almost complete within the first 30 s (Fig. S2). However, the spectra do undergo increased broadening and small frequency shifts for specific bands (e.g., 288–296 cm−1 and 392–397 cm−1) as the air-exposure time is increased from 30 s to 60 min (Fig. S2). This finding suggests that the [2Fe-2S]2+ cluster ligation changes and becomes less homogeneous with prolonged air exposure. As discussed below, MS and CD data indicate that this is most likely a consequence of formation of [2Fe-2S]2+ clusters with one and two cysteine persulfide ligands, with the latter becoming more prevalent as the air-exposure time increases.
Previous electron-paramagnetic-resonance studies have shown that a transient [3Fe-4S]1+ cluster intermediate is formed in the generation of [2Fe-2S]2+-FNR (17, 19, 26). Formation of the [3Fe-4S]1+ cluster is O2-dependent (k = 250 M-1⋅s−1), but its conversion to the [2Fe-2S]2+ cluster product is spontaneous, occurring at a rate (k = 0.008 s−1) that is O2-independent. A small amount (<10%) of [3Fe-4S]1+ cluster fails to convert and is detected as a dead-end product. Cubane-type [3Fe-4S]1+ clusters have intense Raman spectra with 458- and 488-nm excitation that are dominated by a band at ∼347 cm−1 that is attributed primarily to the Fe3-(μ3-S) symmetric stretching mode (27, 28). Hence, it seems likely that the 347-cm−1 band that is clearly observed with 488-nm excitation when the cysteine persulfide-ligated [2Fe-2S]2+ center in FNR is less resonantly enhanced (Fig. S2) arises from a cubane [3Fe-4S]1+ cluster. However, this must be a trace amount of [3Fe-4S]1+ cluster (certainly <10%), because the resonance enhancement for cubane-type [3Fe-4S]1+ clusters, such as the one on Pyrococcus furiosus ferredoxin shown in Fig. S2, is ∼30-times greater than that of the cysteine persulfide-ligated [2Fe-2S]2+ cluster in FNR with 488-nm excitation. Notably, the relative amount of these two types of cluster does not change significantly for 0.5- to 60-min air exposure, suggesting that this is the dead-end product that is resistant to further rearrangement, possibly because of the loss of an Fe from a different subsite. It is likely that the [3Fe-4S]1+ cluster intermediate is formed and degraded within the first 30 s of exposure of the highly concentrated Raman sample to air.
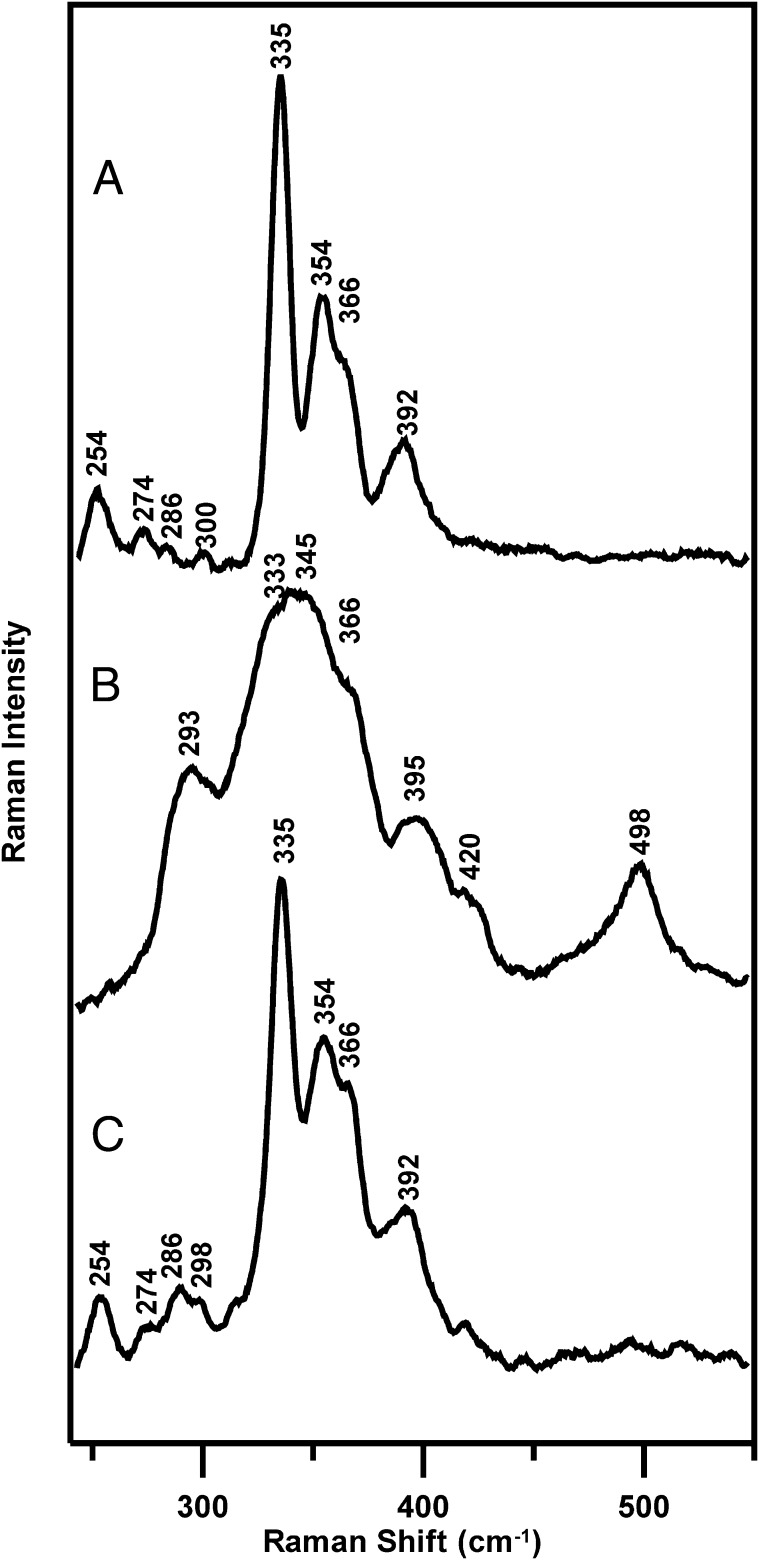
Of particular interest is our observation that O2-induced [4Fe-4S]2+ to cysteine persulfide-ligated [2Fe-2S]2+ cluster conversion in FNR is reversed under anaerobic conditions in the presence of DTT and excess Fe2+. This result is illustrated by resonance Raman studies of FNR in the presence of 3 mM GSH (Fig. 3). After generating the cysteine persulfide-ligated [2Fe-2S]2+ cluster-bound form (Fig. 3B) by exposing the [4Fe-4S]2+ cluster-bound form (∼2 mM in [4Fe-4S]2+ clusters) (Fig. 3A) to air for 20 min, the sample was thawed under anaerobic conditions inside a glove box and incubated for 20 min with 8 mM DTT and Fe2+ before refreezing on the Raman probe. The resulting spectrum (Fig. 3C) indicates substantial conversion back to the original [4Fe-4S]2+ center.
Fig. 3.
Resonance Raman studies of the interconversion between [4Fe-4S]2+ and [2Fe-2S]2+ clusters in FNR. (A) Reconstituted [4Fe-4S]2+-FNR (∼2 mM in [4Fe-4S]2+ clusters) in the presence of 3 mM GSH. (B) [2Fe-2S]2+-FNR obtained by exposing the sample (A) to air for 20 min. (C) After incubation of the sample (B) with 8 mM DTT and 8 mM ferrous ammonium sulfate under anaerobic conditions for 20 min. The Raman experimental conditions are the same as described in Fig. 2.
It is not possible to be more quantitative about the extent of the conversion based on Raman data, as the photon counts that dictate Raman intensity are very dependent on sample alignment. Therefore, the characteristic UV-visible absorption and CD spectra of [2Fe-2S]2+ and [4Fe-4S]2+ cluster-bound forms of FNR (26) were used to provide a quantitative assessment of the extent of [2Fe-2S]2+ to [4Fe-4S]2+ cluster conversion in the presence of 3 mM DTT and the presence or absence of an eightfold excess Fe2+, both before and after repurification of [2Fe-2S]-FNR formed by O2-exposure of [4Fe-4S]-FNR for 2 min (Figs. S3 and S4). The results reveal that DTT is required for [2Fe-2S]2+ to [4Fe-4S]2+ cluster conversion, and that ≥70% of the original [4Fe-4S]2+ clusters are restored within 10 min of addition of an eightfold excess of Fe to the [2Fe-2S]-FNR, even after repurification to remove free sulfides. In contrast, no significant [2Fe-2S]-FNR to [4Fe-4S]-FNR conversion occurred using 3 mM GSH and a eightfold excess of Fe2+. The implication is that DTT facilitates reductive cleavage of two bound cysteine persulfides ligated to the [2Fe-2S]2+ center in O2-exposed FNR under reducing conditions in the presence of Fe2+ to reform a [4Fe-4S]2+ cluster in situ:
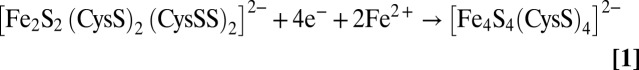 |
The less-than-quantitative yield of [4Fe-4S]2+ centers in these experiments is likely to be a consequence of [2Fe-2S]2+ centers in O2-exposed FNR that are ligated by one cysteine persulfide. In this case, [4Fe-4S]2+ cluster formation in the presence of DTT and Fe2+ would likely require complete cluster degradation and reassembly from Fe2+ and S2−, which is a slow (>2 h) and incomplete (<40%) process in FNR. Hence, we conclude that inter-cluster cannibalization cannot be responsible for the rapid 70–75% [4Fe-4S]2+ cluster recovery observed in these experiments.
MS Characterization of the [4Fe-4S]2+ to [2Fe-2S]2+ Cluster Conversion in FNR.
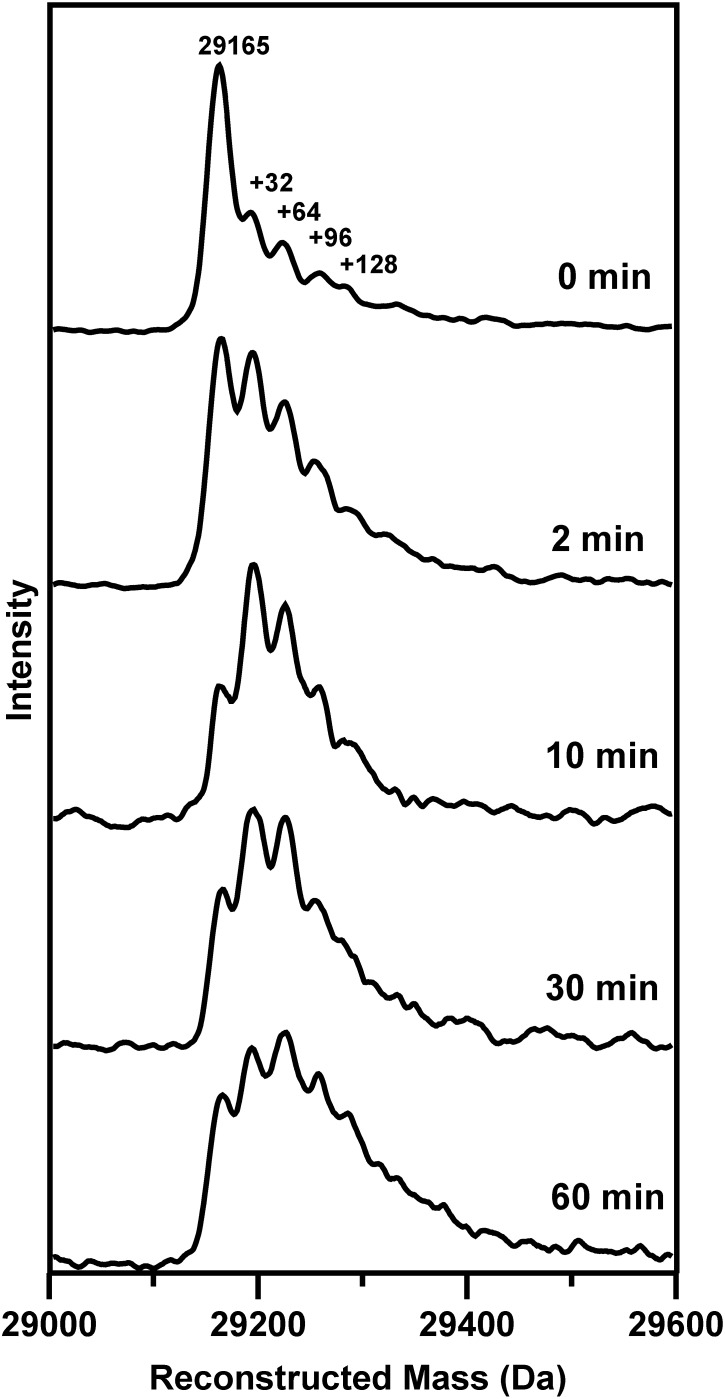
Although liquid-chromatography electrospray ionization (LC-ESI)-quadrupole MS studies of Fe-S proteins invariably result in loss of the Fe-S cluster, our previous studies have demonstrated that cysteine persulfides or covalently attached polysulfides remain intact (29). Hence, this technique provides a direct method for investigating O2-induced S0 generation on [4Fe4S]2+-FNR as a function of time. The results are shown in Fig. 4. Before air exposure, the reconstructed mass spectrum is dominated by the FNR monomer molecular ion peak at 29,165 Da (theoretical mass of 29,165 Da based on the primary sequence). Based on protein and Fe/acid-labile S determinations, the anaerobically reconstituted samples of FNR used in this work contained 0.85–0.99 [4Fe-4S]2+ clusters per monomer immediately after purification. However, the cluster content decreased by up to 10% on anaerobic freezing/thawing and concentration of samples. Hence, the peak at 29,165 Da that persists throughout the air-exposure time course is attributed to the small component of apo-FNR. The time-course experiment indicates that the form of FNR with one cysteine persulfide dominates after 2 and 10 min of air exposure. However, the form of FNR with two cysteine persulfides gradually increases with prolonged air exposure and dominates after 60 min. Hence, the MS data support the resonance Raman data by demonstrating O2-induced S2− to S0 oxidation to form cysteine persulfides and suggest that increased heterogeneity occurs with increasing air exposure, arising primarily from a mixture of [2Fe-2S]2+ clusters with one and two cysteine persulfide ligands.
Fig. 4.
Time course of air-exposure of [4Fe-4S]-FNR monitored by MS. Anaerobically reconstituted FNR (∼1 mM in [4Fe-4S]2+ clusters) was exposed to air for 0, 2, 10, 30, and 60 min before analysis using LC-ESI-quadrupole MS. The peak at 29,165 Da corresponds to the monomer molecular ion peak of FNR and the peaks at +32, +64, +96, and +128 Da correspond to the addition of one, two, three, and four covalently bound sulfur atoms, respectively.
Resonance Raman Evidence for Analogous O2-Induced [4Fe-4S]2+ to [2Fe-2S]2+ Cluster Conversions in Other Proteins.
Anomalous resonance Raman spectra similar to those of the O2-generated [2Fe-2S]2+ clusters in FNR have also been reported for aerobically purified or air-exposed [4Fe-4S]2+ centers in radical S-adenosylmethionine (SAM) enzymes, such as pyruvate formate lyase and ribonucleotide reductase-activating enzymes (30, 31), and biotin synthase (32). In each case the resonance Raman spectra have been attributed to a [2Fe-2S]2+ cluster based on parallel Mössbauer studies. However, these spectra were generally only scanned in the Fe-S stretching region, 200–450 cm−1. Therefore, we have reinvestigated over a wider spectral range the resonance Raman spectrum of air-exposed biotin synthase (BioB), containing only the [4Fe-4S]2+ cluster that is responsible for reductive cleavage of SAM (Fig. 5). The spectrum is almost identical to that of the cysteine persulfide-ligated [2Fe-2S]2+ center in air-exposed [4Fe-4S]-FNR, and exhibits a resonantly enhanced 498-cm−1 band that is the hallmark of a coordinated cysteine persulfide. Moreover, the spectrum is completely different from that of the [2Fe-2S]2+ cluster in the second cluster binding site, which is present in as-purified recombinant BioB (32). Hence, it is likely that the SAM-binding [4Fe-4S]2+ cluster common to all radical SAM enzymes undergoes the same O2-induced [4Fe-4S]2+ to cysteine persulfide-ligated [2Fe-2S]2+ cluster transformation on exposure to O2.
Fig. 5.
Comparison of the resonance Raman spectra of the air-exposed samples of [4Fe-4S]2+ cluster-containing FNR (A) and BioB (B). The experimental conditions for sample preparation, air exposure, and resonance Raman are the same as described in Fig. 2, except that samples of BioB were in 50 mM Hepes buffer, pH 7.5.
Discussion
Implications for the Molecular Mechanism of O2-Sensing by FNR.
The resonance Raman and LC-ESI MS results presented herein clearly point to oxidation of bridging sulfides to generate one or two cysteine persulfide ligands during the reaction of O2 with the [4Fe-4S]2+ cluster in FNR to form a [2Fe-2S]2+ cluster. Such unusual ligation of [2Fe-2S]2+ clusters would appear to be difficult to detect by Mössbauer spectroscopy, based on the similarity of the observed isomer shift (δ = 0.28 mm/s) and quadrupole splitting (ΔEQ = 0.58 mm/s) (10) to those of all cysteinate-ligated [2Fe-2S]2+ clusters. However, this conclusion is consistent with the original analytical and spectroscopic data, which indicated that only 70% of the sulfide in the original [4Fe-4S]2+ cluster was detectable following O2 exposure to give a 60–65% yield of [2Fe-2S]2+ clusters, leaving 30% unaccounted for (10). Indeed, these data led to the original conclusion that the [4Fe-4S]2+ to [2Fe-2S]2+ conversion primarily involved sulfide oxidation, even though there was no direct analytical evidence for S0 (21). Subsequently, this conclusion was challenged by studies that showed that one Fe2+, one Fe3+, and two sulfide ions are released upon stoichiometric O2-induced [4Fe-4S]2+ to [2Fe-2S]2+ cluster conversion (18, 19, 26), leading to the conclusion that conversion occurs via an iron-based oxidation mechanism. In these experiments, released sulfide ion was detected using DTNB [5,5-dithiobis-(2-nitrobenzoic acid)] (Ellman’s reagent), based on the established observation that DTNB reacts with one S2−, generating sulfane (S0) plus the release of two TNB− ions (33). This assay accurately detects sulfide that becomes available for oxidation during or following cluster conversion, distinguishing it from sulfide still bridging iron in the cluster. However, in interpreting the data, we did not consider the possibility that a bridging sulfide and a nearby cysteine might undergo DTNB-induced two-electron oxidation to produce two TBN− ions and a cysteine persulfide, which has now been shown to occur during the oxidative degradation of the [4Fe-4S]2+ cluster in FNR.
The identification of the fate of the sulfide in the O2-induced [4Fe-4S]2+ to [2Fe-2S]2+ cluster transformation in FNR necessitates a reassessment of the mechanism of cluster conversion, in which [2Fe-2S]2+ clusters with one or two cysteine persulfide ligands can be formed. We have previously described the mechanism as a two-step reaction, in which the [4Fe-4S]2+ cluster first undergoes an O2-dependent one-electron oxidation resulting in the formation of a transient cubane-type [3Fe-4S]1+ cluster intermediate (17, 19, 26), with release of Fe2+ and the formation of superoxide, O2− (10, 19). This first step remains as previously proposed, as there is no oxidation of bridging sulfide during this step. The second step involves the conversion of the [3Fe-4S]1+ cluster to the persulfide coordinated [2Fe-2S]2+ form and is therefore more complex than previously envisaged, and may itself involve multiple steps, depending on whether release of iron and sulfide from the cluster occurs simultaneously with sulfide oxidation. It was previously shown that the iron released in this step is Fe3+, although its final oxidation state is influenced by the chelating and redox active species present in the reaction mixture (26). The electrons from sulfide oxidation (either two or four electrons for one and two sulfides, respectively) most likely reduce, directly or indirectly, O2 to either H2O2 or H2O. The overall reactions for the formation of the singly or doubly persulfide-coordinated [2Fe-2S]2+ cluster from the initial [3Fe-4S]1+ intermediate and the nearby free cysteine that are generated in the first step can be written as Scheme 1:
Scheme 1.
Overall one persulfide ligand reaction:
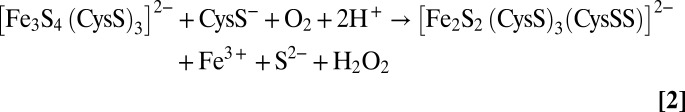 |
Overall two persulfide ligand reaction:
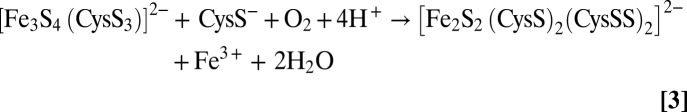 |
The timescale of the appearance of the persulfide coordinated [2Fe-2S]2+ cluster suggests that the oxidation of at least one sulfide occurs simultaneously with release of Fe3+ and sulfide from the [3Fe-4S]1+ intermediate. The formation of a cysteine persulfide by oxidative coupling a μ2-S2− from the trisulfide face of a [Fe3S4(CysS)3]2− cluster with a nearby free cysteine is clearly a plausible mechanism, with parallels in O2-induced disulfide formation. However, as the rate of degradation of [3Fe-4S]1+ intermediate to yield a [2Fe-2S]2+ cluster has been shown to be O2-independent (19), this reaction appears to involve multiple steps with the rate-determining step involving loss of Fe3+/S2− from the intermediate. The overall two-persulfide ligand reaction would most likely result in the four-electron/four-proton reduction of O2 to H2O using two cluster sulfides as the electron donors, and the results presented herein suggest that this is likely to occur via a single cysteine persulfide-ligated intermediate with the production of H2O2, which could provide the oxidizing equivalents for the generation of the second cysteine persulfide.
The observation that [4Fe-4S]2+-to-[2Fe-2S]2+ cluster conversion is rapidly reversed, under anaerobic conditions and in the absence of exogenous sulfide, on addition of Fe2+ and DTT to the [2Fe-2S]2+ cluster-bound form of FNR containing two cysteine persulfide ligands, has important implications both for understanding the FNR mechanism and the mechanism of assembly or repair of O2-sensitive biological [4Fe-4S]2+ clusters in general. For FNR this provides the ability to respond rapidly to changes in cellular O2 levels in a dithiol/disulfide redox-buffering medium. Under aerobic conditions, FNR is inactivated as a transcriptional regulator by O2-induced degradation of the [4Fe-4S]2+ cluster to form a [2Fe-2S]2+ cluster with two cysteine persulfide ligands. However, in many environments encountered by facultative anaerobes, O2 is only transiently available and the data presented herein indicate that the persulfide-coordinated [2Fe-2S]2+ cluster-bound form of FNR could be rapidly reactivated by Fe2+ and dithiol species without needing the intervention of the entire ISC Fe-S cluster biogenesis system. Consequently, [2Fe-2S]-FNR appears to play a key role in the O2-sensing mechanism, as a check point from which the regulator can go back to its transcriptionally active [4Fe-4S]2+ cluster-bound form, or on to its apo-form, depending on the prevailing O2 level. Clearly, the [2Fe-2S]2+ cluster-bound form of FNR should no longer be considered as a passive intermediate in the O2-induced transition from [4Fe-4S]-FNR to apo-FNR.
Implications for the Assembly or Repair of O2-Sensitive Biological [4Fe-4S]2+ Clusters.
Resonance Raman and structural studies indicate that the O2-degradation of [4Fe-4S]2+ clusters to cysteine persulfide-ligated [2Fe-2S]2+ clusters also occurs in radical-SAM enzymes and is not limited to FNR. Moreover, sulfur oxidation on oxidative degradation of Fe-S enzymes is not confined to FNR and radical-SAM enzymes. Kennedy and Beinert first reported multiple (up to three) S0 in the form of persulfides or polysulfides in apo-aconitase on careful ferricyanide oxidation in 1988 (34). Little is currently known about [4Fe-4S]2+ cluster biogenesis. In vitro studies carried out under strictly anaerobic conditions have demonstrated the formation of [4Fe-4S]2+ clusters on the IscU and NifU scaffold proteins, via reductive coupling of two [2Fe-2S]2+ clusters, that can be transferred intact to the apo-forms of aconitase and nitrogenase Fe protein, respectively (35–38). However, currently it is not known how [4Fe-4S]2+ clusters can be assembled under aerobic conditions or repaired in response to oxidative damage. This work suggests an alternative strategy for assembling [4Fe-4S]2+ clusters that has the potential to work under aerobic or microaerobic conditions by trafficking in the more O2-tolerant [2Fe-2S]2+ clusters and for repairing O2-damaged [4Fe-4S]2+ clusters that have been degraded to cysteine persulfide-ligated [2Fe-2S]2+ clusters. For assembly, the first step would be cysteine desulfurase catalyzed cysteine persulfide formation on two active-site cysteine residues of the acceptor protein. The second step would then involve transfer of a [2Fe-2S]2+ cluster to yield a [2Fe-2S]2+ cluster with two cysteine persulfide ligands. The third step, which would mimic the repair mechanism, would involve incorporation of two Fe2+ ions coupled with dithiol-mediated cysteine-persulfide reduction for generating the [4Fe-4S]2+ cluster on the acceptor protein in situ.
Much work needs to be done to test the above hypothesis and identify the putative Fe2+ donor. However, the A-type Fe-S cluster assembly proteins (IscA, NifIscA, and SufA), which are specifically required for the maturation of the [4Fe-4S]2+ cluster under aerobic or oxidative stress conditions (39, 40), are good candidates because they are capable of binding Fe3+ under aerobic conditions and releasing Fe2+ in the presence of cysteine under more reducing conditions (41). Moreover, support for the above hypothesis and the use of A-type proteins as Fe2+ donors comes from recent in vivo studies in Saccharomyces cerevisiae (40). In this work, Mühlenhoff et al. demonstrated that the A-type proteins in yeast mitochondria (Isa1 and Isa2) bind Fe rather than an Fe-S cluster in vivo and are required along with Iba57 (a tetrahydrofolate-dependent protein required for Fe release) and the U-type scaffold proteins (Isu1 and Isu2) for the maturation of protein-bound [4Fe-4S]2+ centers in a step that occurs after cluster assembly on the U-type scaffold proteins (40).
Materials and Methods
Purification and Reconstitution of [4Fe-4S]-FNR with Natural Abundance and 34S Bridging Sulfides.
FNR samples were prepared as previously described (17). Isotopically enriched [4Fe-4S]2+ clusters were prepared using 34S-cysteine synthesized using a thermostable cysteine synthase (44), o-acetylserine and 34S2− (CIL Inc). Samples for spectroscopic studies were in 25 mM Hepes buffer with 2.5 mM CaCl2, 100 mM NaCl, 100 mM NaNO3, at pH 7.5, except resonance Raman samples, which also contained 500 mM KCl. Samples, containing [2Fe-2S]-FNR, were prepared essentially as described previously (18), except the protein fraction from the PD10 column (GE Healthcare) was collected (taking care to ensure no contamination with low molecular weight components), and centrifuged at 14,000 × g for 2 min. The [4Fe-4S]2+ cluster concentration of reconstituted FNR and the [2Fe-2S]2+ cluster concentration of air exposed FNR were determined using ε406 = 16.22 (± 0.14) mM-1⋅cm−1 (26) and ε420 = 7.95 (± 0.06) mM-1⋅cm−1, respectively. The latter was determined by assaying [2Fe-2S] FNR samples for protein, acid-labile sulfide, and iron as previously described (18, 42, 43). These determinations revealed 2.1 and 2.0 iron and sulfide per protein, respectively, consistent with the stoichiometric nature of cluster conversion.
Reconstitution of [4Fe-4S]2+ Centers in E. coli BioB.
BioB containing one [4Fe-4S]2+ cluster per BioB monomer was prepared under strictly anaerobic conditions in 50 mM Hepes buffer, pH 7.5 following the procedure described by Cosper et al. (32). The [4Fe-4S]2+ cluster concentration was based on ε410 = 15.6 mM-1⋅cm−1.
Spectroscopic Methods.
All samples were prepared and handled under Ar or N2 in a glove box at oxygen levels < 2 ppm, unless otherwise stated. Details concerning sampling handling and the spectrometers used in this work are available in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Nick Cull and Dr. Dennis Phillips for technical assistance and Dr. Claudio Vásquez for the gift of CysK. This work was supported by National Institutes of Health Grant GM62524 (to M.K.J.) and Biotechnology and Biological Sciences Research Council Grants BB/G019347/1 and BB/G018960/1 (to N.E.L.B., A.J.T., and J.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208787109/-/DCSupplemental.
References
- 1.Spiro S, Guest JR. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol Rev. 1990;6:399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- 2.Guest JR, Green J, Irvine AS, Spiro S. In: in Regulation of Gene Expression in Escherichia coli. Lin ECC, Lynch AS, editors. Austin, TX: R. G. Landes; 1996. pp. 317–342. [Google Scholar]
- 3.Constantinidou C, et al. A reassessment of the FNR regulon and transcriptomic analysis of the effects of nitrate, nitrite, NarXL, and NarQP as Escherichia coli K12 adapts from aerobic to anaerobic growth. J Biol Chem. 2006;281:4802–4815. doi: 10.1074/jbc.M512312200. [DOI] [PubMed] [Google Scholar]
- 4.Crack JC, et al. Signal perception by FNR: The role of the iron-sulfur cluster. Biochem Soc Trans. 2008;36:1144–1148. doi: 10.1042/BST0361144. [DOI] [PubMed] [Google Scholar]
- 5.Green J, Crack JC, Thomson AJ, Le Brun NE. Bacterial sensors of oxygen. Curr Opin Microbiol. 2009;12:145–151. doi: 10.1016/j.mib.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Kiley PJ, Beinert H. The role of Fe-S proteins in sensing and regulation in bacteria. Curr Opin Microbiol. 2003;6:181–185. doi: 10.1016/s1369-5274(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 7.Fleischhacker AS, Kiley PJ. Iron-containing transcription factors and their roles as sensors. Curr Opin Chem Biol. 2011;15:335–341. doi: 10.1016/j.cbpa.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw DJ, Rice DW, Guest JR. Homology between CAP and Fnr, a regulator of anaerobic respiration in Escherichia coli. J Mol Biol. 1983;166:241–247. doi: 10.1016/s0022-2836(83)80011-4. [DOI] [PubMed] [Google Scholar]
- 9.Green J, Sharrocks AD, Green B, Geisow M, Guest JR. Properties of FNR proteins substituted at each of the five cysteine residues. Mol Microbiol. 1993;8:61–68. doi: 10.1111/j.1365-2958.1993.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 10.Khoroshilova N, Popescu C, Münck E, Beinert H, Kiley PJ. Iron-sulfur cluster disassembly in the FNR protein of Escherichia coli by O2: [4Fe-4S] to [2Fe-2S] conversion with loss of biological activity. Proc Natl Acad Sci USA. 1997;94:6087–6092. doi: 10.1073/pnas.94.12.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiley PJ, Beinert H. Oxygen sensing by the global regulator, FNR: The role of the iron-sulfur cluster. FEMS Microbiol Rev. 1998;22:341–352. doi: 10.1111/j.1574-6976.1998.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 12.Popescu CV, Bates DM, Beinert H, Münck E, Kiley PJ. Mössbauer spectroscopy as a tool for the study of activation/inactivation of the transcription regulator FNR in whole cells of Escherichia coli. Proc Natl Acad Sci USA. 1998;95:13431–13435. doi: 10.1073/pnas.95.23.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazazzera BA, Bates DM, Kiley PJ. The activity of the Escherichia coli transcription factor FNR is regulated by a change in oligomeric state. Genes Dev. 1993;7:1993–2005. doi: 10.1101/gad.7.10.1993. [DOI] [PubMed] [Google Scholar]
- 14.Lazazzera BA, Beinert H, Khoroshilova N, Kennedy MC, Kiley PJ. DNA binding and dimerization of the Fe-S-containing FNR protein from Escherichia coli are regulated by oxygen. J Biol Chem. 1996;271:2762–2768. doi: 10.1074/jbc.271.5.2762. [DOI] [PubMed] [Google Scholar]
- 15.Sutton VR, et al. Superoxide destroys the [2Fe-2S]2+ cluster of FNR from Escherichia coli. Biochemistry. 2004;43:791–798. doi: 10.1021/bi0357053. [DOI] [PubMed] [Google Scholar]
- 16.Mettert EL, Outten FW, Wanta B, Kiley PJ. The impact of O(2) on the Fe-S cluster biogenesis requirements of Escherichia coli FNR. J Mol Biol. 2008;384:798–811. doi: 10.1016/j.jmb.2008.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crack JC, Green J, Thomson AJ. Mechanism of oxygen sensing by the bacterial transcription factor fumarate-nitrate reduction (FNR) J Biol Chem. 2004;279:9278–9286. doi: 10.1074/jbc.M309878200. [DOI] [PubMed] [Google Scholar]
- 18.Crack JC, Green J, Le Brun NE, Thomson AJ. Detection of sulfide release from the oxygen-sensing [4Fe-4S] cluster of FNR. J Biol Chem. 2006;281:18909–18913. doi: 10.1074/jbc.C600042200. [DOI] [PubMed] [Google Scholar]
- 19.Crack JC, Green J, Cheesman MR, Le Brun NE, Thomson AJ. Superoxide-mediated amplification of the oxygen-induced switch from [4Fe-4S] to [2Fe-2S] clusters in the transcriptional regulator FNR. Proc Natl Acad Sci USA. 2007;104:2092–2097. doi: 10.1073/pnas.0609514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jervis AJ, et al. The O2 sensitivity of the transcription factor FNR is controlled by Ser24 modulating the kinetics of [4Fe-4S] to [2Fe-2S] conversion. Proc Natl Acad Sci USA. 2009;106:4659–4664. doi: 10.1073/pnas.0804943106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton VR, Mettert EL, Beinert H, Kiley PJ. Kinetic analysis of the oxidative conversion of the [4Fe-4S]2+ cluster of FNR to a [2Fe-2S]2+ Cluster. J Bacteriol. 2004;186:8018–8025. doi: 10.1128/JB.186.23.8018-8025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czernuszewicz RS, Macor KA, Johnson MK, Gewirth A, Spiro TG. Vibrational mode structure and symmetry in proteins and analogues containing Fe4S4 clusters: Resonance Raman evidence for different degrees of distortion in HiPIP and ferredoxin. J Am Chem Soc. 1987;109:7178–7187. [Google Scholar]
- 23.Han S, Czernuszewicz RS, Spiro TG. Vibrational spectra and normal mode analysis for [2Fe-2S] protein analogues using 34S, 54Fe, and 2H substitution: Coupling of Fe-S stretching and S-C-C bending modes. J Am Chem Soc. 1989;111:3496–3504. [Google Scholar]
- 24.Han S, Czernuszewicz RS, Kimura T, Adams MWW, Spiro TG. Fe2S2 protein resonance Raman revisited: Structural variations among adrenodoxin, ferredoxin, and red paramagnetic protein. J Am Chem Soc. 1989;111:3505–3511. [Google Scholar]
- 25.Fu W, Drozdzewski PM, Davies MD, Sligar SG, Johnson MK. Resonance Raman and magnetic circular dichroism studies of reduced [2Fe-2S] proteins. J Biol Chem. 1992;267:15502–15510. [PubMed] [Google Scholar]
- 26.Crack JC, et al. Influence of the environment on the [4Fe-4S]2+ to [2Fe-2S]2+ cluster switch in the transcriptional regulator FNR. J Am Chem Soc. 2008;130:1749–1758. doi: 10.1021/ja077455+. [DOI] [PubMed] [Google Scholar]
- 27.Johnson MK, Czernuszewicz RS, Spiro TG, Fee JA, Sweeney WV. Resonance Raman spectroscopic evidence for a common [3Fe-4S] structure among proteins containing three-iron clusters. J Am Chem Soc. 1983;103:6671–6678. [Google Scholar]
- 28.Duderstadt RE, Brereton PS, Adams MWW, Johnson MK. Spectroscopic evidence for a new type of [Fe3S4] cluster in a mutant form of Pyrococcus furiosus ferredoxin. J Am Chem Soc. 1998;120:8525–8526. [Google Scholar]
- 29.Smith AD, et al. Sulfur transfer from IscS to IscU: The first step in iron-sulfur cluster biosynthesis. J Am Chem Soc. 2001;123:11103–11104. doi: 10.1021/ja016757n. [DOI] [PubMed] [Google Scholar]
- 30.Broderick JB, et al. Pyruvate formate-lyase activating enzyme is an iron-sulfur protein. J Am Chem Soc. 1997;119:7396–7397. [Google Scholar]
- 31.Ollagnier S, et al. Assembly of 2Fe-2S and 4Fe-4S clusters in the anaerobic ribonucleotide reductase from Escherichia coli. J Am Chem Soc. 1999;121:6344–6350. [Google Scholar]
- 32.Cosper MM, et al. Characterization of the cofactor composition of Escherichia coli biotin synthase. Biochemistry. 2004;43:2007–2021. doi: 10.1021/bi0356653. [DOI] [PubMed] [Google Scholar]
- 33.Nashef AS, Osugo DT, Feeney RE. Determination of hydrogen sulfide with 5,5′-dithiobis-(2-nitrobenzoicacid),N-ethylmaleimide, and parachloromercuribenzoate. Anal Biochem. 2004;79:394–405. doi: 10.1016/0003-2697(77)90413-4. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy MC, Beinert H. The state of cluster SH and S2- of aconitase during cluster interconversion and removal. J Biol Chem. 1988;263:8194–8198. [PubMed] [Google Scholar]
- 35.Unciuleac M-C, et al. In vitro activation of apo-aconitase using a [4Fe-4S] cluster-loaded form of the IscU [Fe-S] cluster scaffolding protein. Biochemistry. 2007;46:6812–6821. doi: 10.1021/bi6026665. [DOI] [PubMed] [Google Scholar]
- 36.Chandramouli K, et al. Formation and properties of [4Fe-4S] clusters on the IscU scaffold protein. Biochemistry. 2007;46:6804–6811. doi: 10.1021/bi6026659. [DOI] [PubMed] [Google Scholar]
- 37.Smith AD, et al. NifS-mediated assembly of [4Fe-4S] clusters in the N- and C-terminal domains of the NifU scaffold protein. Biochemistry. 2005;44:12955–12969. doi: 10.1021/bi051257i. [DOI] [PubMed] [Google Scholar]
- 38.Dos Santos PC, et al. Iron-sulfur cluster assembly: NifU-directed activation of the nitrogenase Fe protein. J Biol Chem. 2004;279:19705–19711. doi: 10.1074/jbc.M400278200. [DOI] [PubMed] [Google Scholar]
- 39.Tan G, Lu J, Bitoun JP, Huang H, Ding H. IscA/SufA paralogues are required for the [4Fe-4S] cluster assembly in enzymes of multiple physiological pathways in Escherichia coli under aerobic growth conditions. Biochem J. 2009;420:463–472. doi: 10.1042/BJ20090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mühlenhoff U, Richter N, Pines O, Pierik AJ, Lill R. Specialized function of yeast Isa1 and Isa2 proteins in the maturation of mitochondrial [4Fe-4S] proteins. J Biol Chem. 2011;286:41205–41216. doi: 10.1074/jbc.M111.296152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding H, Clark RJ. Characterization of iron binding in IscA, an ancient iron-sulphur cluster assembly protein. Biochem J. 2004;379:433–440. doi: 10.1042/BJ20031702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 43.Beinert H. Semi-micro methods for analysis of labile sulfide and of labile sulfide plus sulfane sulfur in unusually stable iron-sulfur proteins. Anal Biochem. 1983;131:373–378. doi: 10.1016/0003-2697(83)90186-0. [DOI] [PubMed] [Google Scholar]
- 44.Saavedra CP, et al. Biochemical characterization of a thermostable cysteine synthase from Geobacillus stearothermophilus V. Biochemie. 2004;86:481–485. doi: 10.1016/j.biochi.2004.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.