Abstract
Interplay between various lymphangiogenic factors in promoting lymphangiogenesis and lymphatic metastasis remains poorly understood. Here we show that FGF-2 and VEGF-C, two lymphangiogenic factors, collaboratively promote angiogenesis and lymphangiogenesis in the tumor microenvironment, leading to widespread pulmonary and lymph-node metastases. Coimplantation of dual factors in the mouse cornea resulted in additive angiogenesis and lymphangiogenesis. At the molecular level, we showed that FGFR-1 expressed in lymphatic endothelial cells is a crucial receptor that mediates the FGF-2–induced lymphangiogenesis. Intriguingly, the VEGFR-3–mediated signaling was required for the lymphatic tip cell formation in both FGF-2– and VEGF-C–induced lymphangiogenesis. Consequently, a VEGFR-3–specific neutralizing antibody markedly inhibited FGF-2–induced lymphangiogenesis. Thus, the VEGFR-3–induced lymphatic endothelial cell tip cell formation is a prerequisite for FGF-2–stimulated lymphangiogenesis. In the tumor microenvironment, the reciprocal interplay between FGF-2 and VEGF-C collaboratively stimulated tumor growth, angiogenesis, intratumoral lymphangiogenesis, and metastasis. Thus, intervention and targeting of the FGF-2– and VEGF-C–induced angiogenic and lymphangiogenic synergism could be potentially important approaches for cancer therapy and prevention of metastasis.
Keywords: neovascularization, growth factors, signaling interplay, cancer spread, antiangiogenic therapy
Metastasis is one of the hallmarks of malignant disease and is responsible for most cancer-related death (1, 2). Metastasis occurs in most cancer types and often affects multiple tissues and organs of the cancer-bearing host. Cancer metastasis is a complex disease that engages tight interactions between malignant cells and the host microenvironment. The metastatic process consists of multiple steps of the tumor–host interaction, including: (i) invasion of tumor cells into the surrounding tissue and dissemination from the primary site (3, 4); (ii) intravasation of malignant cells in the circulation or lymphatic systems (5–9); (iii) transport of tumor cells to the distal tissues and organs; (iv) extravasation of malignant cells from blood or lymphatic vessels (10); (v) formation of the initial metastatic niche and micrometastatic foci in the distal tissues and organs (11); and (vi) regrowth of metastatic foci into a clinical detectable mass. In some cases, tumor cells can escape from the primary metastatic site to further spread to other tissues and organs (5).
At the primary site, intra- and peritumoral blood and lymphatic microvessels are the crucial structures that facilitate tumor cell dissemination; probably both vessel density and structures are key determinants for tumor cell dissemination (5–7). Once arriving at the distal site, tumor cells often form micrometastases caving around the existing blood vessels in which nutrients and O2 can be freely diffused to tumor cells and angiogenesis can be initiated if further growth begins (12, 13). Thus, the entire metastatic process is tightly linked to the intimate interactions between tumor cells and hemvascular and lymphvascular systems. Tumors produce various angiogenic factors to stimulate angiogenesis and lymphangiogenesis (14–21). Both VEGF-C and FGF-2 are commonly expressed in various tumor tissues and their expression levels have been correlated with tumor growth, progression, and metastasis (22, 23). Although the individual roles of angiogenic factors in promoting tumor growth and metastasis are relatively well studied, the cross-talk between various angiogenic factors in the tumor environment remains poorly understood. In this study, we provide a unique example of the intimate interaction between FGF-2 and VEGF-C in promoting lymphangiogenesis and metastasis. Our findings may provide important information for therapeutic development of novel strategies for the treatment of cancer metastasis by intervention of the interaction between FGF-2 and VEGF-C.
Results
FGF-2 and VEGF-C Collaboratively Promote Corneal Angiogenesis and Lymphangiogenesis.
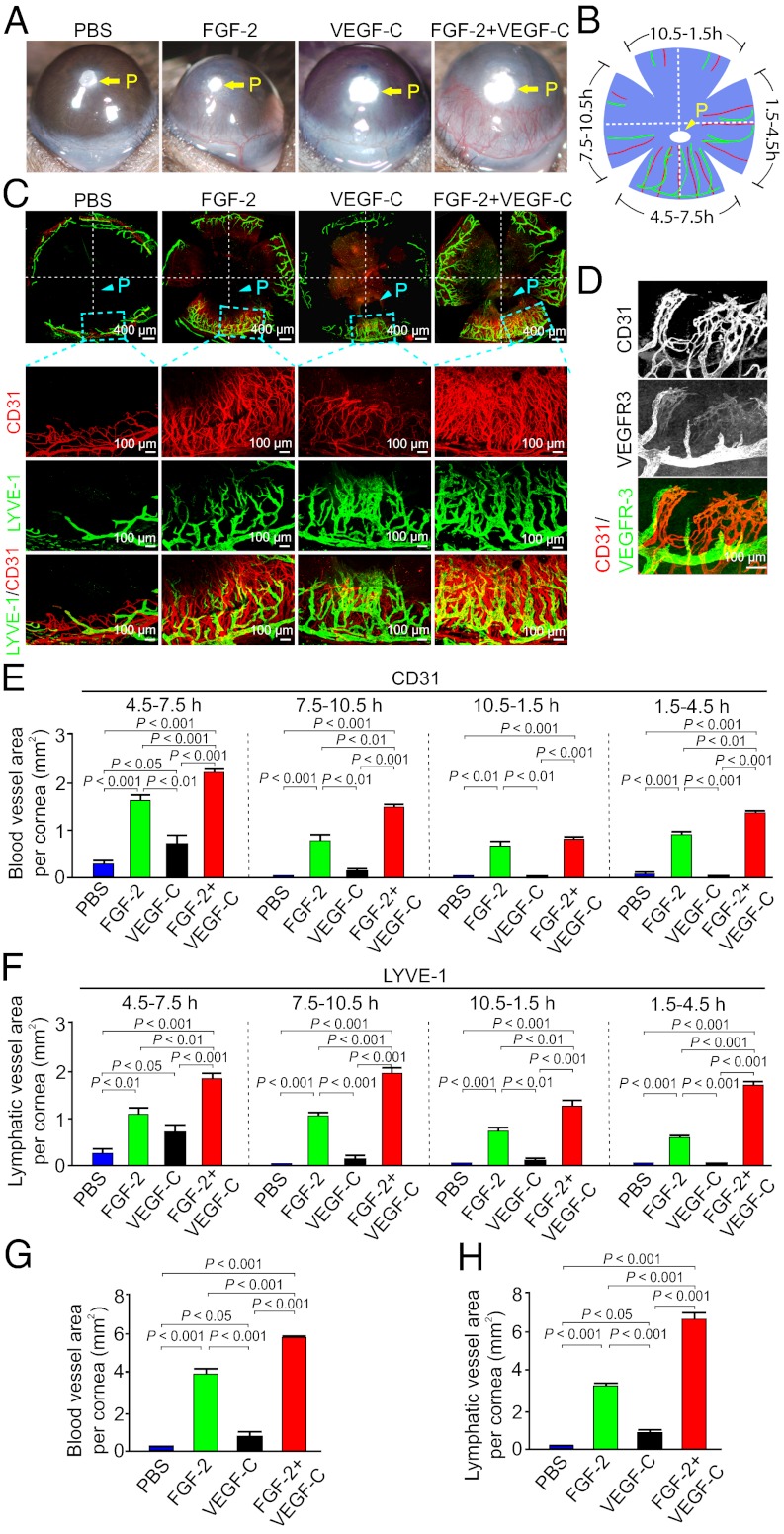
To study interplay between various angiogenic factors in promoting lymphangiogenesis, we chose FGF-2 and VEGF-C, two commonly expressed angiogenic factors, in the tumor microenvironment for our study. First, we tested their angiogenic and lymphangiogenic effects in the mouse corneal micropocket assay (24). As the corneal tissue remains avascular, implantation of angiogenic factors in the cornea would allow us to study a specific factor-induced lymphangiogenesis and vascular structures. FGF-2 and VEGF-C, together with a slow-release polymer composed of sucralfate and hydron, were implanted into the corneal micropockets of C57BL/6 mice. At day 6 postimplantation, gross-examination of corneas showed that FGF-2 displayed a robust angiogenic response (Fig. 1A). As expected, VEGF-C was also able to stimulate corneal angiogenesis, albeit the angiogenic activity was substantially weaker relative to FGF-2 (Fig. 1A). Surprisingly, coimplantation of micropellets containing FGF-2 and VEGF-C resulted in angiogenic synergism (Fig. 1A). The slow-release polymer-implanted corneas without angiogenic factors were used as a negative control and lacked the ability of triggering an angiogenic response (Fig. 1A), suggesting that the implantation procedure per se does not induce angiogenesis.
Fig. 1.
FGF-2 and VEGF-C collaboratively stimulate hem- and lymphangiogensis. (A) Micropellets containing FGF-2, VEGF-C, or FGF-2 plus VEGF-C together with the slow-release polymer were implanted into micropockets of mouse corneas (n = 5–6 per group). At day 6 after implantation, corneal neovascularization was examined and photographed. Slow-release polymer containing PBS was used as a negative control. P indicates the position of the implanted pellet. (B) Schematic diagram demonstrates the flat-mounted cornea and clock-hours of the circumferential cornea. (C) A representative cornea from each group that were double immunostained with CD31 (red) and LYVE-1 (green), which showed no overlapping signals. (D) Double immunostaining of corneal blood and lymphatic vessels using VEGFR-3 (green) and CD31 (red) specific antibodies. (E) Quantification of corneal CD31+ blood vessels at different clock hours (n = 5–6 /group). (F) Quantification of corneal LYVE-1 positive lympghatic vessels at different clockhours (n = 5–6 per group). (G) Quantification of the total CD31+ vessels in the entire of circumferential area of each cornea (n = 5–6 per group). (H) Quantification of the total LYVE-1+ lymphatic vessels in the entire of circumferential area of each cornea (n = 5–6 per group).
To further study synergisms of FGF-2– and VEGF-C–induced angiogenesis and lymphangiogenesis, flat-mounted mouse corneas were immunohistologically stained with CD31 and LYVE-1 (lymphatic vessel endothelial hyaluronan receptor-1), two specific pan-markers for blood vessel endothelial cells and lymphatic endothelial cells (LECs), respectively. To comparatively and optimally quantify the results, the circumferential area of each flat-mounted cornea was divided into clock-hours (24), where the implanted pellet was positioned at the 6 h of the anterior front (Fig. 1B). Angiogenic and lymphangiogenic responses in each cornea of various groups were compared at 4.5–7.5 h, 7.5–10.5 h, 10.5–1.5 h, and 1.5–4.5 h positions. Consistent with gross-examinations, CD31 staining demonstrated that FGF-2 and VEGF-C collaboratively induced corneal neovascularization (Fig. 1 C and E). LYVE-1 staining showed that both FGF-2 and VEGF-C induced relative robust lymphangiogenic responses (Fig. 1 C and F). Interestingly, an additive lympangiogenic effect between FGF-2 and VEGF-C was detected in all clock-hour positions of the cornea (Fig. 1 C and F). The additive angiogenic and lymphangiogenic effects of FGF-2 and VEGF-C were further validated by quantifying the entire circumferential area of each cornea (Fig. 1 G and H). Double immunohistochemical staining showed that CD31+ and LYVE-1+ vessels lacked overlapping signals, demonstrating high specificities of these antibodies. Staining with a VEGFR-3 specific antibody further validated the fact that lymphatic vessels and blood vessels are two independent vascular structures (Fig. 1D). Taken together, FGF-2 and VEGF-C collaboratively stimulated corneal angiogenesis and lymphangiogenesis.
FGF-2 and VEGF-C Independently Stimulate LEC Proliferation and Migration.
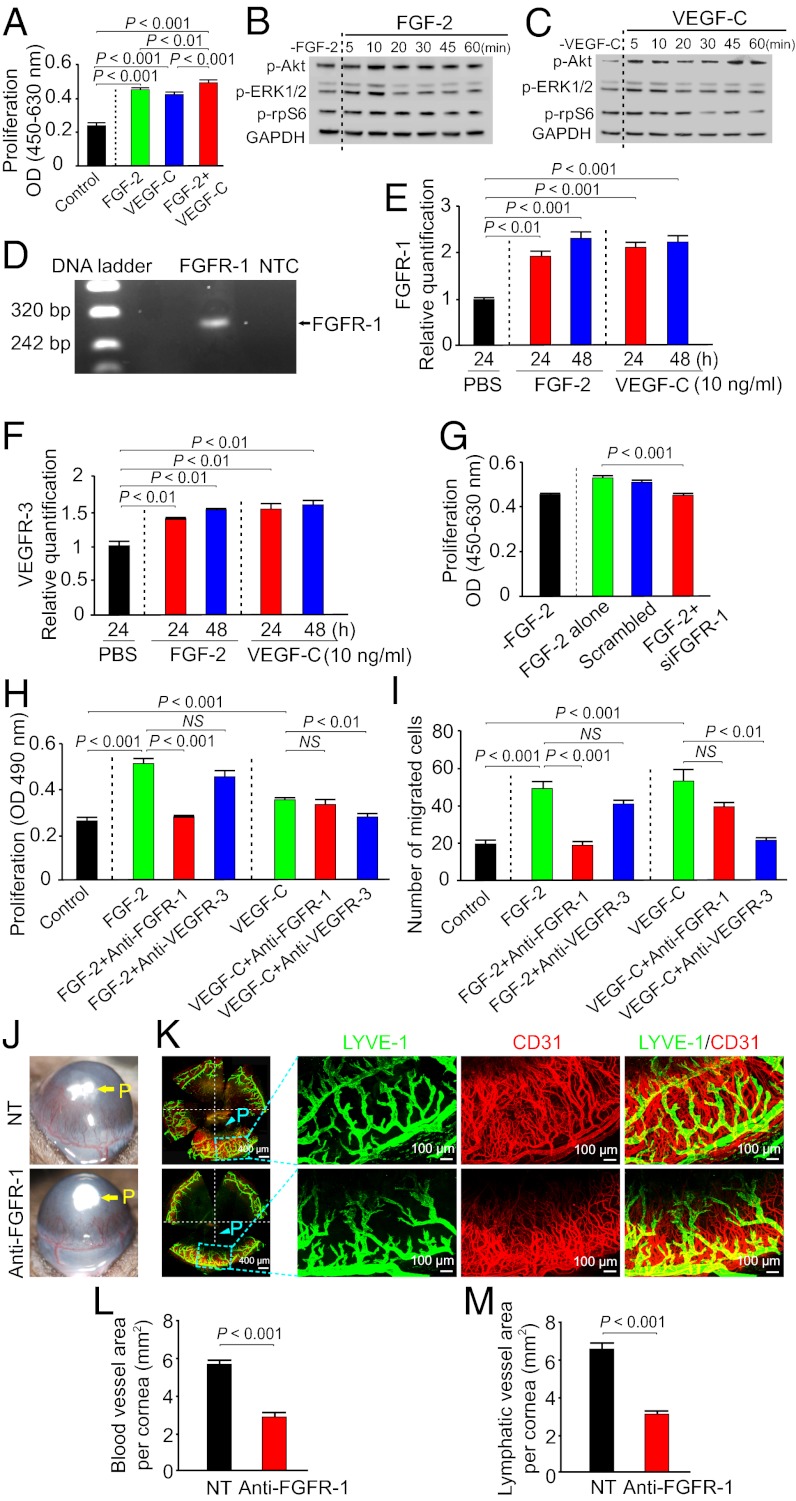
To study the cellular basis of lymphangiogenic synergism between FGF-2 and VEGF-C, primary LECs were used for in vitro proliferation and migration assays. As expected, VEGF-C significantly stimulated human LEC (hLEC) proliferation (Fig. 2A). Interestingly, FGF-2 also potently induced hLEC proliferation, suggesting that FGF-2 directly acts on hLEC to induce lymphangiogenic activity. Costimulation of hLECs with FGF-2 and VEGF-C resulted in significantly increased cell proliferation relative to FGF-2 or VEGF-C alone (Fig. 2A). Consistent with hLEC proliferation, FGF-2 was able to directly induce phosphorylation of several intracellular signaling components in LECs, including Akt, Erk, and ribosomal protein S6, further supporting its direct effect on hLECs (Fig. 2B). Similarly, stimulation of hLECs with VEGF-C also led to activation of these intracellular signaling components (Fig. 2C).
Fig. 2.
In vitro LEC activity and signaling and suppression of hem- and lymphangiogenesis by FGFR-1 blockade. (A) Stimulation of hLEC proliferation by FGF-2, VEGF-C, FGF-2 plus VEGF-C or buffer alone (16 samples per group). (B) Immunoblot analysis of activation of signaling components in hLECs by FGF-2. GDPDH showed the standard level of sample loading. (C) Immunoblot analysis of activation of signaling components in hLECs by VEGF-C. GDPDH showed the standard level of sample loading. (D) Amplification of Fgfr-1 by reverse-transcription PCR using cDNA extracted from LECs. (E) qPCR analysis of Fgfr-1 mRNA expression levels in hLECs stimulated by FGF-2 or VEGF-C. PBS-stimulated cells were used as a control. (F) Quantitative PCR analysis of Vegfr-3 mRNA expression levels in hLECs stimulated by FGF-2 or VEGF-C. PBS-stimulated cells were used as a control. (G) Fgfr-1 specific siRNA substantially inhibited FGF-2–induced LEC proliferation. A scrambled nucleotide sequence was used as a control. (H) Inhibition of FGF-2– or VEGF-C–induced mLEC proliferation by FGFR-1 or VEGFR-3 blockade (six samples per group). (I) Inhibition of FGF-2– or VEGF-C–induced mLEC migration by FGFR-1 or VEGFR-3 blockade (six samples per group). (J) Suppression of FGF-2 plus VEGF-C–induced corneal neovascularization by FGFR-1 blockade. (K) Suppression of FGF-2 plus VEGF-C–induced corneal hemangiogenesis (CD31+ blood vessels) and lymphangiogenesis (LYVE-1+ lymphatic vessels) by FGFR-1 blockade. P, pellet. (L) Quantification of antiangiogenic activity by FGFR-1 blockade (n = 5–6 per group). (M) Quantification of anti-lymphangiogenic activity by FGFR-1 blockade (n = 5–6 per group).
RT-PCR analysis demonstrated that Fgfr-1 was expressed in hLECs (Fig. 2D), whereas other forms of Fgfrs including Fgfr-2, Fgfr-3, and Fgfr-4 were weakly expressed or remained undetectable. To study the molecular mechanism that underlies the lymphangiogenic synergism, reciprocal regulation of Fgfr-1 and Vegfr-3 in hLECs by both VEGF-C and FGF-2 were analyzed. Interestingly, Fgfr-1 expression was up-regulated by both VEGF-C and FGF-2 (Fig. 2E). Similarly, significant up-regulation of Vegfr-3 was detected in FGF-2– and VEGF-C–stimulated LECs (Fig. 2F). These findings suggested that FGF-2 and VEGF-C reciprocally amplify their receptor-mediated signaling systems in LECs, leading to additive lymphangiogenic activity when both factors are coexposed to LECs.
We next used siRNA specific for Fgfr-1 to inhibit FGF-2–induced hLEC proliferation. As expected, siRNA specific for Fgfr-1, but not scrambled siRNA, significantly suppressed FGF-2–induced hLEC proliferation (Fig. 2G). To further study potential involvement of VEGFR-3 and FGFR-1 in mediating FGF-2–induced LEC proliferation and migration, anti–VEGFR-3 and anti-FGFR-1 neutralizing antibodies (hereafter called VEGFR-3 blockade and FGFR-1 blockade) (25, 26) were used to functionally block the VEGFR-3 signaling system or the FGFR-1 signaling system. FGF-2 displayed potent proliferative and migratory effects on primary mouse LECs (mLECs), which were completely neutralized by FGFR-1 blockade, but not by VEGFR-3 blockade (Fig. 2 H and I). Similarly, VEGF-C–induced proliferative and migratory activities were only inhibited by VEGFR-3 blockade, but not by FGFR-1 blockade (Fig. 2 H and I). These findings further support the fact that FGF-2 and VEGFR-3 independently mediate their specific ligand-triggered lymphangiogenic activity.
VEGFR-3–Mediated Lymphatic Tips Cell Formation Are Prerequisite for FGF-2–Induced Lymphangiogenesis.
To study the role of the FGFR-1–mediated signaling system in stimulation of hem- and lymphangiogenesis, a FGFR-1 neutralizing antibody was used for treatment. Interestingly, FGFR-1 blockade significantly inhibited FGFR-2 plus VEGF-C–induced angiogenesis and lymphangiogenesis (Fig. 2 J–M). Quantification of CD31+ vascular structures showed that ∼50% reduction of corneal neovascularization by FGFR-1 blockade (Fig. 2L). A similar potent antilymphangiogenic activity was also observed in FGFR-1 blockade-treated corneas (Fig. 2M).
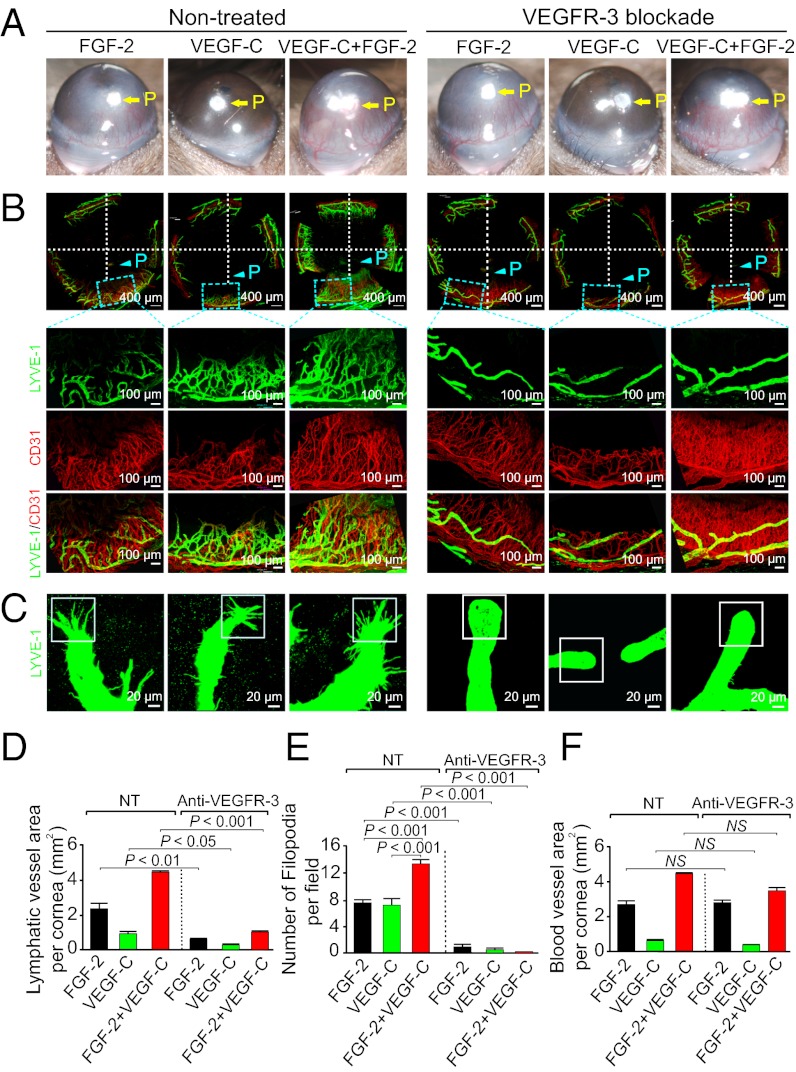
Expectedly, VEGFR-3 blockade completely blocked VEGF-C–induced lymphangiogenesis and hemangiogenesis (Fig. 3 A, B, D, and F), suggesting the VEGFR-3–transduced signals are essential for VEGF-C–stimulated angiogenesis and lymphangiogenesis. Surprisingly, VEGFR-3 blockade almost entirely inhibited FGF-2–induced lymphangiogenesis in this in vivo model (Fig. 3 B and D). These findings are unexpected because the same VEGFR-3 blockade did not significantly affect FGF-2–induced LEC proliferation and migration in vitro.
Fig. 3.
Anti-VEGFR-3 blocks FGF-2–induced lymphangiogenesis and lymphatic tip formation. (A) FGF-2–, VEGF-C–, or FGF-2 plus VEGF-C–induced corneal neovascularization was treated with or without VEGFR-3 blockade. (B) FGF-2–, VEGF-C–, or FGF-2 plus VEGF-C–induced corneas in response to anti–VEGFR-3 treatment were double immunostained with CD31 (red) and LYVE-1 (green). VEGFR-3 blockade almost completely suppressed FGF-2–, VEGF-C–, or FGF-2 plus VEGF-C–induced corneal lymphangiogenesis but not hemangiogenesis. (C) Suppression of FGF-2–, VEGF-C–, or FGF-2 plus VEGF-C–induced tip formation of lymphatic vessels by VEGFR-3 blockade. (D) Quantification of LYVE-1+ lymphatic vessels in various groups treated with or without VEGFR-3 blockade (n = 4–6 per group). (E) Quantification of lymphatic tips in various groups treated with or without VEGFR-3 blockade (n = 4–6 per group). (F) Quantification of CD31+ blood vessels in various groups treated with or without VEGFR-3 blockade (n = 4–6 per group).
To gain a mechanistic insight by which the VEGFR-3 signaling system is a prerequisite for FGF-2–induced lymphangiogenesis, we analyzed the tip cell formation at the leading edge of growing lymphatics. Recent studies have shown that the formation of tip endothelial cells in growing angiogenic and lymphangiogenic vessels is an essential process for development of vascular networks (27–29). Indeed, both FGF-2 and VEGF-C were able to induce endothelial tips at the leading front of the growing lymphatics (Fig. 3C). Quantification analysis showed that coimplantation of FGF-2 and VEGF-C resulted in an additive effect on the formation of LEC tips (Fig. 3 C and E). Surprisingly, VEGFR-3 blockade not only completely inhibited the lymphatic tip cell formation in the VEGF-C–induced lymphatic vessels, but also in FGF-2–stimulated lymphatics (Fig. 3 C and E). Similarly, FGF-2 plus VEGF-C–induced tip cell formation at the leading edge of the lymphatics was also completely suppressed by VEGFR-3 blockade. Unlike antilymphangiogenic activity, VEGFR-3 blockade did not inhibit FGF-2–induced blood vessel growth, suggesting that FGF-2 stimulates angiogenesis via a VEGFR-3 independent pathway. Interestingly, VEGFR-3 blockade did not significantly affect the VEGF-C–induced hemangiogenesis, supporting the fact that the interaction of VEGF-C with VEGFR-2 but not with VEGFR-3 is the key pathway for triggering angiogenesis (13). These findings demonstrate that VEGFR-3–induced tip formation is a prerequisite for FGF-2–stimulated lymphangiogenesis but not for hemangiogenesis.
FGF-2 and VEGF-C Collaboratively Promote Tumor Hemangiogenesis and Lymphangiogenesis.
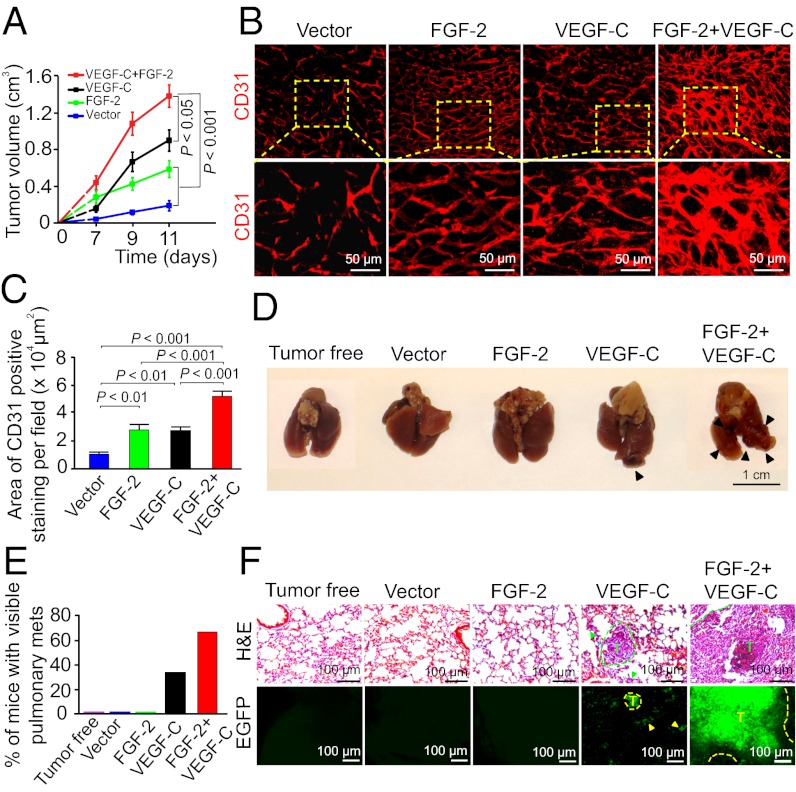
The lymphangiogenic synergism of FGF-2 and VEGF-C in mouse corneas promoted us to study its relevance to tumor lymphatic vessel development and lymphatic metastasis. We transfected a murine fibrosarcoma cell line to express secreted forms of FGF-2 and VEGF-C, as previously described (18, 30). As expected, both FGF-2 and VEGF-C significantly promoted tumor growth when subcutaneously implanted in immunodeficient SCID mice (Fig. 4A). Interestingly, implantation of the same number of tumor cells consisting of 50% FGF-2 and 50% VEGF-C cells resulting in a markedly accelerated tumor growth rate (Fig. 4A), demonstrating that these two factors collaboratively stimulate tumor growth. Consistent with the markedly accelerated tumor growth rate, FGF-2– and VEGF-C–coexpressing tumors contained a higher density of disorganized vascular networks compared with tumors expressing FGF-2, VEGF-C, or vector alone (Fig. 4 B and C). These findings demonstrate that additive stimulation of tumor angiogenesis by FGF-2 and VEGF-C significantly contributes to the accelerated tumor growth rate.
Fig. 4.
FGF-2 and VEGF-C collaboratively promote tumor angiogenesis and hematogenous metastasis. (A) Growth rates of vector, FGF-2–, VEGF-C–, and FGF-2 plus VEGF-C–expressing mouse fibrosarcomas. (B) Detection of tumor microvessels in various tumor groups by CD31 immunostaining. (C) Quantification of CD31+ vessel density in various tumor groups (n = 6 per group). (D) Lung morphology of a representative mouse from each tumor-bearing group (n = 6 per group). Arrowheads point to lung surface metastatic nodules. (E) Quantification of percentage of tumor-bearing mice that had lung metastases (n = 6 per group). (F) Histological examination of pulmonary metastases in mice bearing various tumors. Dashed lines encircle metastatic tumors that express EGFP (green). T, tumor.
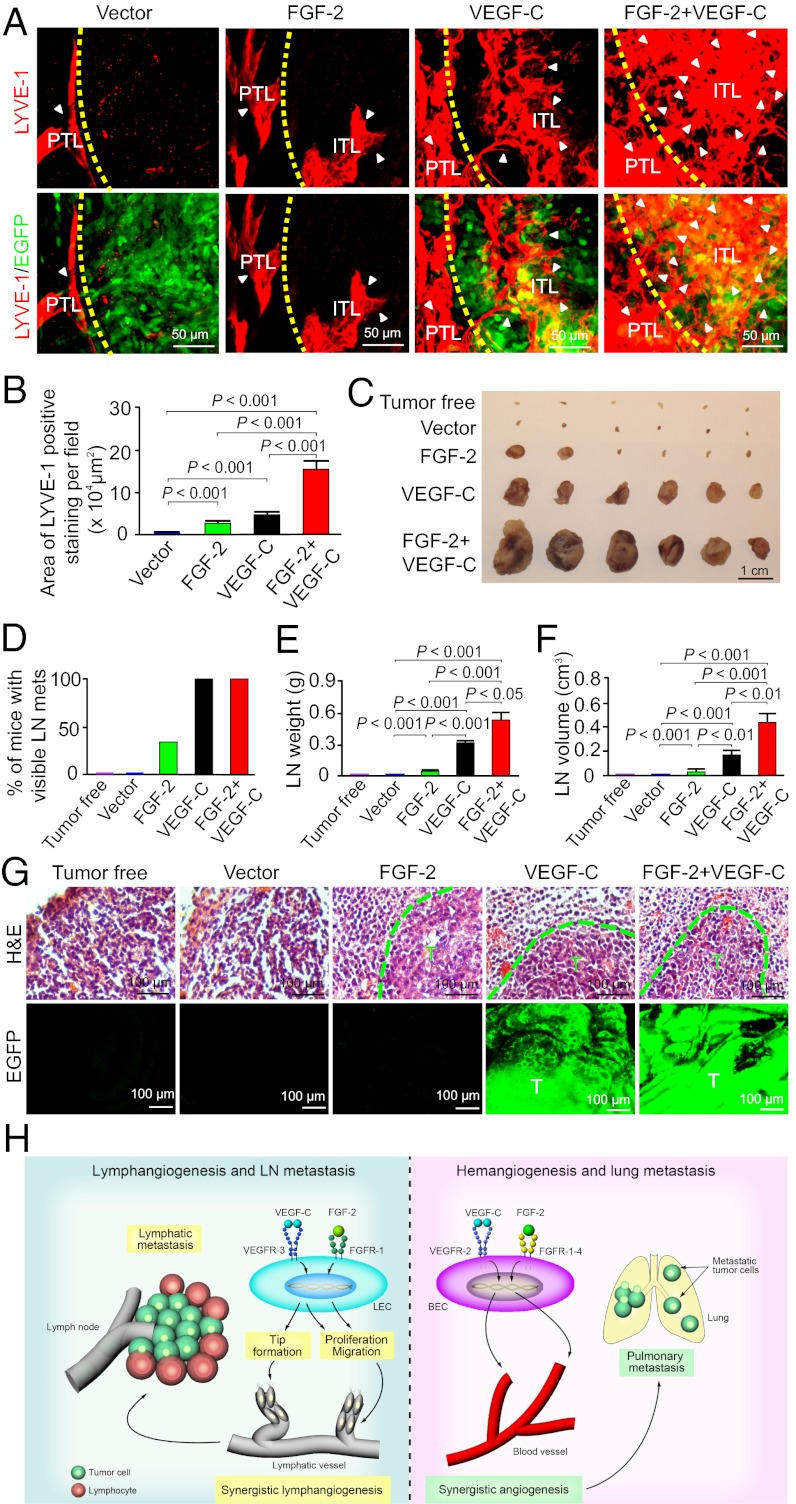
We next analyzed tumor lymphatic vessels using LYVE-1 as a specific marker. In the vector-transfected control tumors, LYVE-1+ lymphatic vessels were primarily distributed in the peritumoral area and intratumoral lymphatics were rarely detectable (Fig. 5A). In contrast, both FGF-2 and VEGF-C tumors significantly stimulated intratumoral lymphatic vessel growth, albeit VEGF-C exhibited more potent lymphangiogenic activity than FGF-2 (Fig. 5A). Surprisingly, tumors expressing both factors contained a markedly higher density of intratumoral lymphatic vessels than tumors expressing FGF-2 or VEGF-C alone (Fig. 5A). Quantification analysis showed that FGF-2 plus VEGF-C collaboratively promoted tumor lymphangiogenesis (Fig. 5B). FGF-2 plus VEGF-C–induced lymphatic vessels appeared as disorganized vascular networks compared with tumors expressing FGF-2, VEGF-C or vector alone (Fig. 5A). Additionally, peritumoral lymphatics became highly dilated and often contained EGFP+ tumor cells (Fig. S1). These findings further validate the mouse corneal data that FGF-2 and VEGF-C collaboratively stimulated lymphangiogenesis, which occurred under pathological conditions such as in the tumor environment.
Fig. 5.
FGF-2 and VEGF-C collaboratively promotes tumor lymphangiogenesis and lymphatic metastasis. (A) Detection of peritumoral and intratumoral lymphatic vessels (PTL and ITL) in various tumor groups by LYVE-1 immunostaining. Dashed line defines the rim between the tumor and peritumoral regions. Arrowheads indicate the tumoral lymphatics. (B) Quantification of intratumoral LYVE-1+ lymphatic vessel density in various tumor groups (n = 6 per group). (C) Sentinel lymph node morphology of various tumor-bearing groups (n = 6 per group). (D) Quantification of percentage of tumor-bearing mice that had sentinel lymph-node metastases (n = 6 per group). (E) Average weight of sentinel lymph nodes in various tumor-bearing mice (n = 6 /group). (F) Average volume of sentinel lymph nodes in various tumor-bearing mice (n = 6 per group). (G) Sentinel lymph node histology showed the presence of lymphatic metastases in FGF-2, VEGF-C, and FGF-2 plus VEGF-C tumor-mice. Dashed lines encircle metastatic tumors that express EGFP (green). T, tumor. (H) Schematic diagram of molecular mechanisms by which FGF-2 and VEGF-C collaboratively induce hem-/lymphangiogenesis and metastasis. VEGF-C activates VEGFR-3 receptor on LECs, leading to LEC tip formation, proliferation, and migration. FGF-2 via activation of FGFR-1 stimulates LEC proliferation and migration. However, VEGFR-3–triggered tip cell formation is a prerequisite for FGF-2–induced lymphangiogenesis. Blockade of the VEGFR-3 signaling system completely inhibits FGF-2–induced lymphangiogenesis. Lymhangiogenic interplay between FGF-2 and VEGF-C in the tumor environment promoted lymphatic metastasis. On blood vessel endothelial cells (BVECs), VEGF-C binds to VEGFR-2 and induced hem-angiogenic signals. FGF-2 directly induces hemangiogenesis by activation of FGFR-1 to -4 expressed on BVECs. The FGF-2 plus VEGF-C–induced high numbers of disorganized tumor blood vessels facilitate hematogenous metastasis.
FGF-2 and VEGF-C Collaboratively Stimulate Hematogenous and Lymphatic Metastasis.
Knowing that FGF-2 and VEGF-C collaboratively stimulated tumor hem- and lymphangiogenesis, and induced disorganization, we next studied their potentials in promoting hematogenous and lymphatic metastasis. Necropathy of tumor-bearing mice showed that ∼70% of FGF-2 plus VEGF-C tumor-bearing mice had pulmonary metastases on the surface of their lungs (Fig. 4 D and E). In contrast, no pulmonary metastasis was detected in FGF-2 tumor-bearing mice (Fig. 4 D and E) and only less than 40% of VEGF-C tumor-bearing mice had lung surface metastases (Fig. 4 D and E). Histological examination further confirmed the presence of large metastatic nodules, whereas only small microscopic micrometastases were found in the VEGF-C tumor-bearing mice (Fig. 4F). Both gross and histological examinations showed that mice with vector tumors completely lacked pulmonary metastases (Fig. 4 E and F). These findings provide compelling evidence demonstrating that FGF-2 and VEGF-C collaboratively promote hematogenous metastasis, most likely mediated by the high density of disorganized vasculatures in dual factor-expressing tumors.
Similar to the synergism of promoting hematogenous metastasis, examination of sentinel lymph nodes of tumor-bearing mice showed that 100% of VEGF-C and FGF-2 plus VEGF-C tumor-bearing mice carried enlarged sentinel lymph nodes (Fig. 5C). Notably, both weights and volumes of lymph nodes in FGF-2 plus VEGF-C tumor-bearing mice were significantly greater than those lymph nodes in VEGF-C tumor-bearing mice (Fig. 5 C–F). In contrast to VEGF-C and FGF-2 plus VEGF-C tumor-bearing mice, only a small number (less than 40%) of FGF-2 tumor-bearing mice suffered from enlargement of lymph nodes, which were marginally greater than those found in tumor-free mice (Fig. 5 C–F). Histology confirmed the presence of sentinel lymph node metastases in FGF-2, VEGF-C and FGF-2 plus VEGF-C tumor-bearing mice (Fig. 5G). Collectively, these data demonstrate that FGF-2 and VEGF-C collaboratively stimulate lymph node metastasis.
Discussion
In this study we provide several unique mechanistic insights on lymphangiogenesis regulated by FGF-2 and VEGF-C that stimulate discrete steps of lymphatic vessel growth (Fig. 5H). First, we show that FGF-2 directly acts on LECs to promote proliferation and migration via activation of the FGFR-1–mediated signaling pathway. These findings demonstrate that FGF-2 is a direct lymphangiogenic factor and have validated other published data (31). Second, we provide evidence that FGF-2 is able to induce tip cell formation at the leading front of growing lymphatic vessels. Third, FGF-2–induced LEC tips are dependent on the VEGFR-3 signaling system. This finding is unexpected because FGF-2 can directly promote VEGFR-3–independent LEC proliferation and migration in vitro. The fact that VEGFR-3 blockade completely inhibited FGF-2–induced lymphangiogenesis in vivo demonstrates the formation of LEC tips is an essential process for lymphatic vessel growth. This process is tightly controlled by the VEGFR-3–mediated signaling system and may not be replaced by other lymphangiogenic signals. If this is the case, the pivotal role of the VEGFR-3 system may reasonably be generalized to other lymphangiogenic factors. For example, hepatocyte growth factor (HGF)-induced lymphangiogenesis is dependent on the VEGFR-3 signaling system (16), although the involvement of the LEC tips in HGF-stimulated lymphatic vessels has not been studied. The essential role of VEGFR-3–induced LEC tips in FGF-2–induced lymphangiogenesis has raised several important issues, including: How does VEGFR-3 become activated? Does FGF-2 induce VEGF-C/-D expression or transactivate the VEGFR-3 signaling in the absence of these ligands? It is also plausible that FGF-2 induces VEGFR-3 expression in LECs, leading to more responsive to VEGF-C/-D stimulation. These interesting questions warrant further investigation. Finally, FGF-2 and VEGF-C collaboratively stimulate lymphangiogenesis in vivo, probably by enhancing the VEGF-C–VEGFR-3–induced LEC tips and FGF-2–triggered proliferative signals.
Unlike lymphangiogenesis, FGF-2–induced hemangiogenesis is completely insensitive to VEGFR-3 blockade, indicating that FGF-2 on its own is sufficiently potent to trigger angiogenic signals. Although the formation of endothelial cell tips at the leading edge of angiogenic vessels is probably also essential for hemangiogenesis, this process does not seem to require activation of VEGFR-3, which is not prominently expressed in blood vessels. However, FGF-2 and VEGF-C also display marked additive activity in stimulation of hemangiogenesis. Because of its binding to VEGFR-2 receptor (13), the angiogenic synergism of FGF-2 and VEGF-C is likely achieved by the intimate signaling interaction between FGFRs and VEGFR-2. Consistent with this notion, previous studies demonstrated that FGF-2 and VEGF-A (another VEGFR-2 binding ligand) collaboratively promote angiogenesis in vivo (32).
Hem- and lymphangiogenic synergisms between FGF-2 and VEGF-C in the tumor microenvironment lead to increased bloodstream and lymphatic metastases. The dual factor-induced angiogenic and lymphangiogenic vessels within tumors appeared to be highly disorganized and premature, a susceptible feature for facilitating tumor-cell dissemination and intravasation. Furthermore, peritumoral lymphatic vessels are highly dilated and may substantially contribute to lymphatic metastasis. Once metastasized to sentinel lymph nodes, the growth of metastases is further dependent on hemangiogenesis. In our tumor models, we show that FGF-2 and VEGF-C not only facilitate the metastatic process, but also further stimulate metastatic growth to become visible metastatic masses. Thus, FGF-2 and VEGF-C accelerate metastasis by acting on several metastatic steps; that is, facilitating tumor cell dissemination via intratumoral disorganized blood or lymphatic vessels, propagating or co-opting surrounding blood or lymphatic vessels, and supporting further growth of metastatic masses in distal organs, where tumor cells can be further spread to other tissues and organs. It is therefore critically important to develop therapeutic agents that interfere with the interplay between various tumor angiogenic factors.
Experimental Procedures
All animal studies were approved by the Northern Stockholm Experimental Animal Ethical Committee. Statistical analyses were performed using a standard two-tail Student's t test. Mean determinants were presented as ± SEM. Details are provided in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Sharon Lim for the artistic work presented in Fig. 5, and ImClone/EliLilly for providing FGFR-1– and VEGFR-3–specific neutralizing antibodies. Y.C.’s laboratory is supported by research grants from the Swedish Research Council, the Swedish Cancer Foundation, the Karolinska Institute Foundation, the Karolinska Institute distinguished professor award, the Torsten Soderbergs Foundation, ImClone Systems/EliLilly, European Union Integrated Project of Metoxia Project 222741, the Nordea Foundation, and the European Research Council advanced Grant ANGIOFAT, Project 250021.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208324109/-/DCSupplemental.
References
- 1.Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79:185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Rouhi P, et al. Hypoxia-induced metastasis model in embryonic zebrafish. Nat Protoc. 2010;5:1911–1918. doi: 10.1038/nprot.2010.150. [DOI] [PubMed] [Google Scholar]
- 4.Lee SL, et al. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc Natl Acad Sci USA. 2009;106:19485–19490. doi: 10.1073/pnas.0909228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y. Opinion: Emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer. 2005;5:735–743. doi: 10.1038/nrc1693. [DOI] [PubMed] [Google Scholar]
- 6.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 7.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–583. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- 8.Kedrin D, et al. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods. 2008;5:1019–1021. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zijlstra A, Lewis J, Degryse B, Stuhlmann H, Quigley JP. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell. 2008;13:221–234. doi: 10.1016/j.ccr.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kienast Y, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y, et al. Expression of angiostatin cDNA in a murine fibrosarcoma suppresses primary tumor growth and produces long-term dormancy of metastases. J Clin Invest. 1998;101:1055–1063. doi: 10.1172/JCI1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Y, et al. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci USA. 1998;95:14389–14394. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y. Why and how do tumors stimulate lymphangiogenesis? Lymphat Res Biol. 2008;6:145–148. doi: 10.1089/lrb.2008.1007. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Zhong W. Tumor-derived lymphangiogenic factors and lymphatic metastasis. Biomed Pharmacother. 2007;61:534–539. doi: 10.1016/j.biopha.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Cao R, et al. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–3536. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- 17.Björndahl M, et al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Y. Direct role of PDGF-BB in lymphangiogenesis and lymphatic metastasis. Cell Cycle. 2005;4:228–230. doi: 10.4161/cc.4.2.1419. [DOI] [PubMed] [Google Scholar]
- 19.Morisada T, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 20.Veikkola T, Alitalo K. Dual role of Ang2 in postnatal angiogenesis and lymphangiogenesis. Dev Cell. 2002;3:302–304. doi: 10.1016/s1534-5807(02)00231-9. [DOI] [PubMed] [Google Scholar]
- 21.Xue Y, et al. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat Med. 2011;18:100–110. doi: 10.1038/nm.2575. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen M, Watanabe H, Budson AE, Richie JP, Folkman J. Elevated levels of the angiogenic peptide basic fibroblast growth factor in urine of bladder cancer patients. J Natl Cancer Inst. 1993;85:241–242. doi: 10.1093/jnci/85.3.241. [DOI] [PubMed] [Google Scholar]
- 23.Van der Auwera I, et al. First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer. 2006;95:1611–1625. doi: 10.1038/sj.bjc.6603445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao R, et al. Mouse corneal lymphangiogenesis model. Nat Protoc. 2011;6:817–826. doi: 10.1038/nprot.2011.359. [DOI] [PubMed] [Google Scholar]
- 25.Kubo H, et al. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pytowski B, et al. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- 27.Tammela T, et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13:1202–1213. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tammela T, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 29.Hellström M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nissen LJ, et al. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117:2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin JW, et al. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: A role for FGF signaling in lymphangiogenesis. Mol Biol Cell. 2006;17(2):576–584. doi: 10.1091/mbc.E05-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nico B, de Falco G, Vacca A, Roncali L, Ribatti D. In vivo absence of synergism between fibroblast growth factor-2 and vascular endothelial growth factor. J Hematother Stem Cell Res. 2001;10:905–912. doi: 10.1089/152581601317211006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.