Abstract
The dominant theories of human placebo effects rely on a notion that consciously perceptible cues, such as verbal information or distinct stimuli in classical conditioning, provide signals that activate placebo effects. However, growing evidence suggest that behavior can be triggered by stimuli presented outside of conscious awareness. Here, we performed two experiments in which the responses to thermal pain stimuli were assessed. The first experiment assessed whether a conditioning paradigm, using clearly visible cues for high and low pain, could induce placebo and nocebo responses. The second experiment, in a separate group of subjects, assessed whether conditioned placebo and nocebo responses could be triggered in response to nonconscious (masked) exposures to the same cues. A total of 40 healthy volunteers (24 female, mean age 23 y) were investigated in a laboratory setting. Participants rated each pain stimulus on a numeric response scale, ranging from 0 = no pain to 100 = worst imaginable pain. Significant placebo and nocebo effects were found in both experiment 1 (using clearly visible stimuli) and experiment 2 (using nonconscious stimuli), indicating that the mechanisms responsible for placebo and nocebo effects can operate without conscious awareness of the triggering cues. This is a unique experimental verification of the influence of nonconscious conditioned stimuli on placebo/nocebo effects and the results challenge the exclusive role of awareness and conscious cognitions in placebo responses.
Keywords: analgesia, hyperalgesia, consciousness
Placebo and nocebo effects are critical components of medical practice and clinical research. Placebo analgesia and nocebo hyperalgesia are the most robust and well studied of these effects. Learning is known to play an important role in placebo and nocebo effects and the dominant theories invoke classical conditioning and expectancies as explanatory tools (1). Both rely on a notion that the conscious perception of sensory or social stimuli, such as the cue that triggers expectancy or the conditioned stimulus in classical conditioning, are needed to obtain placebo responses. In some circumstances, conditioning may be an automatic nonconscious process, but in most cases, it seems to involve the formation of expectations (2–4). However, it is not known whether conscious perception of a conditioned stimulus is needed to elicit a conditioned response.
There is a large literature suggesting that behavior can be motivated by stimuli that are not consciously perceived, because they are presented at low intensities or masked from conscious awareness (5, 6), sometimes referred to as subliminal stimuli. Nonconscious operations are considered a fundamental feature of human cognition, for example in reward processing (7, 8), fear learning (9, 10), and social behavior (11, 12). Furthermore, evidence suggests that conditioned responses may be acquired outside of conscious awareness (13–15). Neuroimaging studies of the human brain suggest that certain structures, such as the striatum and the amygdala, can process incoming stimuli before they reach conscious awareness, and thus they may mediate nonconscious effects on human cognition and behavior (16, 17). It has never been investigated, however, whether learned placebo and nocebo responses can be triggered through this cerebral circuit that bypasses conscious awareness.
Placebo and nocebo may be seen as the behavioral response to signals of reward and threat, respectively. Considering the neurobiological evidence for nonconscious processing of reward and threat signals, placebo and nocebo responses would have the potential to be activated by masked stimuli. Here, we experimentally test this hypothesis and explore the role of nonconscious mental processes in placebo analgesia by investigating whether conditioned placebo and nocebo responses can be activated by masked stimuli (n = 40). Conditioning was performed with clearly visible cues and a subsequent test-sequence–measured placebo and nocebo responses to either visible (unmasked) or nonconscious (masked) cues (see Fig. 1). Our goal was to test whether conditioned placebo and nocebo responses could be activated by both consciously and nonconsciously perceived cues.
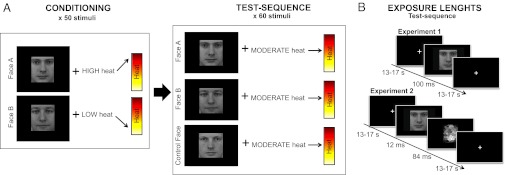
Fig. 1.
Experimental procedure and visual exposures. (A) Overview of the experimental design of experiments 1 and 2. Conditioning sequence was performed where clearly visible images of two male faces were used as visual cues, presented on a computer screen. Each face cue was consistently paired with a rapid high or low heat pain stimulus on the subject’s arm. After the conditioning phase, there was a test sequence in which the high cue, low cue, and a neutral control cue were paired with identical moderate heat stimuli. Subjects were asked to rate their pain intensity in response to each stimulus. (B) Consecutive screenshots displayed during the test sequence of experiment 1 and experiment 2. Duration of each exposure is given in milliseconds. In experiment 1, the face cues were exposed long enough for all subjects to clearly recognize them (100 ms) but in experiment 2, the face cues were exposed for only 12 ms and then followed by a mask to prevent conscious recognition. Faces reprinted with permission from ref. 50. Copyright Karolinska Institutet, Department of Clinical Neuroscience, Section of Psychology, Stockholm, Sweden.
Results
Experiment 1.
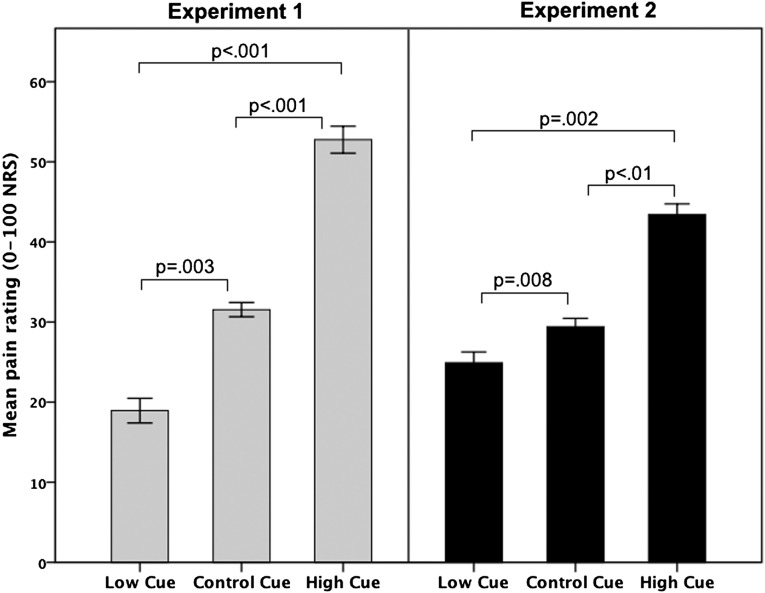
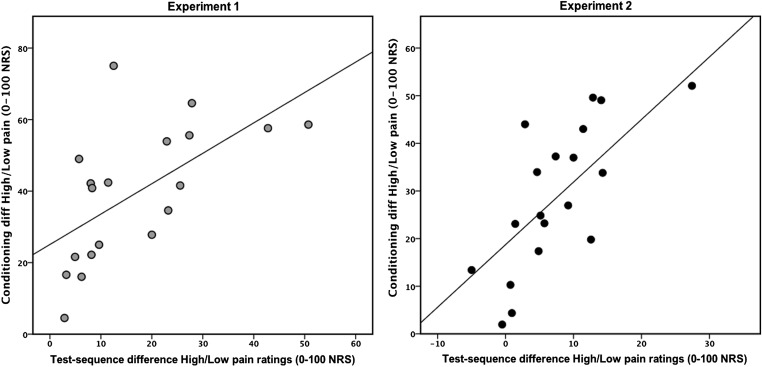
Experiment 1 was designed to ascertain that conditioned placebo and nocebo pain responses could be elicited by consciously perceived stimuli. An initial conditioning sequence, in which high and low thermal pain stimuli were paired with clearly visible exposures of two male faces on a computer screen, produced a mean rating of “high pain” at 63 (± 22.1) on a numeric response scale (NRS), ranging from 0 (no pain) to 100 (worst imaginable pain), and “low pain” at 24 (± 17.4) on the NRS. The test sequence, also using clearly visible stimuli, revealed significantly higher pain ratings in response to the high face and lower ratings for the low face, despite identical moderate temperatures. The control condition, paired with the same moderate temperature, resulted in pain ratings in between the high and low conditions; ANOVA main effect for face type (high/low/control) F(2, 36) = 24, P < 0.001. All pairwise comparisons between the high/low (P < 0.001), high/control (P < 0.001) and low/control (P = 0.003) conditions were significant (Fig. 2) and all subjects reported that they could clearly discriminate between the different faces during the test sequence. Correlations (Pearson’s r) revealed that there was a positive correlation between the difference in high minus low pain ratings during conditioning and the high minus low pain rating during the test sequence of experiment 1; r = 0.608, P = 0.006 (Fig. 3). Analyses of alpha reliability (Chronbach’s alpha) showed that the three conditions of the test sequence in experiment 1 (high face/low face/control face) displayed a reliability of 0.98. The pain ratings during the test sequence were not affected by the factor “time” F(2, 32) = 2.8, P = 0.073 and there was no interaction between pain ratings × time F(2, 32) = 2.2, P = 0.134, validating that pain habituation or sensitization did not confound the placebo/nocebo responses. In conclusion, the results from experiment 1 showed significant placebo and nocebo responses and also indicated that their magnitude was predicted by the difference between high and low pain ratings during the learning phase.
Fig. 2.
Pain ratings in response to identical temperatures during the test sequence. After a conditioning series of high- and low-pain temperatures (paired with a high cue and low cue), a test sequence was performed where identical moderate temperatures were paired with either the high cue, low cue, or a previously unconditioned cue, called the control cue. Graph represents the average pain rating in response to identical moderate temperatures, paired with each of the three different cues. In experiment 1, the cues during the test sequence were clearly recognizable. Experiment 2 was performed in a separate group of subjects and the cues of the test sequence were exposed so quickly that subjects could not consciously recognize them. Error bars represent two intrasubject SEs.
Fig. 3.
Correlation between conditioning and the magnitude of subsequent placebo and nocebo pain responses. The difference between high- and low-pain ratings during the conditioning phase correlated significantly to the magnitude of the placebo- and nocebo-like effects during the test sequence, both for conscious (experiment 1) and nonconscious cues (experiment 2). Scatterplots represent the positive correlation between the difference in high- minus low-pain ratings during conditioning (y axis) and the high- minus low-pain ratings during the test sequence (x axis); experiment 1, r = 0.608, P = 0.006; experiment 2, r = 0.822, P < 0.001.
Experiment 2.
Experiment 2 was designed to test the unique hypothesis that conditioned placebo and nocebo responses could be triggered by masked, nonconscious stimuli. In most respects, the methods in experiment 2 were identical to those used in experiment 1. The only difference was that all faces during the test sequence were presented for 12 ms followed by a visual mask for 84 ms, resulting in comparable visual exposures for both experiments (faces shown for 100 ms in experiment 1). The conditioning sequence in experiment 2 was performed with clearly recognizable images and produced a mean rating of “high pain” at 52 (± 19) and “low pain” at 21 (± 11.8) NRS. The test sequence, however, was performed using masked images, preventing subjects from consciously recognizing the stimuli. As in experiment 1, the results from the test sequence revealed significantly higher pain ratings in response to the high face, lower ratings for the low face, and intermediate ratings for the control face, despite identical moderate temperatures, ANOVA main effect for face type: F(1, 18) = 11, P < 0.001. All pairwise comparisons in experiment 2 were also significant despite subjects’ inability to consciously distinguish the faces from each other; high/low (P = 0.002), high/control (P = 0.011), and low/control (P = 0.008) (Fig. 2). A correlation analysis (Pearson’s) revealed a positive correlation between the difference in high minus low pain ratings during conditioning and the high minus low pain rating during the test sequence (r = 0.822, P < 0.001), indicating that the magnitude of masked placebo/nocebo responses could also be predicted by pain ratings during conditioning. Analyses of alpha reliability (Chronbach’s alpha) showed that the three conditions of the test sequence in experiment 2 (masked high face/low face/control face) displayed a reliability of 0.97. The pain ratings during the test sequence of experiment 2 were not affected by the factor time F(2, 38) = 0.8, P = 0.449 and there was no interaction between pain ratings × time F(2, 32) = 2.2, P = 0.134, validating that pain habituation or sensitization did not confound the placebo/nocebo responses.
All subjects in experiment 2 reported being unable to consciously discriminate between the different faces during the test sequence. To control for any potential variance due to differences in recognition of the faces in experiment 2, the outcome from a subsequent masked face recognition test was used as a covariate in the overall ANOVA, which demonstrated nonsignificance, F(1, 18) = 0.69, P = 0.414. Further validation that the images were indeed nonconscious was demonstrated by an analysis revealing that subjects’ ability to recognize the masked images, represented by the individual recognition rates, was uncorrelated to the effect of high minus low pain during the test sequence (r = −0.253, P = 0.296).
Discussion
Results from the present study demonstrate that placebo and nocebo mechanisms can be triggered by nonconscious cues, operating outside of conscious awareness. As far back as 1885, Peirce and Jastrow suggested that nonconscious cues could influence somatosensory perception (18). Since then, a large literature has suggested that nonconscious signals of threat and relief can be processed by subcortical (but also cortical) structures in the brain and influence behavioral outcomes (8, 19). Recent theoretical analyses have also suggested the possibility that placebo and nocebo responses may be mediated by nonconscious operations (20, 21), what Kihlstrom called the “cognitive unconscious” (22). Previous studies (13–15) have demonstrated that associative learning can be obtained by the use of nonconscious stimuli during the acquisition of conditioned responses. In the present study, we extended the understanding of nonconscious cognitions by showing that explicitly conditioned placebo analgesia and nocebo hyperalgesic responses can be activated by nonconscious cues. Our results thereby translate the investigation of nonconscious effects to the clinical realm, by suggesting that health-related responses can be triggered by cues that are not consciously perceived, not only for pain, which is one of the most common reasons for seeking healthcare (23), but also for other medical problems with demonstrated placebo effects, e.g., asthma (24), depression (25), and irritable bowel syndrome (26). Understanding the role of nonconscious processes in placebo/nocebo opens unique possibilities of enhancing clinical care by attending to the impact of nonconscious cues conveyed during the therapeutic encounter and improving therapeutic decisions.
Common theories of placebos involve expectancy and classical conditioning, and both mechanisms, although they can be hard to separate, involve conscious perception of the stimulus that elicits the placebo or nocebo response (3, 4). Our study is clearly distinguished from previous studies because it focuses on the nonconscious activation of placebo/nocebo responses and demonstrates that placebo/nocebo can be activated even if the conditioned stimulus is not consciously perceived. In traditional placebo studies, conditioning is often used by pairing the administration of an unconditioned stimulus (e.g., effective analgesic pill, cream, or injection) with a conditioned stimulus (e.g., inert placebo pill, cream, or injection), thus producing placebo responses through “associative learning” (27–30). Even if associative learning in such studies has been described in terms of a “nonconscious” mechanism (in which cognition may be an epiphenomenon, rather than part of a causal chain), the activation of the conditioned response has always been obtained by a perceptible conditioned stimulus. Furthermore, much of the evidence for conditioning effects in human placebo experiments demonstrates that a conscious cognitive component plays a significant role in the placebo conditioning. For example, Montgomery and Kirsch (31) replicated Voudouris et al.’s (32, 33) early conditioning placebo experiments and found that conscious awareness of the conditioning process eradicated placebo conditioning. Recent work by Watson et al. (34, 35) also supports the notion that conscious expectation plays a dominant role in conditioned placebo responses. On the other hand, in one of the most compelling experiments of placebo effects and conditioning, Benedetti and colleagues (36) showed that conditioned immune and endocrine placebo responses could still be elicited by saline placebo even if the subjects were given clear verbal directions not to expect any positive change. Given that subjects were aware of the injections of saline (the conditioned stimuli) in this experiment, Benedetti later noted that “a [remaining] key question is: does unconscious conditioning exist in humans?” (ref. 37, p. 53). To the best of our knowledge, our study presents unique evidence that conditioned placebo responses can be activated by cues outside of conscious awareness.
Our results and proposed model, shed light on findings from two previous clinical placebo studies that investigated how pain ratings can be affected by the interaction between patients’ and clinicians’ expectancies of pain relief. In one study, Gracely et al. (38) found, in a double-blind experiment of pain relief, that the clinician’s a priori knowledge of the likelihood of administering active analgesic treatment versus placebo was transmitted to the patient and influenced the placebo response. In another study, Levine and Gordon (39) compared the double-blind administration of morphine/placebo by either a hidden person or a hidden machine and found that the placebo response was significantly lower in response to the machine. We speculate that in both studies, subtle cues that the clinician conveyed to the patient may have been perceived without conscious awareness. The outcomes from these experiments suggest that placebo and nocebo effects in the clinical setting might not only be induced through explicit instructions and explanations, but also through nonconscious cues embedded in the patient–clinician interaction. Nevertheless, neither of these experiments explicitly tested the hypothesis that the findings were due to nonconscious cue mechanisms.
The present study demonstrated successful activation of placebo and nocebo effects in responses to both explicit (experiment 1) and nonconscious cues (experiment 2), suggesting that different levels of brain processing may be involved. Previous placebo and nocebo neuroimaging studies (40–44), using explicit cues to evoke placebo responses, conclude that conscious placebo effects recruit a combination of cortical and subcortical brain regions to modulate pain. Brain imaging studies also suggest that the brain can process environmental cues even if they are not reaching conscious awareness, largely through subcortical regions of the brain such as the amygdala and ventral striatum (8, 10). Thus, we speculate that nonconscious cues may work through the subcortical regions of the brain to produce placebo and nocebo effects. Furthermore, we speculate that nonconscious placebo and nocebo effects may not use the commonly reported cortical regions of the brain, such as the rostral anterior cingulate cortex (40, 43) and the prefrontal cortex (41, 42). Conversely, they are likely to be processed in subcortical parts of the brain that rely on a minimal account of awareness, such as the basal ganglia.
In summary, the present study provides an experimental demonstration of the influence of consciously nonrecognized stimuli on conditioned placebo and nocebo responses. It suggests that cognitive modulation of pain can be exerted without conscious awareness of the triggering cues. Our results point to the importance of a care process where the trajectory toward health is seen as a learning experience that is highly influenced by the activation of nonconscious environmental cues. Future studies will show whether the present findings can be translated into a clinical setting where nonconscious effects on health-related behavior and treatment outcomes can be further validated. In addition to pain responses, conditioned placebo effects are known to affect a variety of clinical symptoms (24, 45–47), suggesting that the present findings could be translated to other disciplines than pain. In addition, future studies should establish whether conditioned placebo and nocebo responses could be obtained with the use of nonconscious stimuli also during the acquisition phase of the conditioning procedure.
Materials and Methods
In total, 40 healthy subjects were included in this study (experiment 1: n = 20 subjects, 13 women and 7 men, mean age 22 ± 4 and experiment 2: n = 20; 11 women and 9 men, mean age 24 ± 4). All subjects were right handed, with no history of medical or psychiatric illness and no previous experience with fast image exposures or backward-masking experiments. Subjects were recruited through posted flyers at several different universities and at free expression boards in residential buildings.
Thermal pain stimuli were delivered using the Pathway CHEPS system from Medoc, with a 27-mm diameter CHEPS thermode. The calibrated goal temperatures were reached with a ramp up time of 300 ms and the duration of each pain stimulus was 3 s. An 85-Hz, 17-inch cathode ray tube monitor (NEC AccuSync) was used for visual presentations and the masked stimulus presentations were synchronized with the refresh rate (12 ms). Screen resolution was 1,024 × 768 pixels and the experiment was programmed in Presentation 13.0 (Neurobehavioral Systems). The images used in the current experiment were taken from The Karolinska Directed Emotional Faces set (KDEF) (48), a set of images specifically developed for use in perception, attention, emotion, memory, and backward masking experiments. The whole set consists of 70 individuals (35 male, 35 female), mean age 25 y (range 20–30) with seven different facial expressions per individual. The images used in the present experiment only represented men in control expressions, i.e., no emotional valence. In total, 24 male faces were used for the purpose of this study.
Subjects were screened for inclusion and exclusion criteria over the telephone and then scheduled for an experiment. Subjects were informed that the study investigated “the influence of implicit and explicit learning on pain perception” but the full purpose of the study was not revealed until the experiment was over. All subjects gave written informed consent and the study was approved by The Institutional Review Board at Massachusetts General Hospital.
The experiment was carried out in a quiet room at constant temperature (23 °C). Subjects were seated in front of a desk with the monitor placed straight in front of them: ∼70 cm from the subject’s face. The desk faced a wall, preventing subjects from visual distractions. The thermode stimulator was placed on the subject’s left volar forearm. Calibration of high and low pain was performed by means of ascending temperatures, starting from 40 °C, followed by a randomized series of mixed high and low temperatures. The goal was to find temperatures that would elicit high pain at ∼60 of 100 on a 0–100 NRS and low pain at ∼20 NRS. The difference between the chosen high and low pain temperature was fixed to 3 °C for all subjects, e.g., high pain/low pain could be represented by 49°/46 °C in one individual and 47°/44 °C in another.
After subjective calibration of heat pain, subjects were given the following instruction for the conditioning sequence “You are about to see some pictures on the screen. Each picture is paired with a pain stimulus on your arm. Your task is to focus on the screen at all times and after each picture I would like you to rate how much pain you felt on your arm, using the same 0–100 verbal scale that you used during the calibration.” To ensure that subjects maintained high attention, the conditioning sequence was divided in two blocks of ∼7 min each. In between the two blocks, subjects had the chance to stretch their legs and look away from the monitor for about 1.5 min or as long as they needed. In total, 50 stimuli were presented during the conditioning sequence: 25 for the high-pain face and 25 for the low-pain face. The exposure rate of each image was 100 ms and the mean stimulus onset asynchrony (SOA) was 15 s (range 13–17 s). In both experiment 1 and experiment 2, the two male faces associated with high or low pain were counterbalanced to reduce the possibility that a certain face would contribute to high or low pain ratings. Immediately after the conditioning sequence, subjects were given the following instruction “You are about to see the same pictures on the screen again and each picture will be paired with a pain stimulus on your arm, just like before. The only difference is that this time there will also be pictures of new guys, that you haven’t been exposed to before. Your task is to focus on the screen at all times and after each picture I would like you to rate how much pain you felt on your arm using the 0–100 verbal scale.” In experiment 2, the following sentence was also added “During this sequence, the pictures will be shown to you much faster than before, and you might not be able to recognize them. This is normal and something that we programmed on purpose. Your only task is to focus on the screen at all times and rate the pain on your arm, even if you can’t see the pictures.” The test sequence consisted of 60 stimuli: 20 for the high-pain condition, 20 for the low-pain condition, and 20 for the control condition. The test sequence was divided in three 6-min runs with a 1.5 min pause in between, allowing subjects to maintain a high level of awareness and lessen straining of the eyes. Two high faces and two low faces in each of the three runs were paired with their original temperatures, to prevent extinction. These “booster trials” were not included in the statistical analysis. In experiment 1, the exposure time of the faces during the test sequence was 100 ms. In experiment 2, the exposure time of the faces during the test sequence was 12 ms (one refresh cycle) and then a mask was exposed for 84 ms (seven refresh cycles). The mask consisted of an abstract image that had the same visual properties as the faces, but it was not representing anything more than a number of small squares put together. The same mask was used for all faces in experiment 2. During the calibration, conditioning, and test sequence, the experimental leader was placed in a chair in the back of the room, facing the subject’s back. The experimental leader repeated each verbal pain rating in a monotonous and control voice before recording the rating in the subject’s protocol. If the subject experienced an incongruency, s(he) was instructed to make a quick correction, e.g., say “No, not fifty, fif-TEEN.” Only in rare cases (<1%), such corrections were required. Moreover, the placement of the experimental leader allowed for constant monitoring to make sure that subjects’ were truly facing the screen and not looking in other directions.
After the last test sequence of experiment 1, subjects were asked whether the exposure time of the pictures allowed them to see each picture properly. All subjects in experiment 1 reported that they could clearly see the content of the picture and discriminate between the different faces. To verify that the stimuli in experiment 2 would be truly nonrecognizable, we first conducted a methodological pilot study in seven healthy individuals who were exposed to the visual paradigm of experiment 2 and then asked to perform a face recognition test. The instruction was: “You are about to see some pictures on the screen again and I would like you to answer if you have seen this face before during the experiment. You can only say “yes” or “no.” The pictures will be exposed to you very quickly so you might not be able to tell if you saw it before or not. In any case, you have to guess “yes” or “no” for each exposure.” The recognition test included 12 exposures of the previously used faces and 12 exposures of new faces and participants were asked to indicate whether the face had been exposed before, or not. Mean accuracy of identification was 53% (± 10), P = 0.466, confirming that the stimuli were indeed nonrecognizable and thus the parameters were used in experiment 2. Immediately after the test sequence of experiment 2, subjects were asked whether they could recognize the images properly. All subjects in experiment 2 answered that they could not consciously discriminate between the masked faces. To verify this, they were also presented with the face recognition test, used in the methodological pilot study. The accuracy of the recognition test, performed at the end of experiment 2, was analyzed using a repeated measures ANOVA. Results validate that there was no significant difference in recognition for any of the 12 faces used in the face recognition test, for any of the two exposures of each face [six new faces, six old faces, with two exposures each = 24 exposures in total. First exposure, main effect for face type F(1, 18) = 0.073, P = 0.790, nonsignificant; second exposure: main effect for face type F(1, 18) = 0.397, P = 0.538, nonsignificant.] The mean accuracy in the recognition test after experiment 2 was 59.9% (± 10), P = 0.003, one-sample t test. Four individuals had a recognition rate >70%, which contributed to a high mean recognition accuracy. However, the main result of experiment 2 was still significant, even if the subjects with the highest recognition accuracy were removed from the statistical analyses: recognition accuracy 52% (±8), P = 0.392, one-sample t test. Significant main effect for face type in a repeated measures ANOVA was: F(2, 22) = 7.25, P = 0.004 and significant pairwise comparisons between the high/low (P < 0.009), high/control (P < 0.047) and low/control condition (P < 0.019).
Acknowledgments
We thank Dr. Jonathan Berrebi who provided valuable technical expertise for this study and Prof. Arne Ohman and Dr. Predrag Petrovic for providing valuable theoretical support for this manuscript. The work is supported by funding from the Swedish Society for Medical Research and the Swedish Council for Working Life and Social Research (to K.B.J.) and Grants R21AT004497 (National Center for Complementary and Alternative Medicine, NCCAM), R03AT218317 (National Institute on Drug Abuse), and R01AT006364 (NCCAM) (to J.K.); K24AT004095 (NCCAM) (to T.J.K.); and R01AT005280 (NCCAM) (to R.L.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Finniss D, Kaptchuk T, Miller F, Benedetti F. Placebo effects: Biological, clinical and ethical advances. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirsch I, Lynn SJ, Vigorito M, Miller RR. The role of cognition in classical and operant conditioning. J Clin Psychol. 2004;60:369–392. doi: 10.1002/jclp.10251. [DOI] [PubMed] [Google Scholar]
- 3.Rescorla RA. Pavlovian conditioning. It’s not what you think it is. Am Psychol. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- 4.Stewart-Williams S, Podd J. The placebo effect: Dissolving the expectancy versus conditioning debate. Psychol Bull. 2004;130:324–340. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- 5.Custers R, Aarts H. The unconscious will: How the pursuit of goals operates outside of conscious awareness. Science. 2010;329:47–50. doi: 10.1126/science.1188595. [DOI] [PubMed] [Google Scholar]
- 6.Dijksterhuis A, Aarts H, Smith PK. The New Unconscious: Social Cognition and Social Neuroscience. New York: Oxford Univ Press; 2005. The power of the subliminal: On subliminal persuasion and other potential applications. [Google Scholar]
- 7.Bijleveld E, Custers R, Aarts H. Unconscious reward cues increase invested effort, but do not change speed-accuracy tradeoffs. Cognition. 2010;115:330–335. doi: 10.1016/j.cognition.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Pessiglione M, et al. Subliminal instrumental conditioning demonstrated in the human brain. Neuron. 2008;59:561–567. doi: 10.1016/j.neuron.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsson K, et al. Fear and the amygdala: Manipulation of awareness generates differential cerebral responses to phobic and fear-relevant (but nonfeared) stimuli. Emotion. 2004;4:340–353. doi: 10.1037/1528-3542.4.4.340. [DOI] [PubMed] [Google Scholar]
- 10.Ohman A, Carlsson K, Lundqvist D, Ingvar M. On the unconscious subcortical origin of human fear. Physiol Behav. 2007;92:180–185. doi: 10.1016/j.physbeh.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 11.Doyen S, Klein O, Pichon CL, Cleeremans A. Behavioral priming: It’s all in the mind, but whose mind? PLoS ONE. 2012;7:e29081. doi: 10.1371/journal.pone.0029081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strahan EJ, Spencer SJ, Zanna MP. Subliminal priming and persuasion: Striking while the iron is hot. J Exp Soc Psychol. 2002;38:556–558. [Google Scholar]
- 13.Bunce SC, Bernat E, Wong PS, Shevrin H. Further evidence for unconscious learning: Preliminary support for the conditioning of facial EMG to subliminal stimuli. J Psychiatr Res. 1999;33:341–347. doi: 10.1016/s0022-3956(99)00003-5. [DOI] [PubMed] [Google Scholar]
- 14.Clark RE, Manns JR, Squire LR. Classical conditioning, awareness, and brain systems. Trends Cogn Sci. 2002;6:524–531. doi: 10.1016/s1364-6613(02)02041-7. [DOI] [PubMed] [Google Scholar]
- 15.Esteves F, Parra C, Dimberg U, Ohman A. Nonconscious associative learning: Pavlovian conditioning of skin conductance responses to masked fear-relevant facial stimuli. Psychophysiology. 1994;31:375–385. doi: 10.1111/j.1469-8986.1994.tb02446.x. [DOI] [PubMed] [Google Scholar]
- 16.LeDoux J. The Emotional Brain. New York: Simon & Schuster; 1990. [Google Scholar]
- 17.Etkin A, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Peirce C, Jastrow J. On small differences in sensation. Memoirs of the National Academy of Sciences. 1885;3:73–83. [Google Scholar]
- 19.Phelps E. The interaction of emotion and cognition: The relation between the human amygdala and cognitive awareness. In: Hassin RR, Uleman JS, Bargh JA, editors. The New Unconscious: Social Cognition and Social Neuroscience. New York: Oxford Univ Press; 2005. [Google Scholar]
- 20.Haug M. Explaining the placebo effect: Aliefs, beliefs, and conditioning. Philos Psychol. 2011;1:1–20. [Google Scholar]
- 21.Kaptchuk TJ. Placebo studies and ritual theory: A comparative analysis of Navajo, acupuncture and biomedical healing. Philos Trans R Soc Lond B Biol Sci. 2011;366:1849–1858. doi: 10.1098/rstb.2010.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kihlstrom JF. The cognitive unconscious. Science. 1987;237:1445–1452. doi: 10.1126/science.3629249. [DOI] [PubMed] [Google Scholar]
- 23.Gerdle B, Björk J, Henriksson C, Bengtsson A. Prevalence of current and chronic pain and their influences upon work and healthcare-seeking: A population study. J Rheumatol. 2004;31:1399–1406. [PubMed] [Google Scholar]
- 24.Wechsler ME, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011;365:119–126. doi: 10.1056/NEJMoa1103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moncrieff J, Kirsch I. Efficacy of antidepressants in adults. BMJ. 2005;331:155–157. doi: 10.1136/bmj.331.7509.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaptchuk TJ, et al. Components of placebo effect: Randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doering BK, Rief W. Utilizing placebo mechanisms for dose reduction in pharmacotherapy. Trends Pharmacol Sci. 2012;33:165–172. doi: 10.1016/j.tips.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Flaten M, Simonsen T, Waterloo K, Olsen H. Pharmacological classical conditioning in humans. Hum Psychopharmacol. 2007;12:367–377. [Google Scholar]
- 29.Giang DW, et al. Conditioning of cyclophosphamide-induced leukopenia in humans. J Neuropsychiatry Clin Neurosci. 1996;8:194–201. doi: 10.1176/jnp.8.2.194. [DOI] [PubMed] [Google Scholar]
- 30.Goebel MU, et al. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 2002;16:1869–1873. doi: 10.1096/fj.02-0389com. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–113. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- 32.Voudouris NJ, Peck CL, Coleman G. Conditioned response models of placebo phenomena: Further support. Pain. 1989;38:109–116. doi: 10.1016/0304-3959(89)90080-8. [DOI] [PubMed] [Google Scholar]
- 33.Voudouris NJ, Peck CL, Coleman G. The role of conditioning and verbal expectancy in the placebo response. Pain. 1990;43:121–128. doi: 10.1016/0304-3959(90)90057-K. [DOI] [PubMed] [Google Scholar]
- 34.Watson A, El-Deredy W, Bentley DE, Vogt BA, Jones AK. Categories of placebo response in the absence of site-specific expectation of analgesia. Pain. 2006;126:115–122. doi: 10.1016/j.pain.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Watson A, El-Deredy W, Vogt BA, Jones AK. Placebo analgesia is not due to compliance or habituation: EEG and behavioural evidence. Neuroreport. 2007;18:771–775. doi: 10.1097/WNR.0b013e3280c1e2a8. [DOI] [PubMed] [Google Scholar]
- 36.Benedetti F, et al. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benedetti F. Placebo Effects: Understanding the Mechanisms in Health and Disease. New York: Oxford Univ Press; 2009. [Google Scholar]
- 38.Gracely R, Dubner R, Deeter W, Wolskee P. Clinicians' expectations influence placebo analgesia. Lancet. 1985;1(8419):43. doi: 10.1016/s0140-6736(85)90984-5. [DOI] [PubMed] [Google Scholar]
- 39.Levine JD, Gordon NC. Influence of the method of drug administration on analgesic response. Nature. 1984;312:755–756. doi: 10.1038/312755a0. [DOI] [PubMed] [Google Scholar]
- 40.Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 41.Kong J, et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wager TD, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 43.Eippert F, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Kong J, et al. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci. 2008;28:13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrovic P, et al. Placebo in emotional processing—induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Vits S, et al. Behavioural conditioning as the mediator of placebo responses in the immune system. Philos Trans R Soc Lond B Biol Sci. 2011;366:1799–1807. doi: 10.1098/rstb.2010.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benedetti F, et al. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci. 2004;7:587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- 48.Lundqvist D, Flykt A, Öhman A. 1998. The Karolinska Directed Emotional Faces (Karolinska Institutet, University of Oslo, Norway)