Abstract
Microscopic polyangiitis is an autoimmune small-vessel vasculitis that often manifests as focal and necrotizing glomerulonephritis and renal failure. Antineutrophil cytoplasmic Abs (ANCAs) specific for myeloperoxidase (MPO) play a role in this disease, but the role of autoreactive MPO-specific CD4+ T cells is uncertain. By screening overlapping peptides of 20 amino acids spanning the MPO molecule, we identified an immunodominant MPO CD4+ T-cell epitope (MPO409–428). Immunizing C57BL/6 mice with MPO409–428 induced focal necrotizing glomerulonephritis similar to that seen after whole MPO immunization, when MPO was deposited in glomeruli. Transfer of an MPO409–428-specific CD4+ T-cell clone to Rag1−/− mice induced focal necrotizing glomerulonephritis when glomerular MPO deposition was induced either by passive transfer of MPO-ANCA and LPS or by planting MPO409–428 conjugated to a murine antiglomerular basement membrane mAb. MPO409–428 also induced biologically active anti-MPO Abs in mice. The MPO409–428 epitope has a minimum immunogenic core region of 11 amino acids, MPO415–426, with several critical residues. ANCA-activated neutrophils not only induce injury but lodged the autoantigen MPO in glomeruli, allowing autoreactive anti-MPO CD4+ cells to induce delayed type hypersensitivity-like necrotizing glomerular lesions. These studies identify an immunodominant MPO T-cell epitope and redefine how effector responses can induce injury in MPO-ANCA–associated microscopic polyangiitis.
Keywords: autoimmunity, lymphocytes, T helper 1 cells, macrophages
Small-vessel vasculitis is often induced by autoimmunity to neutrophil granule proteins, predominantly myeloperoxidase (MPO) and proteinase 3 (Pr3) (1), as well as lysosomal membrane protein-2 (2). Although there is some overlap, autoimmunity to MPO is strongly associated with microscopic polyangiitis and reactivity to Pr3 results in granulomatosis with polyangiitis (GPA). The kidneys are frequently affected by focal and segmental necrotizing glomerulonephritis (FNGN), leading to rapidly progressive glomerulonephritis and end-stage renal failure. Due to the presence of auto-Abs to MPO and Pr3, this disease is also known as antineutrophil cytoplasmic Ab (ANCA)-associated vasculitis (3). Morbidity and mortality rates remain high, with a 5-y survival rate of 46–85% in microscopic polyangiitis (4), and most treatments have limited effectiveness and significant toxicities (5).
Evidence for a pathogenic role for ANCA in microscopic polyangiitis includes the use of plasma exchange as therapy, a case report of lung hemorrhage in a neonate following placental transfer of MPO-ANCA, and other observations in humans (6, 7). Moreover, ANCA can activate neutrophils and promote their adhesion in vitro (8) and in vivo (9–12). The adhesion of neutrophils in target tissues, particularly the kidney, induces injury by means of the release of injurious oxidants and enzymes, including MPO itself (13). In addition, transferred anti-MPO Abs can induce neutrophil- and complement-mediated FNGN (14–18), enhanced by infection-related signals like LPS (14, 19, 20).
Although there is a rationale for autoreactive CD4+ cells contributing to the development of disease in microscopic polyangiitis, their role is less clear. There is evidence that MPO-ANCA production requires antigen-specific CD4+ T cells (21, 22). Furthermore, autoreactive MPO-specific CD4+ T cells can be induced experimentally in animals (23), MPO-specific T cells that produce IFN-γ are present in the peripheral blood of humans with acute ANCA-associated vasculitis (24–26), and urinary CD4+ effector/memory cells reflect disease activity (27). Effector/memory CD4+ T cells, together with macrophages, tissue factor, and fibrin, are present in glomeruli of patients with ANCA-associated glomerulonephritis (28, 29). Finally, in a murine model of anti-MPO FNGN, where autoimmunity to MPO is induced and glomerulonephritis is triggered by injection of sheep anti-mouse glomerular basement membrane (GBM) Ab, CD4+ T-cell depletion during the effector phase attenuated disease (23). Based on this evidence, we hypothesize that MPO-specific effector CD4+ cells are important in disease by localizing to glomeruli and inducing a delayed type hypersensitivity (DTH)-like lesion. Anti-MPO CD4+ cells may localize to glomeruli by recognizing MPO within glomeruli, acting as a planted glomerular autoantigen deposited by ANCA-activated neutrophils that have degranulated and/or formed neutrophil extracellular traps (NETs) (30).
Although MPO’s B-cell epitopes have been the subject of studies mapping them to areas within the heavy chain (31, 32), the T-cell epitopes of MPO are undefined. Identifying MPO’s T-cell epitopes is important in understanding the pathogenesis of anti-MPO disease and would represent progress toward less toxic therapies focused on the autoimmune response. In the current studies, we have defined an immunodominant CD4+ T-cell MPO epitope and then used this epitope to test the hypothesis that antigen-specific CD4+ T cells recognize both this epitope and MPO itself in glomeruli and induce FNGN. This immunodominant T-cell epitope exists across at least three different MHC II alleles and also can induce MPO-ANCA. Our studies redefine our understanding of anti-MPO disease to now include a distinct role for effector CD4+ T cells that recognize MPO, which are planted in glomerular capillaries by MPO-ANCA–activated neutrophils. The pathogenesis of effector responses in microscopic polyangiitis should now be considered to include a sequential mix of types II (Ab-mediated) and IV (DTH-like) hypersensitivity.
Results
MPO409–428 Is the Immunodominant T-Cell Epitope of MPO.
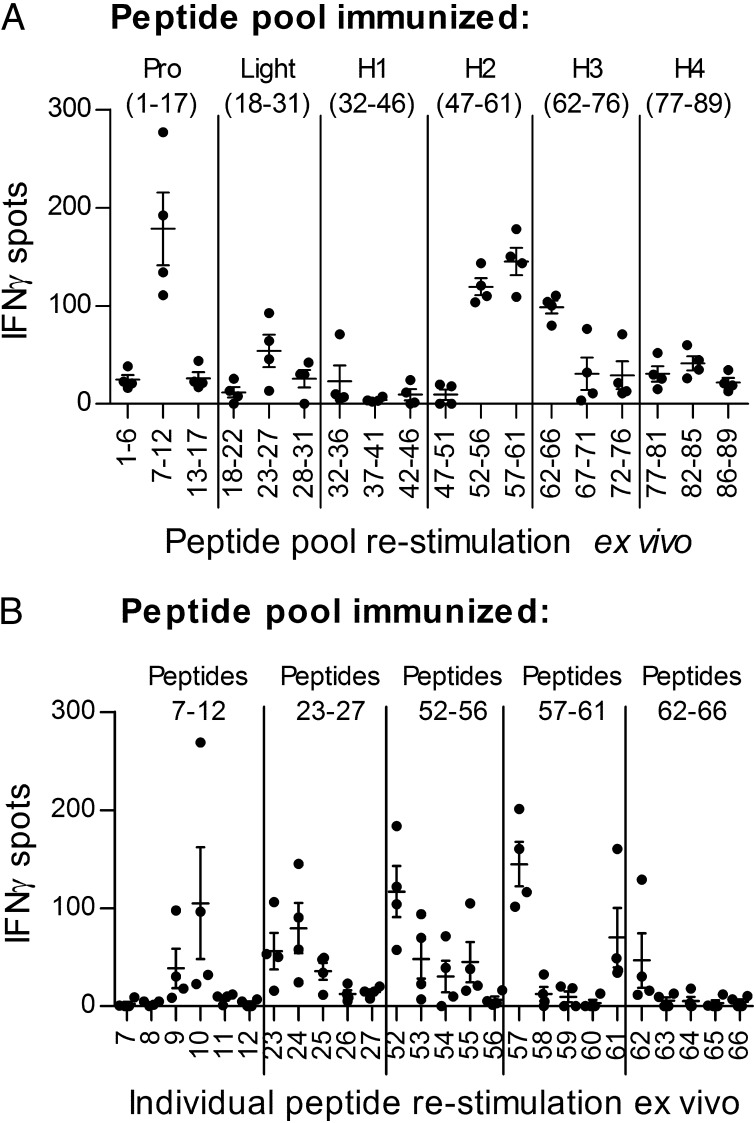
To define CD4+ T-cell epitopes within MPO, we immunized groups of C57BL/6 mice with pools of MPO 20-mers (from the mouse MPO sequence, with each peptide overlapping by 12 aa; Table S1). Separate groups of mice were injected with peptide pools from the proregion of MPO (peptides 1–17), the light chain (peptides 18–31), or one of four quarters (H1–H4) of the heavy chain (peptides 32–46, 47–61, 62–76, or 77–89). Splenocytes were restimulated with each third of the relevant immunizing pool (e.g., splenocytes from the group immunized with peptides 1–17 were restimulated with peptides 1–6, 7–12, or 13–17), and recall responses were measured by IFN-γ enzyme-linked immunospot (ELISPOT) assay. The five peptide groups inducing the strongest responses were peptide pools 7–12, 23–27, 52–56, 57–61, and 62–66 (Fig. 1A). Additional groups of mice were then immunized with one of these groups of peptides, and their draining lymph node (LN) cells were restimulated with each of the individual peptides from within the immunizing pool. The five individual peptides that induced the strongest recall responses were peptides 10, 24, 52, 57, and 61 (Fig. 1B). To determine the immunodominant MPO T-cell epitope, we then immunized separate groups of mice with each of these peptides and restimulated their draining LN cells ex vivo with either the immunizing peptide or recombinant mouse MPO using proliferation assays and ELISPOT assays for IFN-γ and IL-17A. Comparing recall responses, MPO peptide 52 (MPO409–428, PRWNGEKLYQEARKIVGAMV) induced the strongest responses both to itself and to whole recombinant mouse MPO (Table 1). Proliferative responses were significantly higher than those induced by all other peptides. Although proinflammatory cytokine production after peptide 52 immunization was numerically greater than after all other peptides, peptide 61 also induced moderately strong IL-17A and IFN-γ production.
Fig. 1.
MPO T-cell epitopes in C57BL/6 mice. (A) Overlapping peptides (89 20-mers) spanning the entire mouse MPO sequence were used to immunize groups of mice. Pro, proregion peptides (1–17); Light, light chain peptides (18–31). H1 (32–46), H2 (47–61), H3 (62–76), and H4 (77–89) are heavy chain peptides. Splenocytes were restimulated ex vivo with each third of the immunizing pool (x axis), and responses were measured by IFN-γ ELISPOT assay (mean number of spots minus baseline). Each dot represents the mean response from an individual mouse, and results are representative of two independent experiments with a minimum of four mice per group. (B) Groups of mice were immunized with one of the five strongest responding pools of peptides (peptides 7–12, 23–27, 52–56, 57–61, or 62–66) and draining LN cells were restimulated ex vivo with individual peptides (x axis). Each dot represents the mean response from an individual mouse, and results are representative of two independent experiments with a minimum of four mice per group.
Table 1.
Immune responses induced by the five most immunogenic MPO peptides
| Responses to immunizing peptide |
Responses to recombinant MPO |
|||||
| Peptide | Proliferation, SI | IFN-γ, spots | IL-17A, spots | Proliferation, SI | IFN-γ, spots | IL-17A, spots |
| 10 | 2.3 ± 0.5 | 18 ± 4 | 13 ± 1 | 2.2 ± 0.2 | 21 ± 1 | 7 ± 1 |
| 24 | 3.8 ± 1.1 | 14 ± 2 | 12 ± 3 | 3.0 ± 0.3 | 19 ± 3 | 12 ± 1 |
| 52 | 9.2 ± 0.8* | 64 ± 10** | 67 ± 8** | 4.3 ± 0.2* | 39 ± 5*** | 45 ± 5** |
| 57 | 2.4 ± 0.4 | 32 ± 5 | 33 ± 4 | 2.5 ± 0.2 | 22 ± 3 | 20 ± 4 |
| 61 | 5.6 ± 1.0 | 55 ± 10 | 64 ± 13 | 3.0 ± 0.3 | 32 ± 5 | 31 ± 3 |
Groups of mice were immunized with individual peptides, and immune responses to the immunizing peptide or to whole recombinant mouse MPO were measured ex vivo using an [3H]-thymidine proliferation assay and IFN-γ and IL-17A ELISPOT assays. SI, stimulation index; spots, mean number of spots minus baseline. Results are presented as the mean ± SEM and are representative of three independent experiments, each with a minimum of five mice per group.
*P < 0.05 vs. peptides 10, 24, 57, and 61; **P < 0.05 vs. peptides 10, 24, and 57; ***P < 0.05 vs. peptides 10 and 24.
Mice immunized with native mouse MPO (n = 5) developed recall responses to peptide 52 but not to a control peptide, ovalbumin (OVA)323–339 (proliferation assay stimulation index: 2.1 ± 0.2 vs. 1.0 ± 0.1, IFN-γ ELISPOT assay: 8.0 ± 1.8 spots, and IL-17A ELISPOT assay: 10.3 ± 1.7 spots; no samples restimulated with OVA323–339 showed any spots above media alone). Immunization with peptide 53 or 54, both of which overlap with peptide 52 and induce recall responses by IFN-γ ELISPOT assay in mice immunized in peptide pool 52–56 (Fig. 1 A and B), did not induce responses to peptide 52, 53, or 54 or to recombinant MPO (Fig. S1).
T-Cell Autoreactivity to MPO409–428 (MPO Peptide 52) Is Not MHC II-, I-Ab-, Restricted.
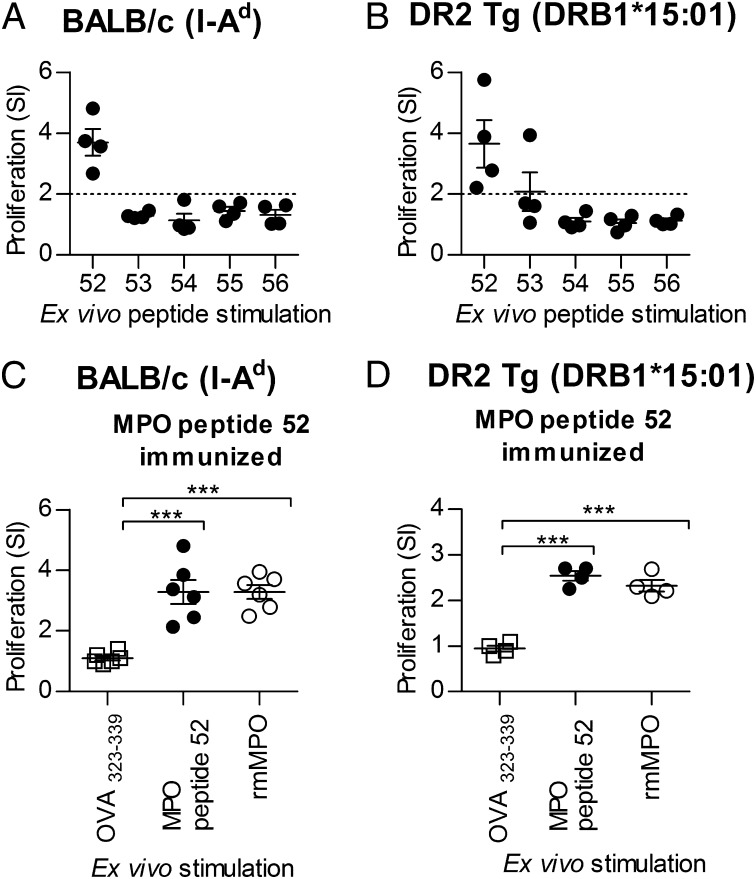
Because there are no clear MHC class II associations with anti-MPO glomerulonephritis, we sought to determine if MPO peptide 52 (MPO409–428) is immunoreactive in mice expressing different MHC II molecules. We immunized BALB/c mice (expressing I-Ad) and humanized HLA-DRB1*15:01 transgenic (Tg) mice (without mouse MHC class II) (33) with the pool of MPO peptides 52–56. In both strains, peptide 52 was the immunogenic peptide (Fig. 2 A and B). BALB/c and HLA-DRB1*15:01 Tg mice immunized with mouse MPO peptide 52 developed autoreactive responses to both MPO peptide 52 and to whole recombinant MPO (Fig. 2 C and D).
Fig. 2.
Immunogenicity of MPO peptide 52 (MPO409–428) is not restricted to MHC II I-Ab. The immunogenicity of MPO peptide 52 was examined in mice expressing I-Ad (BALB/c) or human DR2 (DR2 Tg) (with the absence of murine MHC II and Tg expression of human HLA-DRB1*15:01). When immunized with the pool of peptides 52–56, peptide 52 is capable of inducing recall responses in the context of I-Ad (BALB/c, n = 4) (A) or HLA-DR2 (DR2 Tg, n = 4) (B). When mice were immunized with peptide 52 (MPO409–428) (BALB/c, n = 6; DR2 Tg, n = 4), LN cells from both strains exhibited recall responses to both the peptide itself (MPO peptide 52, ●) and recombinant mouse MPO (rmMPO, ○) compared with control OVA323–339 (□), as measured by proliferation (C and D). Each dot represents the mean response from an individual mouse. ***P < 0.001. Results are representative of three independent experiments with a minimum of four mice per experiment. SI, stimulation index.
Defining the Core and Critical Residues of the MPO409–428 Epitope.
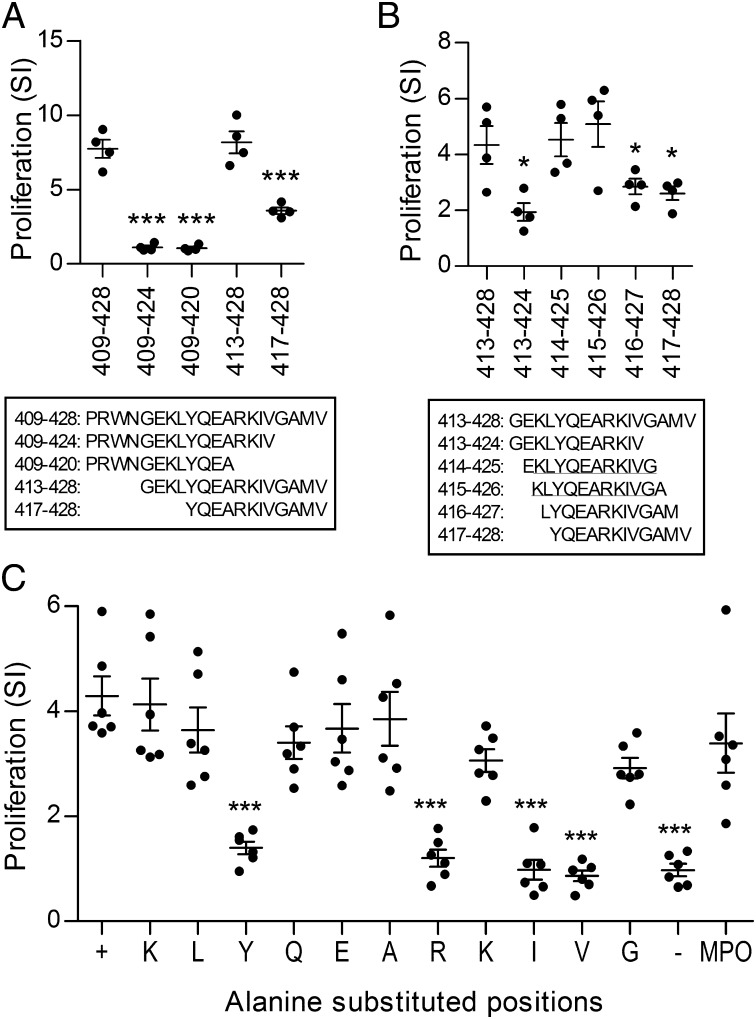
We then further delineated the key areas of this T-cell epitope. We identified the critical peptide length for immunoreactivity by immunizing C57BL/6 mice with the complete 20-aa MPO409–428 (PRWNGEKLYQEARKIVGAMV) and measuring proliferative recall responses to shortened 16-mers and 12-mers of MPO409–428 (Fig. 3A). There were no detectable recall responses when MPO409–428 was shortened from the C terminus to either a 16-mer, amino acids 409–424, or to a 12-mer, amino acids 409–420 (Fig. 3A). However, recall responses with amino acids 413–428, containing the 16 C-terminal amino acids, were comparable to recall responses induced by full-length amino acids 409–428. The recall response to the 12 C-terminal amino acids 417–428, although detectable, was reduced compared with MPO409–428. To delineate the core immunoreactive residues of MPO409–428 further, mice were immunized with the 16-mer amino acids 413–428 and recall responses to sequential 12-mers overlapping by 11 aa were measured. Restimulation of mice immunized with amino acids 413–428 with the 12-mers containing amino acids 414–425 and 415–426 induced recall responses comparable to those obtained by restimulation with the 16-mer immunogen amino acids 413–428 (Fig. 3B). Therefore, the core immunoreactive residues of MPO409–428 are the 11 amino acids (415–425; KLYQEARKIVG). The critical residues within this 11-mer were delineated by immunizing C57BL/6 mice with KLYQEARKIVG and restimulating draining LN cells with individual alanine-substituted 11-mers [alanine itself (MPO420) was substituted by a serine]. Each 11-mer had one residue substituted by alanine in sequential order. An individual amino acid was deemed a critical residue if its substitution by an alanine abrogated a positive ex vivo proliferative response. The substitution of tyrosine, arginine, isoleucine, or valine defined these amino acids as the critical residues within this epitope (Fig. 3C).
Fig. 3.
Core immunogenic residues of MPO409–428. (A) C57BL/6 mice were immunized with MPO409–428 (MPO peptide 52), and reactivity to shortened 16-mers and 12-mers was assessed by [3H]-thymidine proliferation in draining LN cells. Ex vivo reactivity to the shortened 16-mers and 12-mers was compared with reactivity induced by MPO peptide 52. (B) MPO413–428 was used to immunize mice, reactivity to individual 12-mers was assessed by [3H]-thymidine proliferation in draining LN cells, and ex vivo reactivity to the individual 12-mers was compared with MPO413–428. The core 11 amino acid residues are the 11 amino acids shared by MPO414–425 and MPO415–426 (underlined) because both induced comparable reactivity to the immunizing MPO413–428. (C) To determine the critical residues, mice were immunized with the core 11 amino acids, MPO415–425, and draining LN cells were restimulated ex vivo with alanine-substituted 11-mers. Alanine-substituted positions: +, MPO415–425; K, ALYQEARKIVG; L, KAYQEARKIVG; Y, KLAQEARKIVG; Q, KLYAEARKIVG; E, KLYQAARKIVG; A, KLYQESRKIVG; R, KLYQEAAKIVG; second K, KLYQEARAIVG; I, KLYQEARKAVG; V, KLYQEARKIAG; G, KLYQEARKIVA; −, OVA323–339; MPO, whole recombinant mouse MPO. Reactivity to individual alanine-substituted 11-mers was compared with reactivity to MPO415–425 and showed that tyrosine (Y), arginine (R), isoleucine (I), and valine (V) were critical to the response. *P < 0.05; ***P < 0.001. Results are representative of two independent experiments, each with a minimum of four mice per group. Each dot represents the mean response from an individual mouse. SI, stimulation index.
MPO409–428 Immunization Induces Nephritogenic T-Cell Responses.
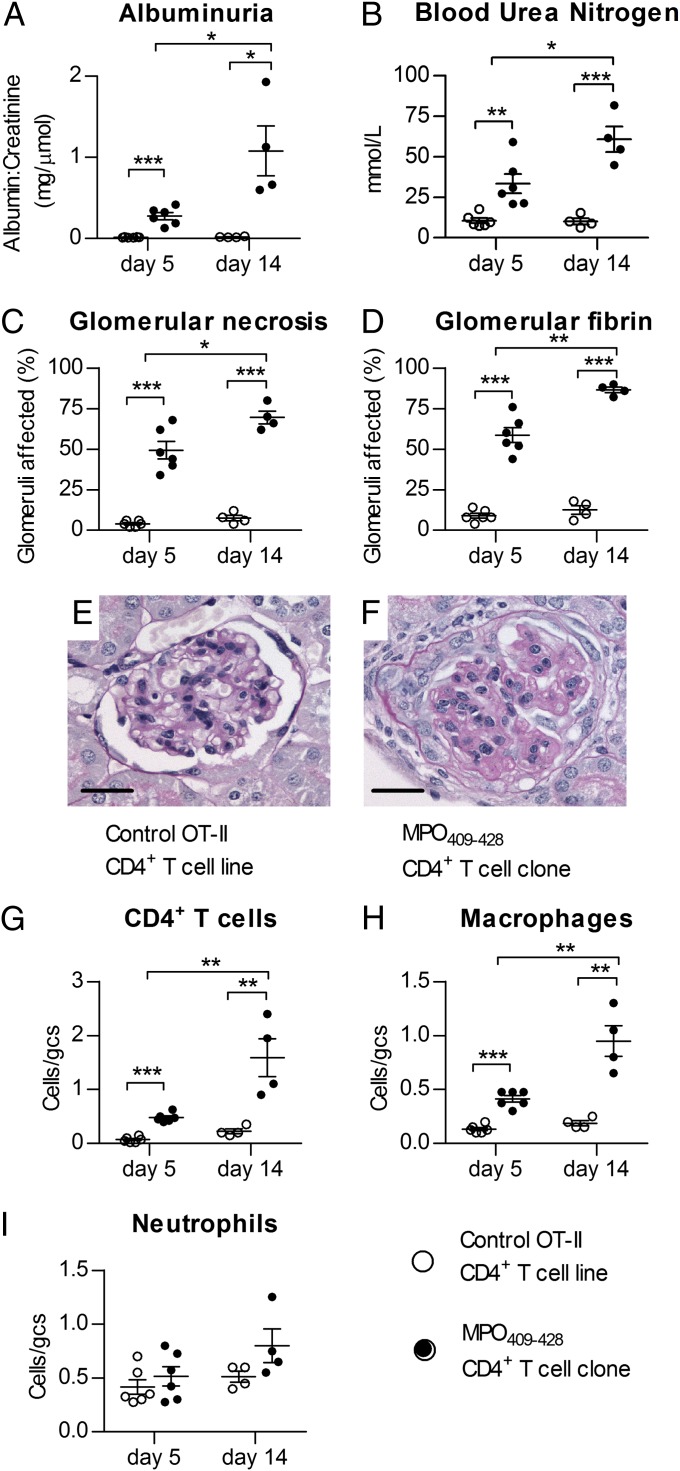
To determine the nephritogenicity of the anti-MPO T-cell responses generated by MPO409–428 immunization, a previously established model of anti-MPO–directed glomerulonephritis was used (23, 34). In this model, C57BL/6 mice immunized with mouse MPO lose tolerance to MPO, but this autoimmunity, in itself, is not sufficient to induce glomerulonephritis, which is triggered by injecting low-dose sheep anti-mouse GBM Ab. This Ab induces transient glomerular neutrophil recruitment with MPO deposition in glomeruli. MPO409–428-immunized mice developed renal injury with increased albuminuria and blood urea nitrogen to a similar degree as mice immunized with whole native mouse MPO (Fig. 4 A and B). MPO409–428-immunized mice developed FNGN comparable to mice immunized with whole native mouse MPO, characterized by segmental glomerular necrosis and fibrin deposition within the glomerular tuft. Mice immunized with OVA323–339 and given anti-GBM Ab exhibited only mild lesions (Fig. 4 C–J). Analysis of glomerular cellular effectors showed increased infiltration of CD4+ T cells and macrophages compared with control mice, similar to that seen after whole native mouse MPO immunization (Fig. 4 K–M). Concurrently, a group of mice (n = 6) immunized with the next highest responding MPO peptide from Table 1 (peptide 61, MPO481–500) and given anti-GBM Ab did not develop more proteinuria or histological glomerular injury than OVA323–339-immunized mice (6.1 ± 1.1 vs. 5.3 ± 0.7 mg over 24 h and 16.1 ± 2.4 vs. 16.2 ± 2.0% of glomeruli affected by necrosis, respectively).
Fig. 4.
MPO409–428 immunization induces nephritogenic autoimmunity. C57BL/6 mice were immunized with either OVA323–339 [control (n = 5), □], MPO409–428 (n = 6, ●), or native mouse MPO (n = 4, ○), and disease was triggered by recruiting neutrophils to glomeruli with low-dose sheep anti-mouse GBM Ab. Mice immunized with an irrelevant antigen (OVA323–339) and injected with low-dose sheep anti-mouse GBM Ab developed minimal injury, whereas mice immunized with MPO409–428 developed FNGN similar to that induced by immunization with whole-mouse MPO. Functional indices of glomerular injury, albuminuria (A) and blood urea nitrogen (B), were increased after MPO-specific immunization. Some glomeruli from MPO409–428- and whole MPO–immunized mice exhibited segmental necrosis (H&E) (C and E–G) with glomerular fibrin deposition (fibrin as the brown reaction product) (D and H–J). Photomicrographs of glomeruli and fibrin deposition are taken at a magnification of 400×. (Scale bars: E–J, 10 μm.) Glomerular infiltrates of CD4+ T cells (K) and macrophages (L) but not neutrophils (M) were increased after MPO409–428 immunization. gcs, glomerular cross section. *P < 0.05; **P < 0.01; ***P < 0.001.
Transfer of MPO409–428-Specific CD4+ T Cells into Rag1−/− Mice Induces FNGN.
To determine definitively whether T-cell autoreactivity to this dominant MPO epitope is nephritogenic, we generated mouse MPO409–428-specific T-cell clones from C57BL/6 mice. CD4+ lines from OT-II Tg mice (i.e., OVA323–339-specific) derived under the same conditions were control cells. We transferred T-cell clones into naive Rag1−/− mice (MPO409–428-specific or OVA323–339-specific: 5 × 106 cells per mouse) and then immunized mice with the clones’ cognate peptide. As before, MPO was deposited in glomeruli using low-dose sheep anti-mouse GBM Ab and mice were culled 5 or 14 d after disease induction. At 5 d, mice that received the MPO409–428-specific T-cell clone developed a DTH response to MPO injected intradermally 24 h before the end of the experiment, which was not detected in control mice (footpad swelling: 0.4 ± 0.1 vs. 0.0 ± 0.0 mm). Splenocytes harvested at 14 d from mice that received MPO409–428-specific clones showed recall responses to both MPO409–428 and MPO (IFN-γ ELISPOT assay: 156 ± 33 and 170 ± 40 spots, respectively).
Mice receiving the MPO409–428-specific T-cell clone developed progressive FNGN with increased albuminuria, blood urea nitrogen, segmental glomerular necrosis, and fibrin deposition compared with mice that received OVA323–339-specific T cells and anti-GBM Ab (Fig. 5 A–F). There were increased CD4+ T cells and macrophages, but not neutrophils, within glomeruli compared with control clone recipients (Fig. 5 I–K). The degree of perivascular and tubulointerstitial inflammation was also increased, with more CD4+ T cells and macrophages but similar numbers of neutrophils in the tubulointerstitium (Fig. S2 and Table S2). Glomerular crescent formation was observed in mice receiving the MPO409–428-specific T-cell clone at day 14 (6 ± 2% of glomeruli affected) but not in mice receiving OVA323–339-specific T cells. In some mice, pulmonary histology showed signs of interstitial pneumonitis and lymphocytic bronchiolitis, but these findings were inconsistent and not present in all clone transfer studies.
Fig. 5.
Transfer of T-cell clones specific for MPO409–428 induces progressive FNGN. T-cell clones specific for MPO409–428 (5 × 106) were transferred into Rag1−/− mice (●), disease was triggered 7 d later with low-dose sheep anti-mouse GBM Ab, and experiments were ended after a further 5 d (n = 6) or 14 d (n = 4). Control Rag1−/− mice that received sheep anti-mouse GBM Ab with a CD4+ T-cell line specific for OVA323–339 (○) (n = 6, 5 d; n = 4, 14 d) developed minimal injury, but mice given MPO409–428-specific cells developed progressive functional injury with pathological albuminuria (A), increased blood urea nitrogen levels (B), and FNGN (C) with glomerular fibrin deposition (D). Histologically, glomeruli from control mice at 14 d were near normal (E) compared with those of mice receiving MPO409–428-specific cells (F). (Scale bars: E and F, 20 μm.) Progressive increases in glomerular CD4+ T cells (G) and macrophages (H) but not neutrophils (I) over time were seen in recipients of MPO-specific T cells. gcs, glomerular cross section. *P < 0.05; **P < 0.01; ***P < 0.001.
Localizing MPO409–428 to Glomeruli Induces CD4+ T-Cell Clone-Mediated FNGN.
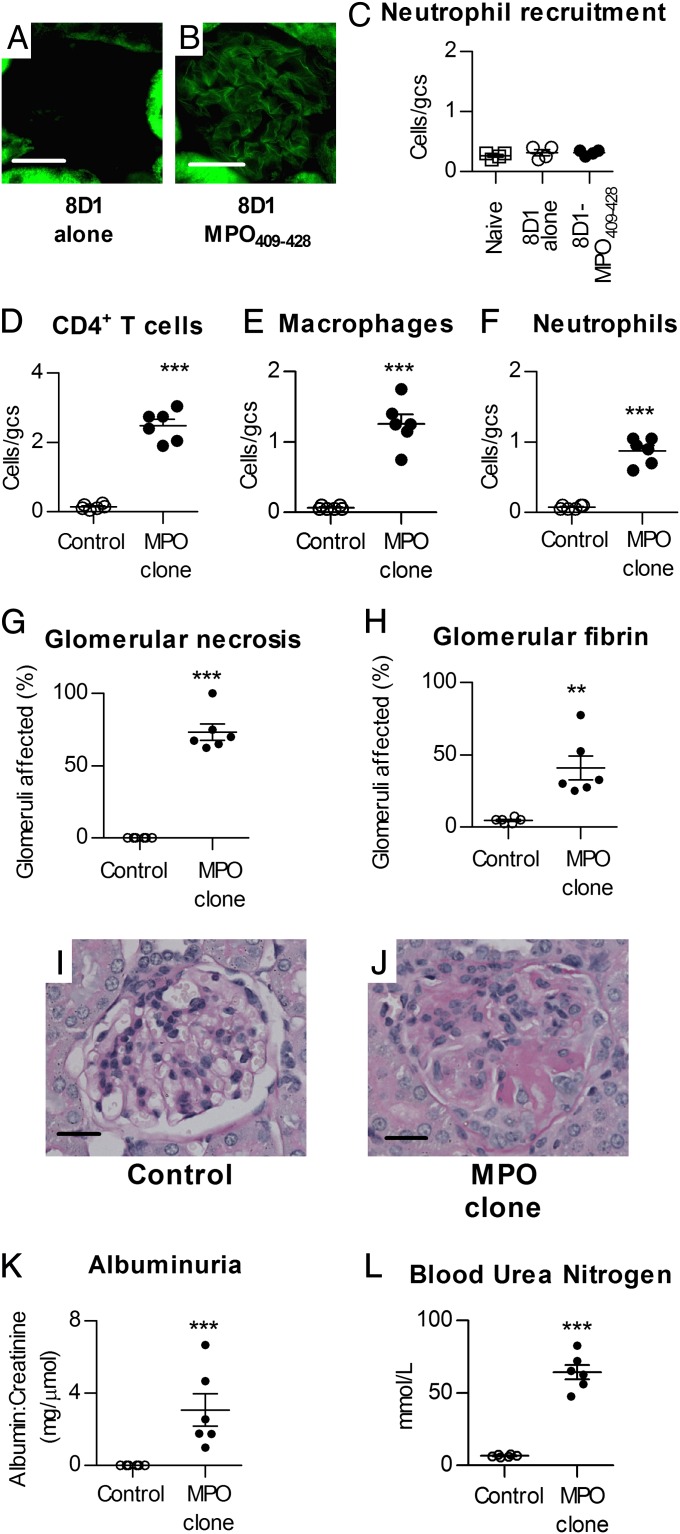
To determine whether the presence of the T-cell epitope MPO409–428 itself in glomeruli can induce T-cell–mediated renal injury in mice with MPO409–428 CD4+ T cells, we injected a biotinylated MPO409–428 peptide conjugated to a mouse anti-mouse monoclonal IgG1 (clone 8D1) specific for the mouse noncollagenous domain of the α3 chain of type IV collagen, which does not induce pathological albuminuria or major histological changes (35). Glomerular deposition of biotinylated MPO409–428 was demonstrated by direct immunofluorescence using streptavidin-FITC Ab (Fig. 6 A and B). Injection of the MPO409–428-8D1 conjugate itself did not increase glomerular neutrophil recruitment (Fig. 6C) or renal MPO activity (naive mice: 1.8 ± 0.2, 8D1 alone: 2.2 ± 0.5, 8D1-MPO409–428: 2.0 ± 0.4; expressed as ΔA460/min × 10−3) at a 4-h time point.
Fig. 6.
Planting MPO409–428 in glomeruli induces CD4+ cell-mediated FNGN. Injecting a mouse anti-mouse α3 chain of type IV collagen [α3(IV)NC1] IgG1 mAb (clone 8D1) conjugated to biotinylated MPO409–428 into naive Rag1−/− mice results in deposition of biotinylated MPO409–428 peptide in glomeruli. After 4 h, a streptavidin-FITC Ab showed no biotin in glomeruli of mice injected with unconjugated 8D1 (n = 6) (A), but clear biotin signal was observed within glomeruli of mice injected with the 8D1-biotinylated MPO409–428 conjugate (n = 6) (B). The tubular fluorescence in both panels is endogenous biotin within renal tubules. (C) Injection of 8D1-biotinylated MPO409–428 conjugate (●) did not result in an increase in glomerular neutrophils compared with naive (□) or unconjugated 8D1 mAb alone in recipient (○) Rag1−/− mice at 4 h. MPO409–428-specific CD4+ T-cell clones (25 × 106) were transferred into Rag1−/− mice (●), and disease was triggered 7 d later by targeting MPO409–428 to glomeruli via MPO409–428 conjugated to clone 8D1. Mice were culled 14 d (n = 6) after triggering disease. Control Rag1−/− mice were injected with the OVA323–339-specific CD4+ T-cell line followed by the 8D1-MPO409–428 conjugate (○) (n = 6). The presence of MPO409–428 in glomeruli resulted in glomerular localization of MPO-specific but not OVA-specific T cells (D), with macrophage and neutrophils in glomeruli (E and F). Recruitment of these leukocytes resulted in FNGN with glomerular fibrin deposition (G–J). The 8D1-MPO409–428 conjugate with OVA-specific CD4+ cells did not induce functional renal injury, but MPO409–428-specific CD4+ cells resulted in both albuminuria (K) and renal impairment (L). **P < 0.01; ***P < 0.001. (Scale bars: A, B, I, and J, 20 μm.)
Compared with control Rag1−/− mice receiving OVA323–339-specific cells and MPO409–428-8D1, mice receiving the MPO-specific clone (25 × 106 cells) followed by MPO409–428-8D1 developed glomerular infiltrates of CD4+ T cells, macrophages, and neutrophils (Fig. 6 A–C) with FNGN (Fig. 6 H–K). Glomerular crescent formation was observed in four of six mice receiving the MPO409–428-specific clone (3%, 5%, 5%, and 10% of glomeruli affected) but not in mice receiving OVA323–339-specific cells. These pathological abnormalities translated into functional injury with increased albuminuria and blood urea nitrogen levels (Fig. 6 L and M). Similarly, perivascular inflammation, tubulointerstitial injury, and infiltration of effector cells were observed in mice that received the MPO409–428-specific clone (Table S2). These experiments show that MPO409–428 itself in glomeruli, without the initial presence of neutrophils or endogenous MPO, is sufficient to induce effector MPO-specific T-cell localization and injury.
LPS and MPO-ANCA Recruit Neutrophils and Deposit MPO, Leading to CD4+ T-Cell–Mediated FNGN.
Relapse of ANCA-associated vasculitis is associated with infections (36, 37). At least part of the explanation for these clinical observations may come from experimental evidence showing that infection primes neutrophils [and potentially other cells (20)], allowing ANCA to bind to and activate neutrophils (9, 19). This results in their recruitment to target tissues, especially the kidney (9, 15). Modeling this process involves injecting LPS and transferring anti-MPO IgG (generated in Mpo−/− mice) into mice. Although these Abs can induce injury (14), recruiting neutrophils into glomeruli may also result in the deposition of MPO within glomeruli, where effector CD4+ cells could potentially recognize MPO as a planted glomerular antigen. Having shown that MPO-specific CD4+ cells can recognize the immunodominant MPO T-cell epitope, MPO409–428, in the glomerulus, we next tested the hypothesis that MPO from anti-MPO Ab-activated neutrophils acts as an autoantigen in target tissues, resulting in effector CD4+ cell-mediated injury. Mice were injected with LPS and anti-MPO IgG (generated by immunizing Mpo−/− mice with murine MPO). Histological analyses 3 h after injection showed [as previously demonstrated (20)] glomerular neutrophil recruitment after LPS and anti-MPO IgG (Fig. 7 A, C, and D). There was also increased renal MPO activity (Fig. 7B). Confocal microscopy using anti-CD45 and MPO-specific Abs revealed the presence of leukocyte-associated MPO as well as extracellular MPO that had been deposited in the glomerulus (Fig. 7E).
Fig. 7.
Coinjection of LPS and anti-MPO Abs deposits MPO in glomeruli and triggers cell-mediated glomerular injury and FNGN. Injecting LPS and anti-MPO Abs into naive Rag1−/− mice (n = 4 in each group) results in neutrophil recruitment (A, C, and D) and increased renal MPO activity after 4 h (B). (Scale bars: C and D, 20 μm.) (E) When assessed by confocal microscopy, free [nonleukocyte (CD45)-associated] MPO could be detected in glomeruli (CD45, red; MPO, green; nonleukocyte-associated MPO in green). (Scale bar: 5 μm.) The dotted line shows the outline of the glomerulus. MPO409–428-specific CD4+ T-cell clones were transferred into Rag1−/− mice (5 × 106, n = 6; 25 × 106, n = 6; ●), and disease was triggered 7 d later by injecting LPS and anti-MPO Abs. Mice were culled after a further 14 d. Control groups were Rag1−/− mice injected with LPS and anti-MPO Abs, followed by an OVA323–339-specific CD4+ T-cell line (5 × 106, n = 4; 25 × 106, n = 5; ○). Mice with OVA-specific T cells injected with LPS and anti-MPO Abs showed only mild injury with modest albuminuria (F), no renal impairment (G), and low proportions of glomeruli affected by segmental necrosis (H) and fibrin deposition (I). Renal injury was markedly increased by MPO409–428-specific CD4+ T cells in a dose-dependent manner. Representative photomicrographs of glomeruli show only mild glomeruli hypercellularity in control mice (J) but FNGN in mice receiving MPO-specific CD4+ cells (K). A similar pattern was seen with glomerular fibrin deposition [control cells (L) and MPO-specific CD4+ cells (M)]. (Scale bars: J–M, 25 μm.) Glomerular leukocyte recruitment showed a dose-dependent increase in CD4+ cells (N) and antigen-specific increases in macrophages (O) and neutrophils (P). gcs, glomerular cross section. *P < 0.05; **P < 0.01; ***P < 0.001.
Injecting LPS and anti-MPO Abs after transferring either 5 × 106 or 25 × 106 MPO409–428-specific CD4+ T-cell clones induced significant renal disease compared with transfer of LPS and OVA323–399-specific cells. MPO-specific effector T cells resulted in increased albuminuria, blood urea nitrogen, and FNGN with glomerular necrosis and fibrin deposition (Fig. 7 F–M). Renal injury was more severe after 25 × 106 MPO-specific cells compared with 5 × 106 MPO-specific cells. Glomerular crescent formation was observed in three of six mice that received 25 × 106 MPO-specific cells (with 10%, 15%, and 45% of glomeruli affected) but not in mice receiving 5 × 106 MPO-specific cells. There were also increases in glomerular infiltration of CD4+ T cells, macrophages, and neutrophils, similar to those observed when using the MPO409–428-8D1 conjugate to plant the T-cell epitope in glomeruli (Fig. 7 N–P). Perivascular and tubulointerstitial injury followed a similar pattern (Table S2). Transfer of 50 × 106 MPO409–428-specific cells resulted in markedly accelerated renal disease specific to mice receiving MPO-specific cells, and experiments were terminated at 6 d (Fig. S3).
MPO409–428 Induces Biologically Active MPO-ANCA.
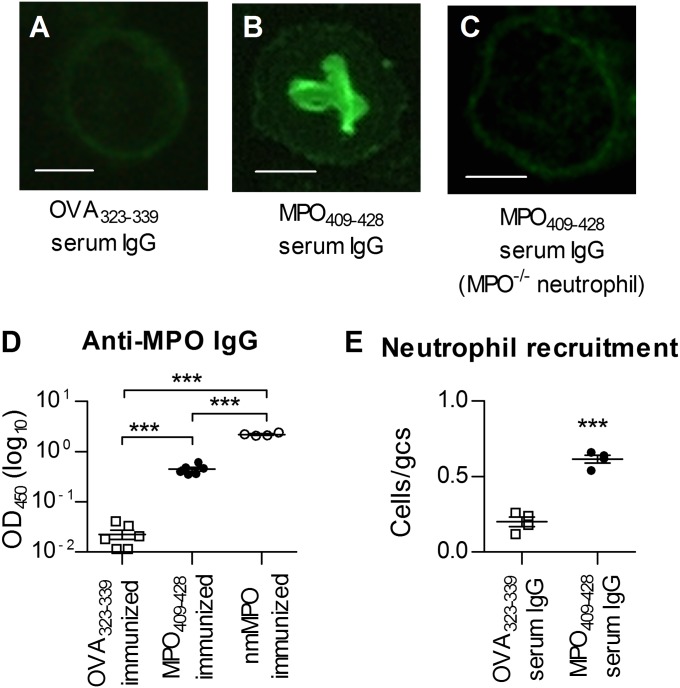
Indirect immunofluorescence using purified IgG on ethanol-fixed peritoneal mouse neutrophils showed a p-ANCA pattern specific to MPO409–428-immunized mice and not seen in serum IgG from OVA323–339-immunized mice or when IgG from MPO409–428-immunized mice was applied to neutrophils from Mpo−/− mice (Fig. 8 A–C). To determine if immunization with MPO409–428 induced MPO-ANCA, we tested sera from MPO409–428-immunized C57BL/6 mice for MPO-specific IgG by ELISA. Sera from OVA323–339- or whole native mouse MPO-immunized mice served as negative and positive controls. MPO409–428-immunized mice developed MPO-ANCA IgG, albeit at lower titers compared with whole native mouse MPO immunization (Fig. 8D). To determine whether MPO-ANCA induced by MPO409–428 had functional activity, we transferred purified MPO-ANCA into LPS-primed C57BL/6 recipient mice. Compared with IgG from OVA323–339-immunized mice, serum IgG from MPO409–428-immunized mice resulted in increased glomerular neutrophil recruitment at 3 h, (Fig. 8E), showing that these auto-Abs are biologically active.
Fig. 8.
MPO409–428-immunized mice develop biologically active MPO-ANCA. (A–C) Representative micrographs (400×) of ethanol-fixed peritoneal neutrophils stained by indirect immunofluorescence with purified serum IgG. (Scale bars: 1 μm.) The result is representative of two individual experiments with IgG purified from pooled sera. (D) Serum from C57BL/6 mice immunized with MPO409–428 develops detectable levels of MPO-specific IgG, as measured by serum IgG ELISA (n = 6 per group). nmMPO, native mouse myeloperoxidase. (E) Purified serum IgG was transferred into LPS-primed syngeneic recipients, and glomerular recruitment of neutrophils was assessed 3 h later (n = 4 per group). □, serum from OVA323-339 immunized C57BL/6 mice; ●, serum from MPO409-428 immunized C57BL/6 mice; ○, serum from native mouse myeloperoxidase immunized mice. ***P < 0.001.
Discussion
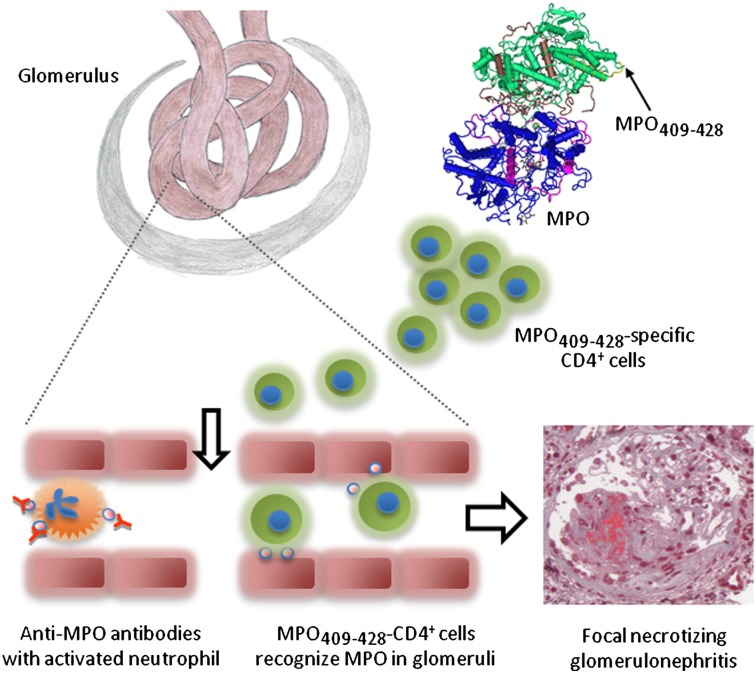
The glomerulus is a common target in MPO-ANCA–associated microscopic polyangiitis, with the severity of renal disease often defining the outcome. Although ANCA- and neutrophil-induced injury is important in microscopic polyangiitis, the presence of CD4+ T cells (26) and the MPO itself (28, 30) within lesions implies that MPO-specific autoreactive T cells may localize to glomeruli and cause injury. The current studies demonstrate a role for MPO-specific CD4+ cells in experimental ANCA-associated FNGN and define an immunodominant CD4+-cell epitope of MPO (MPO409–428). Our findings support a model whereby both ANCA and autoreactive effector CD4+ cells are important in microscopic polyangiitis. After tolerance is lost to MPO (with MPO409–428 as a potential key epitope), antigen-specific CD4+ T cells provide T-cell help to autoreactive B cells in producing MPO-ANCA. In many people who develop MPO-ANCA, these auto-Abs interact with and activate neutrophils, causing the neutrophils to be recruited to glomerular capillaries. Neutrophils mediate injury but, critically, also release the autoantigen MPO, which then is present in glomeruli and can be recognized by autoreactive MPO-specific CD4+ cells. Glomerular localization of these damaging MPO-specific effector T cells results in the recruitment of effector leukocytes, the development of FNGN, and renal impairment.
In contrast to other autoimmune diseases, there have been no consistent MHC class II associations in microscopic polyangiitis. HLA typing studies in patients with ANCA-associated vasculitis have focused largely on GPA (most frequently marked by autoimmunity to Pr3). In this disease, a variety of MHC class II associations have been found (reviewed in 38), with DPB1*04:01 being the most consistent. There are, however no clear positive associations with microscopic polyangiitis, which usually features autoimmunity to MPO with MPO-ANCA. We therefore tested the immunogenicity of MPO409–428 in mice with I-Ad and mice with HLA-DRB1*15:01, finding that this peptide could induce immune responses in more than one MHC II haplotype. Further studies in C57BL/6 mice identified a minimum immunogenic peptide, MPO415–425, and several critical amino acids within this peptide.
When glomerular disease was triggered with low-dose anti-GBM Ab, MPO409–428 peptide-immunized mice (but not OVA323–339-immunized mice) developed significant FNGN, showing that immunity to this peptide induced FNGN. MPO is a major constituent of neutrophils and is released when neutrophils induce inflammation, contributing to tissue injury by generating damaging oxidants (13, 39). However, in microscopic polyangiitis following ANCA-induced glomerular neutrophil localization, neutrophils release MPO that is deposited in glomeruli (28, 30). Immunologically, the glomerulus becomes a site where MPO, the autoantigen, is present and available for antigen recognition by effector T cells.
Therefore, after generating T helper (Th) 1 IFN-γ–secreting MPO409–428-specific clones that induced dermal DTH in vivo, we performed a series of transfer experiments demonstrating that MPO-specific CD4+ cells induce FNGN after MPO has been deposited in the glomerular microvasculature. This antigen-specific disease (not found in recipients of OVA-specific CD4+ T cells) was characterized by segmental glomerular necrosis; glomerular fibrin deposition; CD4+ T-cell, macrophage, and neutrophil recruitment; and functional renal injury (albuminuria and raised blood urea nitrogen). Tubulointerstitial infiltrates and injury, in the form of tubular dilation, necrosis, and protein cast formation, as well as perivascular inflammation were present in mice receiving MPO-specific CD4+ cells.
Because injection of anti-GBM Ab induces neutrophil recruitment, increased renal MPO activity, and free MPO within the glomerulus, we initially injected these Abs to trigger disease (34, 39–41). However, this strategy of inducing glomerular MPO deposition is confounded by anti-GBM Ab-induced neutrophil recruitment and the enzymatic effects of MPO, which, together, complicate the study of MPO as an autoantigen. By defining an immunodominant MPO peptide, we have defined a role for MPO as a planted autoantigen distinct from its effects as an injurious effector molecule. To determine whether MPO409–428 itself could be recognized as a nephritogenic peptide within glomeruli, we used a modification of our previously published delivery system (35). By conjugating MPO409–428 to a nonimmunogenic mouse anti-mouse GBM monoclonal IgG1 Ab, we could plant MPO409–428 in glomeruli. Compared with some other murine IgG subclasses, the murine IgG1 subclass has limited capacity to cause injury itself, because it fixes complement poorly and has a relatively low affinity for leukocyte-activating Fcγ receptors (42). At the dose that we used, the Ab itself induces minimal leukocyte recruitment and no albuminuria (35). Therefore, compared with polyclonal sheep IgG or anti-MPO Abs, it served as a relatively inactive carrier that could localize MPO409–428 to glomeruli. When effector CD4+ cells were transferred into mice, only MPO-specific cells localized to glomeruli and induced significant FNGN with renal impairment.
Studies in patients with ANCA-associated vasculitis show associations between infection and disease relapse and activity, suggesting a role for infection in the pathogenesis of these diseases (36, 37). Having shown that MPO-specific T cells could recognize MPO409–428 within glomeruli to induce injury, we triggered disease with transferred anti-MPO Abs and LPS, a prototypic infection-related signal. This is likely to be more analogous to the “clinical” situation, where enzymatically active and autoantigenic MPO lodges in glomeruli when it is released by MPO-ANCA–activated neutrophils. LPS has activating effects on both neutrophils and intrinsic glomerular cells, including induction of the production of neutrophil chemoattractants by glomerular endothelial cells (20). Although, as expected, there was some relatively mild injury induced by the initial LPS and anti-MPO Ab-induced glomerular neutrophil recruitment, MPO-specific T cells caused significant and dose-dependent FNGN, with the highest dose of cells resulting in rapidly progressive renal failure in an antigen-specific manner and premature termination of experiments. These experiments show that the humoral and cellular arms of the autoimmune response to MPO collaborate to induce ANCA-associated vasculitis.
There are several ways by which MPO, an abundant protein in neutrophils, can become lodged in glomeruli in ANCA-associated glomerulonephritis. Although ANCA-induced neutrophil recruitment results in neutrophil degranulation, ANCA also promotes NET formation (30). These NETs are extracellular, include both MPO and Pr3, and are present in glomeruli in ANCA-associated glomerulonephritis. In addition, neutrophil microparticles, which are present in ANCA-associated vasculitis, contain MPO and can localize to endothelial cells (43). It remains to be determined which cell type presents MPO409–428 to effector CD4+ cells in glomeruli, allowing antigen-specific local MPO recognition. Leukocytes traditionally present antigens to effector CD4+ cells. Dendritic cells (rare in glomeruli) or monocyte/macrophages may be important in ingesting MPO present within glomeruli to allow glomerular T-cell recognition of antigen, whereas neutrophils themselves can also express MHC II and present antigens under some circumstances (44). Alternatively, intrinsic glomerular cells could be important, because they can both internalize MPO (45) and be in contact with intracapillary MPO-specific CD4+ T cells. Studies in murine glomerulonephritis induced by a planted foreign antigen support a role for glomerular cells in both antigen recognition and activation of effector CD4+ cells (46–48). Chimeric mice lacking MHC II expression (46) or the costimulatory molecule CD40 (47) in renal tissue cells did not develop severe T cell-mediated glomerulonephritis, and in the same model, glomerular CD80 and CD86 contributed to injury (48).
Both effector Th1 cells and Th17 cells are important in experimental glomerulonephritis induced by a planted foreign antigen (35, 49–51), whereas in autoimmune diseases, cell-mediated injury can be mediated by either Th1 cells, Th17 cells, or both Th1 and Th17 cells (52–54). We have shown that either Th1 cells or Th17 cells can induce injury in glomerulonephritis (35), and evidence exists for the involvement of both Th1 and Th17 cells in human immune renal injury (55, 56). Although some human observational studies and our recent experimental data support a role for Th17 cells and IL-17A in ANCA-associated glomerulonephritis (34, 57, 58), other studies implicate Th1 cells (59). The current studies, in showing that injury can be mediated by IFN-γ–secreting Th1 cells, support a model wherein both Th1 and Th17 cells mediate injury in anti-MPO disease.
The MPO409–428 epitope can also induce MPO-ANCA with some biological activity, because transfer of pooled IgG from MPO409–428-immunized mice with LPS could induce modest glomerular neutrophil recruitment. The target areas for human MPO-ANCA have been identified in regions within the MPO heavy chain (MPO387–745, corresponding to mouse MPO360–718) (31), which include our T-cell epitope, although it is not necessary for autoreactive T-cell and B-cell epitopes to be from similar parts of the autoantigen. Targeted antigen-specific therapy is a long-term aim in treating autoimmune disease. The identification of an important epitope within MPO provides a platform for further work aimed at developing antigen-specific therapies, given the apparently relatively restricted range of autoantigens in microscopic polyangiitis with MPO-ANCA.
When considered with published data on the role of ANCA in disease, our studies demonstrate that tissue injury in microscopic polyangiitis is mediated by the following series of events. Following neutrophil priming and activation by the coordinate action of infection-related signals and MPO-ANCA, neutrophils are recruited to glomeruli. Here, they initiate injury not only by releasing injurious mediators but by depositing MPO, the autoantigen, in glomerular capillaries. The presence of MPO within these small vessels allows effector MPO-specific CD4+ T cells to localize to glomeruli and induce a DTH-like necrotizing glomerulonephritis. Therefore, the pathogenesis of microscopic polyangiitis includes distinct and important roles for both MPO-ANCA–activated neutrophils and autoreactive effector CD4+ T cells that recognize MPO within glomeruli.
Materials and Methods
MPO Peptides, Proteins, and Mice.
Peptide libraries for MPO T-cell epitope screening assays were synthesized as PepSets (Mimotopes). Peptides were 20 aa long and overlapped by 12 aa. Peptide sequences are based on National Center for Biotechnology Information reference NP_034954 (mouse MPO; Table S1). Individual peptide immunization and restimulation assays were performed with >90% pure peptides by HPLC (Mimotopes or AusPep). OVA323–339 was purchased from Auspep. Native mouse MPO was purified from a mouse cell line, 32Dcl3, and recombinant mouse MPO was generated using a baculovirus system, as previously described (60). C57BL/6 and BALB/c mice were obtained from Monash Animal Services, Monash University. Rag1−/−, humanized MHC class II−/− HLA-DRB1*15:01 Tg (33), Mpo−/−, and OT-II mice were bred at Monash Medical Centre Animal Facilities. Male mice, aged 6–8 wk, were used for experiments and kept in specific pathogen-free conditions at Monash Medical Centre Animal Facilities.
Identification of the MPO T-Cell Epitopes.
To identify the T-cell immunogenic regions of MPO, groups of C57BL/6 mice were initially immunized s.c. with peptide pools that correspond to the proregion of the molecule (peptides 1–17), the light chain (peptides 18–31), or one of four quarters (H1–H4) of the heavy chain (peptides 32–46, 47–61, 62–76, and 77–89; 10 μg of peptide per mouse) in Freund’s incomplete adjuvant (Sigma). Spleens were harvested 6 d postimmunization. Splenocytes were stimulated ex vivo with each third of the immunizing pool, and responses were measured by IFN-γ ELISPOT assay. Groups of mice were then immunized s.c. in the base of the tail with one of the peptide pools 7–12, 23–27, 52–56, 57–61, or 62–66 (10 μg of peptide per mouse) in Freund’s complete adjuvant. Draining LN cells were harvested 10 d postimmunization. Finally, C57BL/6 mice were immunized s.c. in the base of tail with one of the five peptides that induced the strongest responses (peptides 10, 24, 52, 57, and 61; 10 μg per mouse) in Freund’s complete adjuvant. Draining LN cells were harvested 10 d postimmunization. Reactivity to individual peptides and to whole recombinant MPO was compared by [3H]-thymidine proliferation and IFN-γ and IL-17A ELISPOT assays.
Murine Model of anti-MPO–Directed Glomerulonephritis.
Mice were immunized s.c. in the base of tail with 100 μg of MPO409–428, OVA323–339, or 40 μg of native mouse MPO in Freund’s complete adjuvant, followed by a similar s.c. injection 7 d later in the back of the neck in Freund’s incomplete adjuvant. Seven days later, mice received an i.v. injection of 1 mg of sheep anti-mouse GBM Ab on 2 d consecutively. Injury was assessed 4 d after the last i.v. injection.
T-Cell Clone Transfer Models.
Each Rag1−/− mouse received 5–50 × 106 (MPO409–428- or OVA323–339-specific cells) T cells, followed by s.c. injection of 100 μg of the cognate peptide in Freund’s complete adjuvant. MPO was deposited in glomeruli 7 d posttransfer by (i) i.v. injection of 1 mg of sheep anti-mouse GBM Ab, (ii) i.v. injection of 150 μg of 8D1 mAb conjugated to MPO409–428, or (iii) i.p. injection of LPS (0.5 μg/g) and i.v. injection of protein G-purified anti-MPO IgG Ab (50 μg/g) derived from immunizing Mpo−/− mice (20). Injury was assessed at day 5 or at day 14 posttransfer. To conjugate MPO409–428 to 8D1 mAb, 5 mg/mL (grown in-house) was reacted with 0.1 mg/mL N-succinimidyl-6-maleimido-caproate (Sigma) for 2 h. MPO409–428 (10 mg/mL) was combined with 8D1 mAb at 10-fold molar excess for 3 h, the reaction was stopped by adding 2 mM cysteine, and unconjugated peptide was removed by dialysis in PBS. Conjugation was confirmed by Western blot using streptavidin-HRP Abs (BD Biosciences).
Additional methods are detailed in SI Materials and Methods.
Statistics.
Where there were three or more groups (the majority of experiments), including experiments across different time points, one-way ANOVA followed by Tukey’s posttest was used to assess differences. Where there were only two groups, a Student t test was used. Means and SEMs are shown (*P < 0.05, **P < 0.01, and ***P < 0.001).
Study Approval.
These studies were conducted in strict accordance with the Australian code of practice for the care and use of animals for scientific purposes by the National Health and Medical Research Council of Australia. Animal studies were approved by the Monash University Animal Ethics Committee.
Supplementary Material
Acknowledgments
We thank Prof. J. Dowling for advice on pulmonary histology; Prof. F. Carbone for the anti-Vα Ab; Dr. C. Lo, A. Li, and C. Lo for technical assistance; and Dr. H. Braley (Commonwealth Serum Laboratories, Australia) for technical advice on 8D1 conjugation. These studies were funded by National Health and Medical Research Council of Australia Program Grant 334067 and Project Grant 1008849.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 15547 (volume 109, number 39).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210147109/-/DCSupplemental.
References
- 1.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 2.Kain R, et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med. 2008;14:1088–1096. doi: 10.1038/nm.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–1523. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 4.Corral-Gudino L, Borao-Cengotita-Bengoa M, Del Pino-Montes J, Lerma-Márquez JL. Overall survival, renal survival and relapse in patients with microscopic polyangiitis: A systematic review of current evidence. Rheumatology (Oxford) 2011;50:1414–1423. doi: 10.1093/rheumatology/ker112. [DOI] [PubMed] [Google Scholar]
- 5.Bosch X, Guilabert A, Espinosa G, Mirapeix E. Treatment of antineutrophil cytoplasmic antibody associated vasculitis: A systematic review. JAMA. 2007;298:655–669. doi: 10.1001/jama.298.6.655. [DOI] [PubMed] [Google Scholar]
- 6.Bansal PJ, Tobin MC. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann Allergy Asthma Immunol. 2004;93:398–401. doi: 10.1016/S1081-1206(10)61400-7. [DOI] [PubMed] [Google Scholar]
- 7.Jayne DR, et al. European Vasculitis Study Group Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180–2188. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 8.Williams JM, et al. Antineutrophil cytoplasm antibody-stimulated neutrophil adhesion depends on diacylglycerol kinase-catalyzed phosphatidic acid formation. J Am Soc Nephrol. 2007;18:1112–1120. doi: 10.1681/ASN.2006090973. [DOI] [PubMed] [Google Scholar]
- 9.Kuligowski MP, et al. Antimyeloperoxidase antibodies rapidly induce alpha-4-integrin-dependent glomerular neutrophil adhesion. Blood. 2009;113:6485–6494. doi: 10.1182/blood-2008-12-192617. [DOI] [PubMed] [Google Scholar]
- 10.Little MA, et al. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood. 2005;106:2050–2058. doi: 10.1182/blood-2005-03-0921. [DOI] [PubMed] [Google Scholar]
- 11.Nolan SL, et al. Mechanisms of ANCA-mediated leukocyte-endothelial cell interactions in vivo. J Am Soc Nephrol. 2008;19:973–984. doi: 10.1681/ASN.2007111166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreiber A, Xiao H, Falk RJ, Jennette JC. Bone marrow-derived cells are sufficient and necessary targets to mediate glomerulonephritis and vasculitis induced by anti-myeloperoxidase antibodies. J Am Soc Nephrol. 2006;17:3355–3364. doi: 10.1681/ASN.2006070718. [DOI] [PubMed] [Google Scholar]
- 13.van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: Molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 14.Xiao H, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao H, et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol. 2005;167(1):39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170(1):52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huugen D, et al. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 2007;71:646–654. doi: 10.1038/sj.ki.5002103. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber A, et al. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20:289–298. doi: 10.1681/ASN.2008050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huugen D, et al. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: Role of tumor necrosis factor-alpha. Am J Pathol. 2005;167(1):47–58. doi: 10.1016/s0002-9440(10)62952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summers SA, et al. Intrinsic renal cell and leukocyte-derived TLR4 aggravate experimental anti-MPO glomerulonephritis. Kidney Int. 2010;78:1263–1274. doi: 10.1038/ki.2010.327. [DOI] [PubMed] [Google Scholar]
- 21.Brouwer E, et al. Predominance of IgG1 and IgG4 subclasses of anti-neutrophil cytoplasmic autoantibodies (ANCA) in patients with Wegener’s granulomatosis and clinically related disorders. Clin Exp Immunol. 1991;83:379–386. doi: 10.1111/j.1365-2249.1991.tb05647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida M, et al. In vitro production of myeloperoxidase anti-neutrophil cytoplasmic antibody and establishment of Th1-type T cell lines from peripheral blood lymphocytes of patients. Clin Exp Rheumatol. 2005;23:227–230. [PubMed] [Google Scholar]
- 23.Ruth AJ, et al. Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:1940–1949. doi: 10.1681/ASN.2006020108. [DOI] [PubMed] [Google Scholar]
- 24.Griffith ME, Coulthart A, Pusey CD. T cell responses to myeloperoxidase (MPO) and proteinase 3 (PR3) in patients with systemic vasculitis. Clin Exp Immunol. 1996;103:253–258. doi: 10.1046/j.1365-2249.1996.d01-629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavele KM, et al. Regulation of myeloperoxidase-specific T cell responses during disease remission in antineutrophil cytoplasmic antibody-associated vasculitis: The role of Treg cells and tryptophan degradation. Arthritis Rheum. 2010;62:1539–1548. doi: 10.1002/art.27403. [DOI] [PubMed] [Google Scholar]
- 26.Brouwer E, Stegeman CA, Huitema MG, Limburg PC, Kallenberg CG. T cell reactivity to proteinase 3 and myeloperoxidase in patients with Wegener’s granulomatosis (WG) Clin Exp Immunol. 1994;98:448–453. doi: 10.1111/j.1365-2249.1994.tb05511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdulahad WH, Kallenberg CG, Limburg PC, Stegeman CA. Urinary CD4+ effector memory T cells reflect renal disease activity in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2009;60:2830–2838. doi: 10.1002/art.24747. [DOI] [PubMed] [Google Scholar]
- 28.Brouwer E, et al. Neutrophil activation in vitro and in vivo in Wegener’s granulomatosis. Kidney Int. 1994;45:1120–1131. doi: 10.1038/ki.1994.149. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham MA, Huang XR, Dowling JP, Tipping PG, Holdsworth SR. Prominence of cell-mediated immunity effectors in “pauci-immune” glomerulonephritis. J Am Soc Nephrol. 1999;10:499–506. doi: 10.1681/ASN.V103499. [DOI] [PubMed] [Google Scholar]
- 30.Kessenbrock K, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erdbrügger U, et al. Mapping of myeloperoxidase epitopes recognized by MPO-ANCA using human-mouse MPO chimers. Kidney Int. 2006;69:1799–1805. doi: 10.1038/sj.ki.5000354. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, et al. Analysis of risk epitopes of anti-neutrophil antibody MPO-ANCA in vasculitis in Japanese population. Microbiol Immunol. 2007;51:1215–1220. doi: 10.1111/j.1348-0421.2007.tb04017.x. [DOI] [PubMed] [Google Scholar]
- 33.Rich C, et al. Myelin oligodendrocyte glycoprotein-35-55 peptide induces severe chronic experimental autoimmune encephalomyelitis in HLA-DR2-transgenic mice. Eur J Immunol. 2004;34:1251–1261. doi: 10.1002/eji.200324354. [DOI] [PubMed] [Google Scholar]
- 34.Gan PY, et al. Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol. 2010;21:925–931. doi: 10.1681/ASN.2009070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Summers SA, et al. Th1 and Th17 cells induce proliferative glomerulonephritis. J Am Soc Nephrol. 2009;20:2518–2524. doi: 10.1681/ASN.2009030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stegeman CA, et al. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994;120(1):12–17. doi: 10.7326/0003-4819-120-1-199401010-00003. [DOI] [PubMed] [Google Scholar]
- 37.de Lind van Wijngaarden RA, et al. Hypotheses on the etiology of antineutrophil cytoplasmic autoantibody associated vasculitis: The cause is hidden, but the result is known. Clin J Am Soc Nephrol. 2008;3:237–252. doi: 10.2215/CJN.03550807. [DOI] [PubMed] [Google Scholar]
- 38.Willcocks LC, Lyons PA, Rees AJ, Smith KG. The contribution of genetic variation and infection to the pathogenesis of ANCA-associated systemic vasculitis. Arthritis Res Ther. 2010;12:202. doi: 10.1186/ar2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odobasic D, Kitching AR, Semple TJ, Holdsworth SR. Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J Am Soc Nephrol. 2007;18:760–770. doi: 10.1681/ASN.2006040375. [DOI] [PubMed] [Google Scholar]
- 40.Kitching AR, et al. The requirement for granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in leukocyte-mediated immune glomerular injury. J Am Soc Nephrol. 2002;13:350–358. doi: 10.1681/ASN.V132350. [DOI] [PubMed] [Google Scholar]
- 41.Kuligowski MP, Kitching AR, Hickey MJ. Leukocyte recruitment to the inflamed glomerulus: A critical role for platelet-derived P-selectin in the absence of rolling. J Immunol. 2006;176:6991–6999. doi: 10.4049/jimmunol.176.11.6991. [DOI] [PubMed] [Google Scholar]
- 42.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 43.Hong Y, et al. Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles. J Am Soc Nephrol. 2012;23:49–62. doi: 10.1681/ASN.2011030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Culshaw S, Millington OR, Brewer JM, McInnes IB. Murine neutrophils present Class II restricted antigen. Immunol Lett. 2008;118(1):49–54. doi: 10.1016/j.imlet.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang JJ, et al. Internalization of proteinase 3 is concomitant with endothelial cell apoptosis and internalization of myeloperoxidase with generation of intracellular oxidants. Am J Pathol. 2001;158:581–592. doi: 10.1016/S0002-9440(10)64000-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S, Kurts C, Köntgen F, Holdsworth SR, Tipping PG. Major histocompatibility complex class II expression by intrinsic renal cells is required for crescentic glomerulonephritis. J Exp Med. 1998;188:597–602. doi: 10.1084/jem.188.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruth AJ, Kitching AR, Semple TJ, Tipping PG, Holdsworth SR. Intrinsic renal cell expression of CD40 directs Th1 effectors inducing experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14:2813–2822. doi: 10.1097/01.asn.0000091381.60059.fb. [DOI] [PubMed] [Google Scholar]
- 48.Odobasic D, et al. Glomerular expression of CD80 and CD86 is required for leukocyte accumulation and injury in crescentic glomerulonephritis. J Am Soc Nephrol. 2005;16:2012–2022. doi: 10.1681/ASN.2004060437. [DOI] [PubMed] [Google Scholar]
- 49.Kitching AR, Holdsworth SR, Tipping PG. IFN-gamma mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J Am Soc Nephrol. 1999;10:752–759. doi: 10.1681/ASN.V104752. [DOI] [PubMed] [Google Scholar]
- 50.Paust HJ, et al. The IL-23/Th17 axis contributes to renal injury in experimental glomerulonephritis. J Am Soc Nephrol. 2009;20:969–979. doi: 10.1681/ASN.2008050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinmetz OM, et al. The Th17-defining transcription factor RORγt promotes glomerulonephritis. J Am Soc Nephrol. 2011;22:472–483. doi: 10.1681/ASN.2010040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 53.Luger D, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holdsworth SR, Kitching AR, Tipping PG. Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999;55:1198–1216. doi: 10.1046/j.1523-1755.1999.00369.x. [DOI] [PubMed] [Google Scholar]
- 56.Kitching AR, Holdsworth SR. The emergence of TH17 cells as effectors of renal injury. J Am Soc Nephrol. 2011;22:235–238. doi: 10.1681/ASN.2010050536. [DOI] [PubMed] [Google Scholar]
- 57.Abdulahad WH, Stegeman CA, Limburg PC, Kallenberg CG. Skewed distribution of Th17 lymphocytes in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 2008;58:2196–2205. doi: 10.1002/art.23557. [DOI] [PubMed] [Google Scholar]
- 58.Nogueira E, et al. 2010. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol Dial Transplant 25:2209–2217.
- 59.Lúdvíksson BR, et al. Active Wegener’s granulomatosis is associated with HLA-DR+ CD4+ T cells exhibiting an unbalanced Th1-type T cell cytokine pattern: Reversal with IL-10. J Immunol. 1998;160:3602–3609. [PubMed] [Google Scholar]
- 60.Apostolopoulos J, Ooi JD, Odobasic D, Holdsworth SR, Kitching AR. The isolation and purification of biologically active recombinant and native autoantigens for the study of autoimmune disease. J Immunol Methods. 2006;308(1–2):167–178. doi: 10.1016/j.jim.2005.10.011. [DOI] [PubMed] [Google Scholar]